Molecular Characterization of Wilson’s Disease in Liver Transplant Patients: A Five-Year Single-Center Experience in Iran
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort and Ethics
2.2. Clinical Diagnosis
2.3. Biochemical Diagnosis
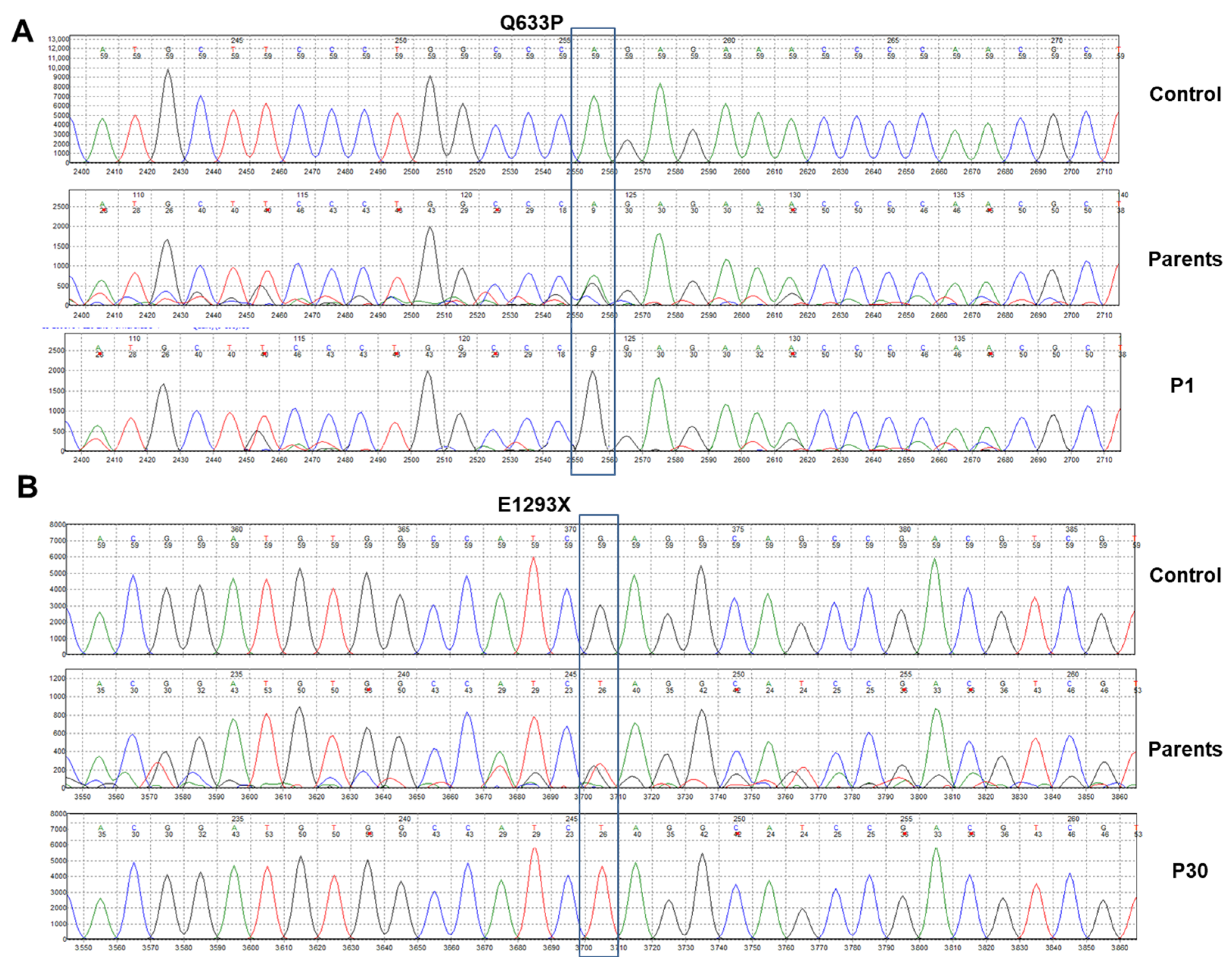
2.4. Mutation Screening in ATP7B
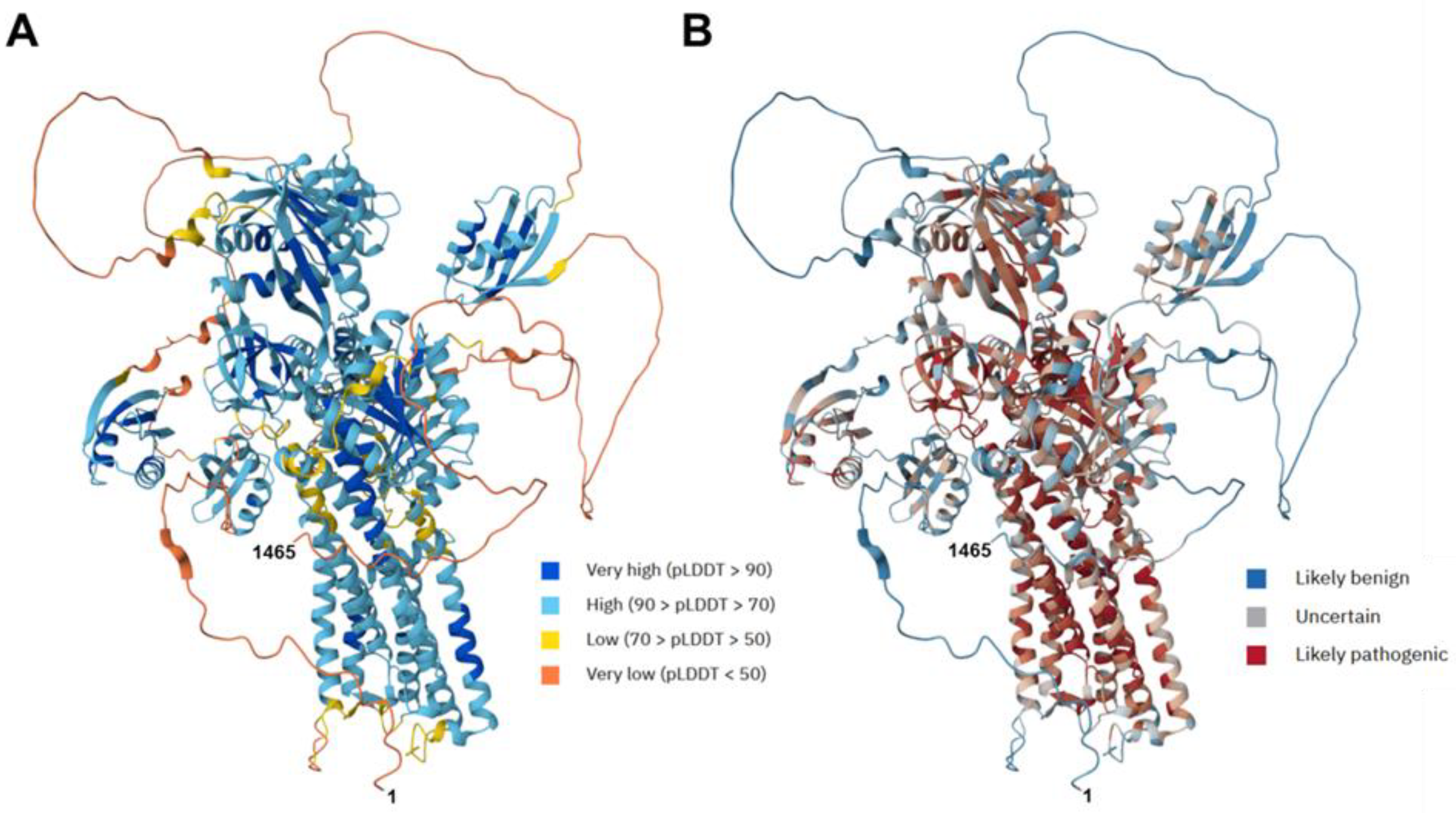
2.5. Protein Modeling
3. Results
3.1. Demographic and Clinical Data
3.2. Biochemical Results
3.3. Genomic Diagnostic Results
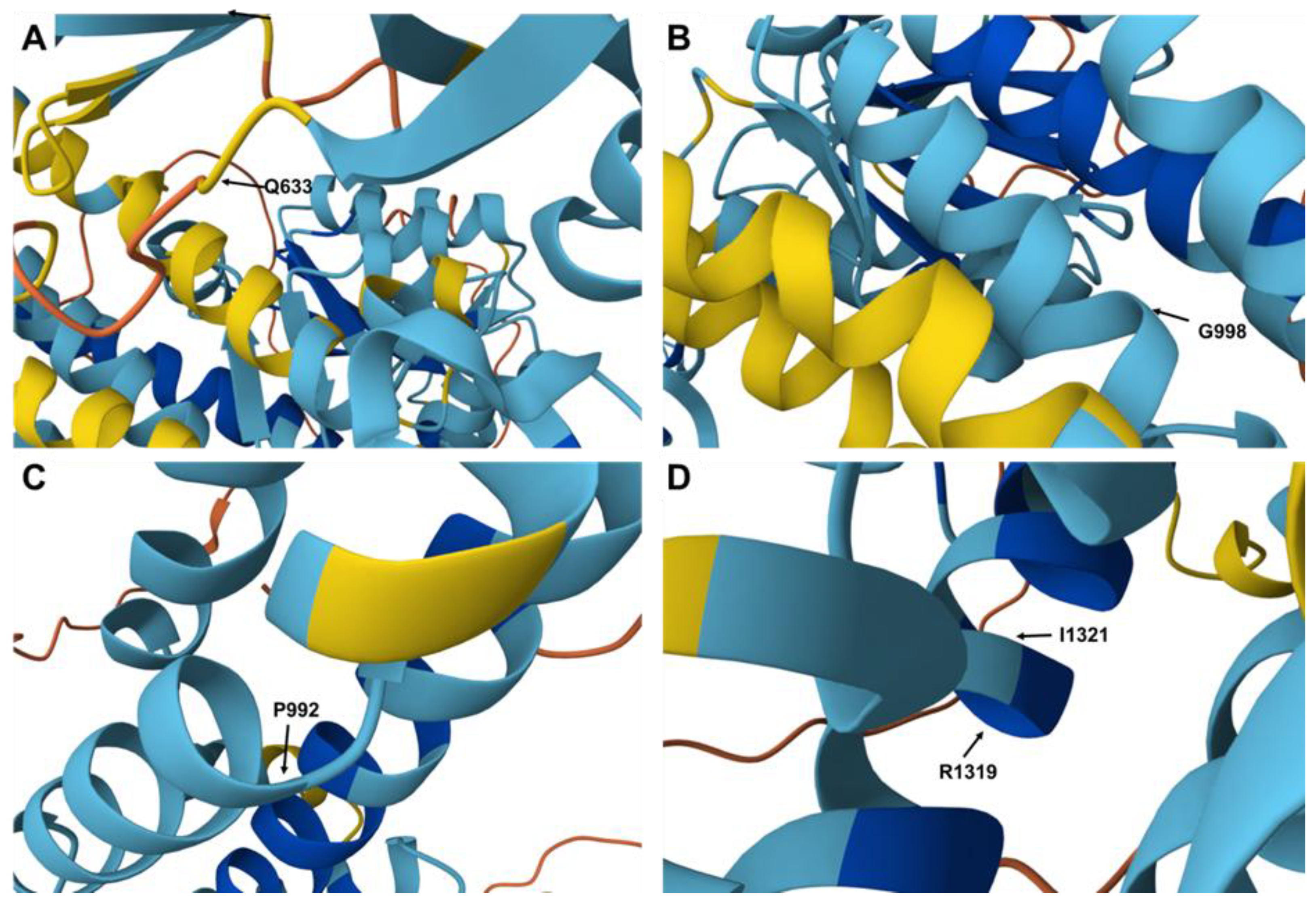
3.4. Structure and Functional Implications of ATP7B (P-Type ATPase): In Silico Analysis
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| ALF | acute hepatic failure |
| AST | aspartate aminotransferase |
| BUN | blood urea nitrogen |
| DDLT | deceased donor liver transplantation |
| LDLT | living donor liver transplantation |
| LT | liver transplantation |
| VUS | variant of uncertain significance |
| WD | Wilson disease |
References
- Ala, A.; Walker, A.P.; Ashkan, K.; Dooley, J.S.; Schilsky, M.L. Wilson’s disease. Lancet 2007, 369, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Bull, P.C.; Thomas, G.R.; Rommens, J.M.; Forbes, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993, 5, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Petrukhin, K.; Fischer, S.G.; Pirastu, M.; Tanzi, R.E.; Chernov, I.; Devoto, M.; Brzustowicz, L.M.; Cayanis, E.; Vitale, E.; Russo, J.J. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat. Genet. 1993, 5, 338–343. [Google Scholar] [CrossRef]
- Sazinsky, M.H.; Mandal, A.K.; Arguello, J.M. Structure of the ATP binding domain from the Archaeoglobus fulgidus Cu+-ATPase. J. Biol. Chem. 2006, 281, 11161–11166. [Google Scholar] [CrossRef]
- Bennett, J.; Hahn, S.H. Clinical molecular diagnosis of Wilson disease. Semin. Liver. Dis. 2011, 31, 233–238. [Google Scholar] [CrossRef]
- Ralle, M.; Huster, D.; Vogt, S. Wilson disease at a single cell level: Intracellular copper trafficking activates compartment-specific responses in hepatocytes. J. Biol. Chem. 2010, 285, 30875–30883. [Google Scholar] [CrossRef]
- Roberts, E.A.; Schilsky, M.L. American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: An update. Hepatology 2008, 47, 2089–2111. [Google Scholar] [CrossRef]
- Schoen, R.E.; Sternlieb, I. Clinical aspects of Wilson’s disease. Am. J. Gastroenterol. 1990, 85, 1453–1457. [Google Scholar] [PubMed]
- Chanpong, A.; Dhawan, A. Wilson disease in children and young adults—State of the art. Saudi J. Gastroenterol. 2022, 28, 21–31. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Bale, G.; Vishnubotla, R.; Rambhatla, A.; Sharma, M.; Sabhapandit, S.; Chintam, A.; Alla, M.; Venishetty, S.; Iyengar, S.; et al. Clinical and Genetic Profile of Pediatric and Adult Wilson’s Disease in India. Gastro Hep Adv. 2025, 4, 100717. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Huang, X.; Ye, Y.; Yang, D.; Xie, L.; Fu, D.; Peng, L.; Zhou, D.; Liao, J. Role of gender and age in features of Wilson’s disease. Front. Neurol. 2023, 14, 1176946. [Google Scholar] [CrossRef]
- The Human Gene Mutation Database. Available online: https://www.hgmd.cf.ac.uk/ac/gene.php?gene=ATP7B (accessed on 18 August 2025).
- Chang, I.J.; Hahn, S.H. The genetics of Wilson disease. Handb. Clin. Neurol. 2017, 142, 19–34. [Google Scholar] [CrossRef]
- Medici, V.; LaSalle, J.M. Genetics and epigenetic factors of Wilson disease. Ann. Transl. Med. 2019, 7, S58. [Google Scholar] [CrossRef]
- Beyzaei, Z.; Mehrzadeh, A.; Hashemi, N.; Geramizadeh, B. The mutation spectrum and ethnic distribution of Wilson disease, a review. Mol. Genet. Metab. Rep. 2023, 38, 101034. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M.; Ansari-Lari, M.; Farhud, D.D. Consanguineous marriage in Iran. Ann. Hum. Biol. 2004, 31, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ghosh, S.; Ray, J.; Ray, K.; Sengupta, M. Missing heritability of Wilson disease: A search for the uncharacterized mutations. Mamm. Genome. 2023, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Socha, P.; Janczyk, W.; Dhawan, A.; Baumann, U.; D’Antiga, L.; Tanner, S.; Iorio, R.; Vajro, P.; Houwen, R.; Fischler, B.; et al. Wilson’s Disease in Children: A Position Paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 334–344. [Google Scholar] [CrossRef]
- Stättermayer, A.F.; Entenmann, A.; Gschwantler, M.; Zoller, H.; Hofer, H.; Ferenci, P. The dilemma to diagnose Wilson disease by genetic testing alone. Eur. J. Clin. Investig. 2019, 49, e13147. [Google Scholar] [CrossRef]
- Espinós, C.; Ferenci, P. Are the new genetic tools for diagnosis of Wilson disease helpful in clinical practice? JHEP Rep. 2020, 2, 100114. [Google Scholar] [CrossRef]
- Ferenci, P.; Steindl-Munda, P.; Vogel, W.; Jessner, W.; Gschwantler, M.; Stauber, R.; Datz, C.; Hackl, F.; Wrba, F.; Bauer, P.; et al. Diagnostic value of quantitative hepatic copper determination in patients with Wilson’s Disease. Clin. Gastroenterol. Hepatol. 2005, 3, 811–818. [Google Scholar] [CrossRef]
- Ryan, A.; Nevitt, S.J.; Tuohy, O.; Cook, P. Biomarkers for diagnosis of Wilson’s disease. Cochrane. Database. Syst. Rev. 2019, 11, CD012267. [Google Scholar] [CrossRef] [PubMed]
- Caprai, S.; Loudianos, G.; Massei, F.; Gori, L.; Lovicu, M.; Maggiore, G. Direct diagnosis of Wilson disease by molecular genetics. J. Pediatr. 2006, 148, 138–140. [Google Scholar] [CrossRef]
- Ferenci, P. Regional distribution of mutations of the ATP7B gene in patients with Wilson disease—impact on genetic testing. Hum. Genet. 2006, 120, 151–159. [Google Scholar] [CrossRef]
- Gomes, A.; Dedoussis, G.V. Geographic distribution of ATP7B mutations in Wilson disease. Ann. Hum. Biol. 2016, 43, 1–8. [Google Scholar] [CrossRef]
- Fattahi, Z.; Beheshtian, M.; Mohseni, M.; Poustchi, H.; Sellars, E.; Nezhadi, S.H.; Amini, A.; Arzhangi, S.; Jalalvand, K.; Jamali, P.; et al. Iranome: A catalog of genomic variations in the Iranian population. Hum. Mutat. 2019, 40, 1968–1984. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]
- Den Dunnen, J.T.; Antonarakis, S.E. Mutation nomenclature extensions and suggestions to describe complex mutations: A discussion. Hum. Mutat. 2000, 15, 7–12. [Google Scholar] [CrossRef]
- AlphaFold Protein Structure Database. Available online: https://alphafold.ebi.ac.uk/ (accessed on 18 August 2025).
- Mariani, V.; Biasini, M.; Barbato, A.; Schwede, T. lDDT: A local superposition-free score for comparing protein structures and models using distance difference tests. Bioinformatics 2013, 29, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Novati, G.; Pan, J.; Bycroft, C.; Žemgulytė, A.; Applebaum, T.; Pritzel, A.; Wong, L.H.; Zielinski, M.; Sargeant, T.; et al. Accurate proteome-wide missense variant effect prediction with AlphaMissense. Science 2023, 381, eadg7492. [Google Scholar] [CrossRef]
- WilsonGen. Available online: https://clingen.igib.res.in/WilsonGen/ (accessed on 18 August 2025).
- ClinVar. Available online: https://www.ncbi.nlm.nih.gov/clinvar/ (accessed on 18 August 2025).
- GnomAD. Available online: https://gnomad.broadinstitute.org/ (accessed on 18 August 2025).
- NIH. National Library of Medicine. Homo Sapiens ATPase Copper Transporting Beta (ATP7B9), Transcript Variant 1, mRNA. Available online: https://www.ncbi.nlm.nih.gov/nuccore/NM_000053.4 (accessed on 18 August 2025).
- Seo, J.K. Diagnosis of Wilson disease in young children: Molecular genetic testing and a paradigm shift from the laboratory diagnosis. Pediatr. Gastroenterol. Hepatol. Nutr. 2012, 15, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shen, B.; Xiao, J.J.; Wu, R.; Duff Canning, S.J.; Wang, X.P. Currently Clinical Views on Genetics of Wilson’s Disease. Chin. Med. J. 2015, 128, 1826–1830. [Google Scholar] [CrossRef]
- Litwin, T.; Bembenek, J.; Antos, A.; Przybyłkowski, A.; Skowrońska, M.; Kurkowska-Jastrzębska, I.; Członkowska, A. Liver transplantation as a treatment for Wilson’s disease with neurological presentation: A systematic literature review. Acta. Neurol. Belg. 2022, 122, 505–518. [Google Scholar] [CrossRef]
- Immergluck, J.; Grant, L.M.; Anilkumar, A.C. Wilson Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Beyzaei, Z.; Ghatei, K.; Shamsaeefar, A.; Geramizadeh, B. Liver transplantation in Wilson disease: A single-center experience. Orphanet. J. Rare. Dis. 2025, 20, 292. [Google Scholar] [CrossRef]
- Xu, W.Q.; Wang, R.M.; Dong, Y.; Wu, Z.Y. Emerging neurological symptoms after liver transplantation: A 6-year follow-up of an adolescent patient with Wilson’s disease. CNS Neurosci. Ther. 2022, 28, 788–791. [Google Scholar] [CrossRef]
- Yagci, M.A.; Tardu, A.; Karagul, S. Influence of liver transplantation on neuropsychiatric manifestations of Wilson disease. Transplant. Proc. 2015, 47, 1469–1473. [Google Scholar] [CrossRef]
- Olsson, K.S.; Wålinder, O.; Kindmark, A.; Williams, R. Common local founder effects for Wilson’s disease and hereditary hemochromatosis; mutation studies of a large family. Scand. J. Gastroenterol. 2012, 47, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Khdair Ahmad, F.; Aburizeg, D.; Rayyan, Y.; Tamimi, T.A.; Burayzat, S.; Ghanma, A.; Barbar, M.; Azab, B. Genetic profiling of Wilson disease reveals a potential recurrent pathogenic variant of ATP7B in the Jordanian population. J. Pediatr. Gastroenterol. Nutr. 2025, 80, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Kalinsky, H.; Funes, A.; Zeldin, A.; Pel-Or, Y.; Korostishevsky, M.; Gershoni-Baruch, R.; Farrer, L.A.; Bonne-Tamir, B. Novel ATP7B mutations causing Wilson disease in several Israeli ethnic groups. Hum. Mutat. 1998, 11, 145–151. [Google Scholar] [CrossRef]
- Calvo, J.S.; Heger, T.; Kabin, E.; Mowrey, W.R.; Del Angel, G.; Ding, W.; Lutsenko, S. Functional Screen of Wilson Disease ATP7B Variants Reveals Residual Transport Activities. Hum. Mutat. 2025, 2025, 7485658. [Google Scholar] [CrossRef] [PubMed]
- Guttmann, S.; Bernick, F.; Naorniakowska, M.; Michgehl, U.; Groba, S.R.; Socha, P.; Zibert, A.; Schmidt, H.H. Functional Characterization of Novel ATP7B Variants for Diagnosis of Wilson Disease. Front. Pediatr. 2018, 6, 106. [Google Scholar] [CrossRef]

| Variable | Overall Patients (n = 20) |
|---|---|
| Gender (M/F) | 13/7 |
| Current Mean age (years) | 27.1 ± 0.6 |
| Mean age at transplantation (years) | 21.2 ± 4.5 |
| Parental consanguinity n (%) | 8 (40) |
| Type of donor, n (%) | |
| Deceased donor (whole) | 14 (70%) |
| Living donor (partial) | 6 (30%) |
| Outcome | |
| Alive | 12 (60%) |
| Dead | 8 (40%) |
| Main clinical presentations, n | |
| Hepatological | Liver cirrhosis 9, Jaundice 5, Acute liver injury 2 |
| Neurological | Tremor 2, Ataxia 2 |
| Psychiatric | - |
| Chelation therapy, n (%) | |
| Penicillamine | 17 (85) |
| Trientine | 3 (15) |
| Follow-up duration (years) | 3.5 ± 0.6 |
| Parameter | Pre-Transplant (Mean ± SD) | Post-Transplant (Mean ± SD) |
|---|---|---|
| ALT (U/L) | 90 ± 65 | 32 ± 21 |
| AST (U/L) | 95 ± 70 | 28 ± 18 |
| BUN (mg/dL) | 12 ± 3 | 13 ± 4 |
| CREA (mg/dL) | 0.9 ± 0.5 | 1.0 ± 0.4 |
| ALB (g/dL) | 3.2 ± 0.9 | 4.2 ± 0.4 |
| SCER | 0.49 ± 0.80 | - |
| Urine copper (µg/24 h) | 350 ± 200 | – |
| Patients/ Gender | Region of Protein * | Chr: Loc (hg19) | Exome/ Intron | Nucleotide Change | Protein Change | Variant Type | Zygosity | Previous Definition and Pathogenicity in HGMD | WilsonGen [32] | ClinVar [33] | gnomAD (Allele Frequency) [34] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1/M | - | 13:52536021 | E5 | c.1898A > C | p.Q633P | Missense | Homozygous | ND, P, Da | ND | ND | ND |
| P2/M | - TM4 | 13:52532554 13:52532491 | E8 E8 | c.2248G > A c.2311T > G | p.V750I p.F771V | Missense Missense | Heterozygous Heterozygous | ND, B, Da ND, P, Da | ND ND | ND ND | ND ND |
| P4/F | COOH | 13:52508954 | E21 | c.4336G > T | p.D1446Y | Missense | Homozygous | ND, B | ND | ND | ND |
| P5/M | TM6 TM6 | 13:52520506 13:52520488 | E13 E13 | c.2974C > T c.2992G > T | p.P992S p.G998C | Missense Missense | Heterozygous Heterozygous | ND, LP, Da ND, LP, Da | ND ND | ND ND | ND ND |
| P16/M | - | 13:52524464 | E10 | c.2519C > T | p.P840L | Missense | Homozygous | D, P, Da | P | P | P, 6.20 × 10−7 |
| P18/M | HMA5 - | 13:52542642 13:52524258 | E5 E11 | c.1645C > G c.2615T > A | p.L549V p.V872E | Missense Missense | Heterozygous Heterozygous | D, P, Da ND, P, Da | ND ND | ND ND | ND ND |
| P23/M | - | 13:52548673 | E2 | c.683A > G | p.Q228R | Missense | Homozygous | ND, B | ND | ND | ND |
| P24/M | HMA5 | 13:52542642 | E5 | c.1645C > G | p.L549V | Missense | Homozygous | D, P, Da | ND | ND | ND |
| P30/F | - | 13:52511638 | E18 | c.3877G > T | p.E1293X | Nonsense | Homozygous | ND, P, Da | ND | ND | ND |
| P31/M | - TM7 | 13:52524258 13:52511471 | E11 E19 | c.2614G > A c.3962T > G | p.V872E p.I1321T | Missense Missense | Heterozygous Heterozygous | ND, P, Da ND, P, Da | ND ND | ND ND | ND ND |
| P32/F | TM3 | 13:52532610 | E8 | c.2192T > A | p.V731E | Missense | Homozygous | D, LP, Da | ND | ND | ND |
| P33/M | - | 13:52516689 | E14 | c.3245A > C | p.E1082A | Missense | Homozygous | ND, P, Da | ND | ND | ND |
| P36/F | - | 13:52548763 | E2 | c.593G > A | p.R198G | Missense | Homozygous | D, P, Da | ND | ND | ND |
| P37/M | - | 13:52524258 | E11 | c.2614T > A | p.V872E | Missense | Homozygous | ND, P, Da | ND | ND | ND |
| P39/F | - | 13:52548596 | E2 | c.760A > C | p.S254R | Missense | Homozygous | ND, B | ND | ND | ND |
| P40/F | - | 13:51937342 | E19 | c.3955C > T | p.R1319X | Nonsense | Homozygous | D, P, Da | P | P | P, 1.40 × 10−4 |
| P41/M | - HMA3 | 13:52509813 13:52548560 | E19 E2 | c.4040G > T c.953A > C | p.G1347V p.M266L | Missense Missense | Heterozygous Heterozygous | ND, P, Da ND, P, Da | ND ND | ND ND | ND ND |
| P45/M | - | 13:52532542 | E8 | c.2260G > A | p.E754K | Missense | Homozygous | D, P, Da | VUS | VUS | VUS |
| P49/M | TM6 | 13:52520505 | E13 | c.2975C > T | p.P992L | Missense | Homozygous | D, P, Da | LP | LB | LB, 6.20 × 10−7 |
| P52/M | - | 13:52548977 | E2 | c.379G > A | p. E127K | Missense | Homozygous | ND, B | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyzaei, Z.; Majed, M.; Dehghani, S.M.; Imanieh, M.H.; Khazaee, A.; Geramizadeh, B.; Weiskirchen, R. Molecular Characterization of Wilson’s Disease in Liver Transplant Patients: A Five-Year Single-Center Experience in Iran. Diagnostics 2025, 15, 2504. https://doi.org/10.3390/diagnostics15192504
Beyzaei Z, Majed M, Dehghani SM, Imanieh MH, Khazaee A, Geramizadeh B, Weiskirchen R. Molecular Characterization of Wilson’s Disease in Liver Transplant Patients: A Five-Year Single-Center Experience in Iran. Diagnostics. 2025; 15(19):2504. https://doi.org/10.3390/diagnostics15192504
Chicago/Turabian StyleBeyzaei, Zahra, Melika Majed, Seyed Mohsen Dehghani, Mohammad Hadi Imanieh, Ali Khazaee, Bita Geramizadeh, and Ralf Weiskirchen. 2025. "Molecular Characterization of Wilson’s Disease in Liver Transplant Patients: A Five-Year Single-Center Experience in Iran" Diagnostics 15, no. 19: 2504. https://doi.org/10.3390/diagnostics15192504
APA StyleBeyzaei, Z., Majed, M., Dehghani, S. M., Imanieh, M. H., Khazaee, A., Geramizadeh, B., & Weiskirchen, R. (2025). Molecular Characterization of Wilson’s Disease in Liver Transplant Patients: A Five-Year Single-Center Experience in Iran. Diagnostics, 15(19), 2504. https://doi.org/10.3390/diagnostics15192504







