The Association Between Naples Prognostic Score and Coronary Collateral Circulation in Patients with Chronic Coronary Total Occlusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Data Collection and Variables
2.6. Calculation of the Naples Prognostic Score (NPS)
- Serum albumin (≥4 g/dL scored as 0; <4 g/dL scored as 1),
- Total cholesterol (TC) (≥180 mg/dL scored as 0; <180 mg/dL scored as 1),
- Neutrophil-to-lymphocyte ratio (NLR) (≤2.96 scored as 0; >2.96 scored as 1),
- Lymphocyte-to-monocyte ratio (LMR) (>4.44 scored as 0; ≤4.44 scored as 1).
2.7. Assessment of Coronary Collateral Circulation
- Grade 0: No visible collateral vessels,
- Grade 1: Filling of side branches of the artery to be perfused without visualization of the epicardial segment,
- Grade 2: Partial filling of the epicardial segment via collateral channels,
- Grade 3: Complete filling of the epicardial segment of the occluded artery [23].
3. Statistical Analysis
4. Results
5. Discussion
5.1. Future Perspectives
- Prospective, multicenter studies are necessary to incorporate systemic biomarkers, such as the NPS and HDL-C, into comprehensive risk prediction models alongside angiographic and clinical variables.
- Mechanistic studies examining the influence of inflammation, nutrition, and lipid metabolism on arteriogenesis could clarify causal pathways. Therapeutic interventions that target these mechanisms also merit investigation.
- For instance, structured exercise programs and anti-inflammatory therapies could enhance collateral growth by improving endothelial function, and dietary and metabolic strategies could optimize nutritional and lipid profiles. Additionally, emerging approaches focused on HDL functionality, such as interventions designed to increase cholesterol efflux capacity, should be evaluated for their ability to promote vascular remodeling.
- Finally, the integration of advanced imaging and hemodynamic techniques, including quantitative perfusion imaging and invasive indices of collateral flow, could enable a more precise assessment of collateral function and response to therapy. These research directions show promise in translating biomarker-based risk assessment into personalized therapeutic strategies for CTO patients.
5.2. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fefer, P.; Knudtson, M.L.; Cheema, A.N.; Galbraith, P.D.; Osherov, A.B.; Yalonetsky, S.; Gannot, S.; Samuel, M.; Weisbrod, M.; Bierstone, D.; et al. Current perspectives on coronary chronic total occlusions: The Canadian Multicenter Chronic Total Occlusions Registry. J. Am. Coll. Cardiol. 2012, 59, 991–997. [Google Scholar] [CrossRef]
- Jeroudi, O.M.; Alomar, M.E.; Michael, T.T.; El Sabbagh, A.; Patel, V.G.; Mogabgab, O.; Fuh, E.; Sherbet, D.; Lo, N.; Roesle, M.; et al. Prevalence and management of coronary chronic total occlusions in a tertiary Veterans Affairs hospital. Catheter. Cardiovasc. Interv. 2014, 84, 637–643. [Google Scholar] [CrossRef]
- Christakopoulos, G.E.; Christopoulos, G.; Carlino, M.; Jeroudi, O.M.; Roesle, M.; Rangan, B.V.; Abdullah, S.; Grodin, J.; Kumbhani, D.J.; Vo, M.; et al. Meta-analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am. J. Cardiol. 2015, 115, 1367–1375. [Google Scholar] [CrossRef]
- Simsek, B.; Kostantinis, S.; Karacsonyi, J.; Alaswad, K.; Megaly, M.; Karmpaliotis, D.; Masoumi, A.; Jaber, W.A.; Nicholson, W.; Rinfret, S.; et al. A Systematic Review and Meta-Analysis of Clinical Outcomes of Patients Undergoing Chronic Total Occlusion Percutaneous Coronary Intervention. J. Invasive Cardiol. 2022, 34, E763–E775. [Google Scholar] [CrossRef]
- Seiler, C.; Stoller, M.; Pitt, B.; Meier, P. The human coronary collateral circulation: Development and clinical importance. Eur. Heart J. 2013, 34, 2674–2682. [Google Scholar] [CrossRef]
- Regieli, J.J.; Jukema, J.W.; Nathoe, H.M.; Zwinderman, A.H.; Ng, S.; Grobbee, D.E.; van der Graaf, Y.; Doevendans, P.A. Coronary collaterals improve prognosis in patients with ischemic heart disease. Int. J. Cardiol. 2009, 132, 257–262. [Google Scholar] [CrossRef]
- Choo, G.H. Collateral circulation in chronic total occlusions—An interventional perspective. Curr. Cardiol. Rev. 2015, 11, 277–284. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.Y.; Kim, N.; Jang, S.Y.; Bae, M.H.; Yang, D.H.; Cho, Y.; Chae, S.C.; Park, H.S. Coronary Collaterals Function and Clinical Outcome Between Patients with Acute and Chronic Total Occlusion. JACC Cardiovasc. Interv. 2017, 10, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.S.; Wang, Y.X.; Wu, Z.M.; Ding, F.H.; Lu, L.; Shen, W.F.; Dai, Y.; Shen, Y. Elevated systemic immune-inflammatory index predicts poor coronary collateralization in type 2 diabetic patients with chronic total occlusion. Front. Cardiovasc. Med. 2024, 11, 1490498. [Google Scholar] [CrossRef] [PubMed]
- Adali, M.K.; Buber, I.; Sen, G.; Yilmaz, S. Relationship Between Systemic Immune-Inflammation Index and Coronary Collateral Circulation in Patients with Chronic Total Occlusion. Arq. Bras. Cardiol. 2022, 119, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Galizia, G.; Lieto, E.; Auricchio, A.; Cardella, F.; Mabilia, A.; Podzemny, V.; Castellano, P.; Orditura, M.; Napolitano, V. Naples Prognostic Score, Based on Nutritional and Inflammatory Status, Is an Independent Predictor of Long-Term Outcome in Patients Undergoing Surgery for Colorectal Cancer. Dis. Colon. Rectum. 2017, 60, 1273–1284. [Google Scholar] [CrossRef]
- Galizia, G.; Auricchio, A.; de Vita, F.; Cardella, F.; Mabilia, A.; Basile, N.; Orditura, M.; Lieto, E. Inflammatory and nutritional status is a predictor of long-term outcome in patients undergoing surgery for gastric cancer. Validation of the Naples prognostic score. Ann. Ital. Chir. 2019, 90, 404–416. [Google Scholar]
- Lieto, E.; Auricchio, A.; Tirino, G.; Pompella, L.; Panarese, I.; Del Sorbo, G.; Ferraraccio, F.; De Vita, F.; Galizia, G.; Cardella, F. Naples Prognostic Score Predicts Tumor Regression Grade in Resectable Gastric Cancer Treated with Preoperative Chemotherapy. Cancers 2021, 13, 4676. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Lu, W.; Peng, Y.; Chen, L.; Lin, Y. The Prognostic Role of Naples Prognostic Score in Patients with Coronary Artery Disease. J. Inflamm. Res. 2025, 18, 6999–7012. [Google Scholar] [CrossRef] [PubMed]
- Nacar, A.B.; Erayman, A.; Kurt, M.; Buyukkaya, E.; Karakas, M.F.; Akcay, A.B.; Buyukkaya, S.; Sen, N. The relationship between coronary collateral circulation and neutrophil/lymphocyte ratio in patients with coronary chronic total occlusion. Med. Princ. Pract. 2015, 24, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Aktas, I.; Bolayir, H.A.; Karasu, M. Neutrophil Percentage-to-Albumin Ratio as a Predictor of Collateral Circulation in Chronic Total Occlusion. Anatol. J. Cardiol. 2025, 29, 489–495. [Google Scholar] [CrossRef]
- Saygi, M.; Tanalp, A.C.; Tezen, O.; Pay, L.; Dogan, R.; Uzman, O.; Karabay, C.Y.; Tanboga, I.H.; Kacar, F.O.; Karagoz, A. The prognostic importance of the Naples prognostic score for in-hospital mortality in patients with ST-segment elevation myocardial infarction. Coron. Artery Dis. 2024, 35, 31–37. [Google Scholar] [CrossRef]
- Keskin, B.; Hakgor, A.; Olgun, F.E.; Duman, A.B.; Cakal, B.; Tanyeri, S.; Kultursay, B.; Yildiz, C.E.; Dervis, E.; Karaca, I.O.; et al. Prognostic impact of Naples prognostic score on long-term mortality in non-ST-elevation myocardial infarction. Biomark. Med. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, W.; Shi, L.; Pei, M.; Zhou, Y.; Wei, Y.; Tang, Y.; Qiu, G.; Duan, W.; Chen, S.; et al. Impact of the Naples Prognostic Score at admission on long-term prognosis among patients with coronary artery disease. Front. Immunol. 2025, 16, 1529779. [Google Scholar] [CrossRef]
- Mao, L.S.; Geng, L.; Wang, Y.X.; Qi, Y.; Wang, M.H.; Ding, F.H.; Dai, Y.; Lu, L.; Zhang, Q.; Shen, W.F.; et al. Clinical risk score to predict poor coronary collateralization in type 2 diabetic patients with chronic total occlusion. BMC Cardiovasc. Disord. 2025, 25, 250. [Google Scholar] [CrossRef]
- Saylik, F.; Cinar, T.; Sarikaya, R.; Akbulut, T.; Selcuk, M.; Ozbek, E.; Tanboga, H.I. The association of serum uric acid/albumin ratio with the development of coronary collateral circulation in patients with chronic total occluded coronary arteries. J. Cardiovasc. Thorac. Res. 2023, 15, 14–21. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Sandoval, Y.; Azzalini, L.; Leibundgut, G.; Garbo, R.; Hall, A.B.; Davies, R.E.; Mashayekhi, K.; Yamane, M.; Avran, A.; et al. Chronic Total Occlusion Percutaneous Coronary Intervention: Present and Future. Circ. Cardiovasc. Interv. 2025, 18, e014801. [Google Scholar] [CrossRef]
- Rentrop, K.P.; Cohen, M.; Blanke, H.; Phillips, R.A. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J. Am. Coll. Cardiol. 1985, 5, 587–592. [Google Scholar] [CrossRef]
- Stoller, M.; Seiler, C. Salient features of the coronary collateral circulation and its clinical relevance. Swiss Med. Wkly. 2015, 145, w14154. [Google Scholar] [CrossRef]
- Meier, P.; Hemingway, H.; Lansky, A.J.; Knapp, G.; Pitt, B.; Seiler, C. The impact of the coronary collateral circulation on mortality: A meta-analysis. Eur. Heart J. 2012, 33, 614–621. [Google Scholar] [CrossRef]
- Allahwala, U.K.; Nour, D.; Bhatia, K.; Ward, M.R.; Lo, S.; Weaver, J.C.; Bhindi, R. Prognostic impact of collaterals in patients with a coronary chronic total occlusion: A meta-analysis of over 3000 patients. Catheter. Cardiovasc. Interv. 2021, 97, E771–E777. [Google Scholar] [CrossRef] [PubMed]
- Gitmez, M.; Ekingen, E.; Zaman, S. Predictive Value of the Naples Prognostic Score for One-Year Mortality in NSTEMI Patients Undergoing Selective PCI. Diagnostics 2025, 15, 640. [Google Scholar] [CrossRef]
- Kerner, A.; Gruberg, L.; Goldberg, A.; Roguin, A.; Lavie, P.; Lavie, L.; Markiewicz, W.; Beyar, R.; Aronson, D. Relation of C-reactive protein to coronary collaterals in patients with stable angina pectoris and coronary artery disease. Am. J. Cardiol. 2007, 99, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, H.; Gunturk, E.E.; Akkaya, F.; Karabiyik, U.; Gunturk, I.; Yilmaz, S. Assessment of the Relationship Between the Adropin Levels and the Coronary Collateral Circulation in Patients with Chronic Coronary Syndrome. Arq. Bras. Cardiol. 2022, 119, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Esenboga, K.; Kurtul, A.; Yamanturk, Y.Y.; Kozluca, V.; Tutar, E. Prognostic Nutritional Index is Associated with the Degree of Coronary Collateral Circulation in Stable Angina Patients with Chronic Total Occlusion. Arq. Bras. Cardiol. 2024, 121, e20230765. [Google Scholar] [CrossRef]
- Chen, L.; Ma, C.; Xiao, H.; Kong, F. Association between NPS and all-cause and cardiovascular mortality in US adults with diabetes mellitus. Nutr. Hosp. 2025, 42, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.S.; Aydemir, S.; Ozmen, M.; Aksakal, E.; Sarac, I.; Aydinyilmaz, F.; Altinkaya, O.; Birdal, O.; Tanboga, I.H. The importance of Naples prognostic score in predicting long-term mortality in heart failure patients. Ann. Med. 2025, 57, 2442536. [Google Scholar] [CrossRef] [PubMed]
- Dundar, C.; Tanalp, A.C.; Yagmur, A.; Karaaslan, M.B. The impact of the Naples prognostic score in long-term outcomes after percutaneous coronary intervention for chronic total occlusions. Coron. Artery Dis. 2025, 36, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.M.; Ann, S.J.; Kang, M.; Cheon, E.J.; An, D.B.; Choi, Y.R.; Lee, C.J.; Oh, J.; Park, S.; et al. Cholesterol Efflux and Collateral Circulation in Chronic Total Coronary Occlusion: Effect-Circ Study. J. Am. Heart Assoc. 2021, 10, e019060. [Google Scholar] [CrossRef]
- Lee, J.J.; Chi, G.; Fitzgerald, C.; Kazmi, S.H.A.; Kalayci, A.; Korjian, S.; Duffy, D.; Shaunik, A.; Kingwell, B.; Yeh, R.W.; et al. Cholesterol Efflux Capacity and Its Association with Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 774418. [Google Scholar] [CrossRef]
- Dong, S.; Qiao, J.; Gao, A.; Zhao, Z.; Huang, X.; Kan, Y.; Yang, Z.; Ma, M.; Fan, C.; Han, H.; et al. Association between the atherogenic index of plasma and coronary collateral circulation in patients with chronic total occlusion. BMC Cardiovasc. Disord. 2024, 24, 360. [Google Scholar] [CrossRef]
| Variable | Poor Collateral (n = 208, 64.2%) | Good Collateral (n = 116, 35.8%) | p-Value |
|---|---|---|---|
| Age (years) | 63.51 ± 10.39 | 62.26 ± 8.8 | 0.275 |
| Gender (male, %) | 84.6% | 83.6% | 0.814 |
| BMI (kg/m2) | 26.92 (24.95–29.36) | 27.58 (26.14–30.01) | 0.04 ** |
| WBC (103/µL) | 8.29 (6.90–10.25) | 8.50 (7.21–9.98) | 0.076 |
| Hb (g/dL) | 13.51 ± 1.96 | 13.92 ± 1.89 | 0.07 |
| GFR (mL/min/1.73m2) | 89.09 (70.51–100.2) | 84.66 (69.07–98.33) | 0.31 |
| Total Cholesterol (mg/dL) | 152.5 (130–182) | 160.1 (131–204.53) | 0.384 |
| LDL (mg/dL) | 84.30 (64–106.75) | 82.6 (64.1–119.86) | 0.970 |
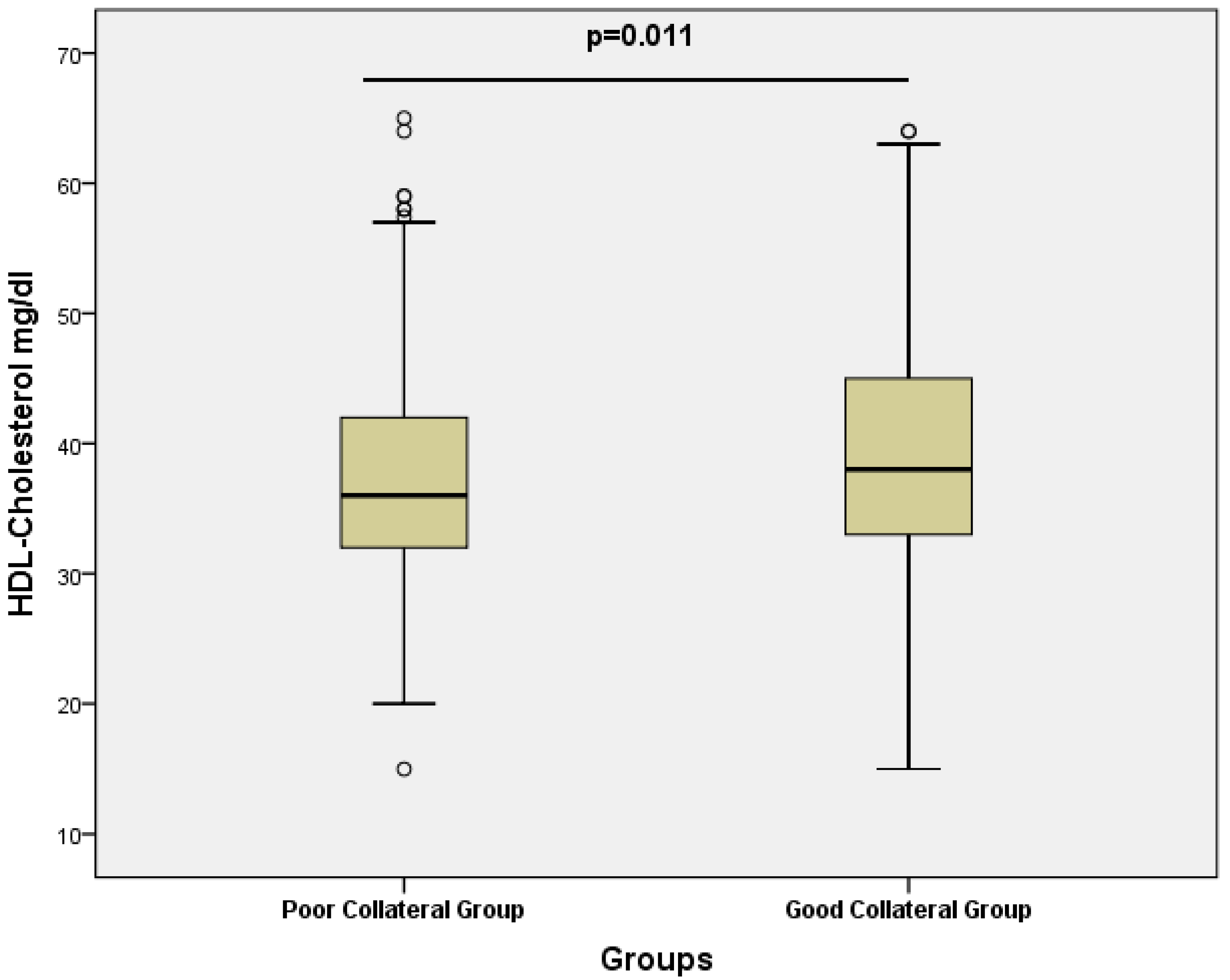
| HDL (mg/dL) | 36 (32–42) | 38 (33–45) | 0.042 ** |
| Triglycerides (mg/dL) | 147.5 (105–197.5) | 165 (116.25–228.5) | 0.040 ** |
| CRP (mg/L) | 4.47 (3–7) | 4.12 (3–6.95) | 0.792 |
| Systolic BP (mmHg) | 110 (100–120) | 110 (110–120) | 0.364 |
| Diastolic BP (mmHg) | 70 (65–80) | 70 (70–75) | 0.741 |
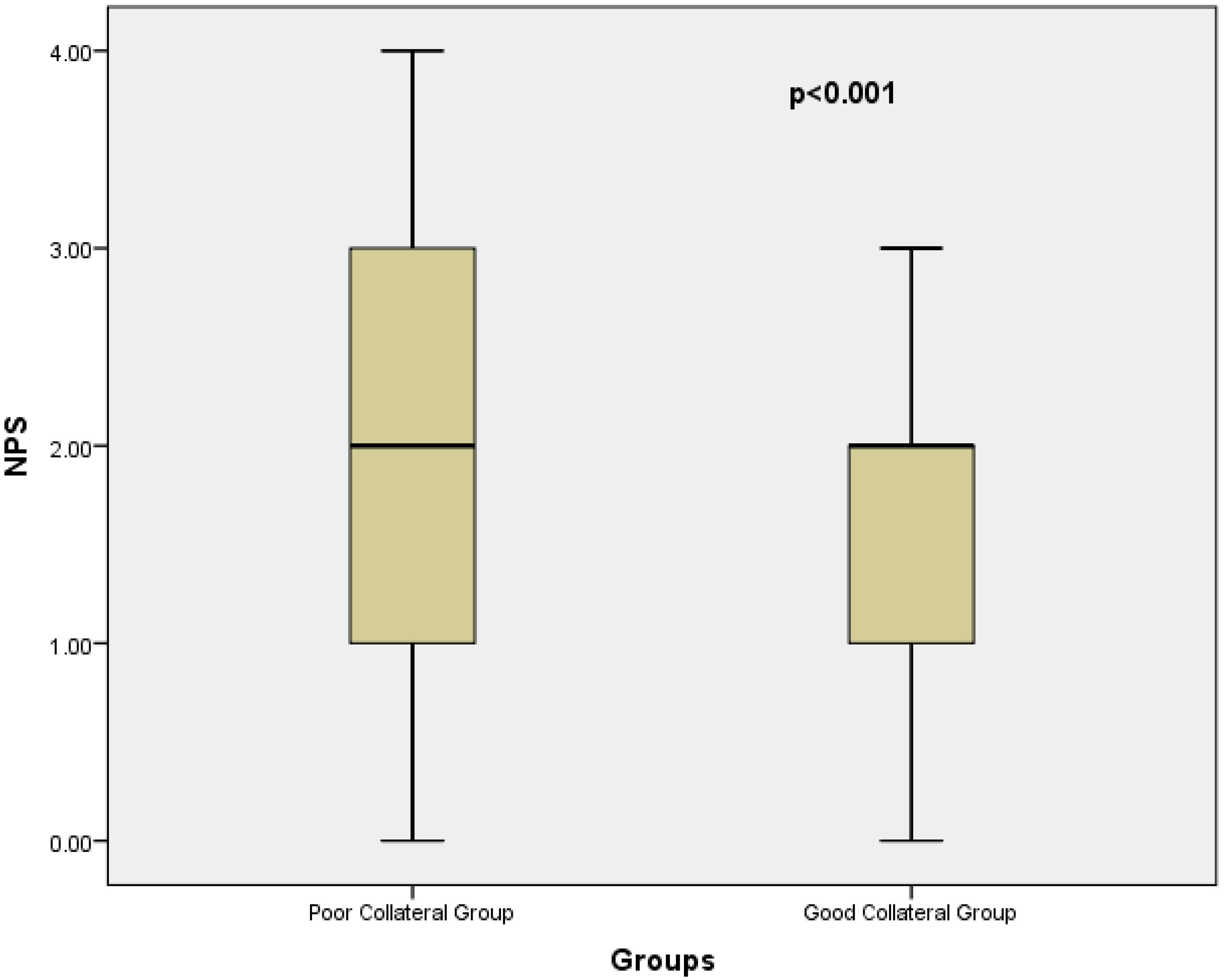
| NPS | 2 (2–3) | 2 (1–2) | <0.001 ** |
| Statin use (%) | 84.6% | 85.3% | 0.861 |
| LVEF (%) | 58 (53–62) | 59 (52–64) | 0.883 |
| Diabetes mellitus (%), n | 38.5% (80) | 41.4% (48) | 0.482 |
| Hypertension (%), n | 61.5% (128) | 69.8% (81) | 0.147 |
| Smoking (%), n | 49.5% (103) | 53.4% (62) | 0.767 |
| ACE inhibitor-ARB use (%), n | 56.7% (118) | 61.2% (71) | 0.481 |
| Beta-blocker use (%), n | 72.6% (151) | 72.4% (84) | 1.000 |
| Variables | Univariate OR (95% CI) | p | Multiple OR (95% CI) | p |
|---|---|---|---|---|
| BMI (kg/m2) | 1.050 (0.991–1.112) | 0.098 | - | - |
| Hb (g/dL) | 1.116 (0.991–1.257) | 0.071 | - | - |
| WBC (103/µL) | 0.989 (0.898–1.088) | 0.818 | - | - |
| HDL cholesterol (mg/dL) | 1.029 (1.003–1.055) | 0.027* | 1.035 (1.008–1.063) | 0.011 * |
| Triglyceride (mg/dL) | 1.001 (0.998–1.003) | 0.478 | - | - |
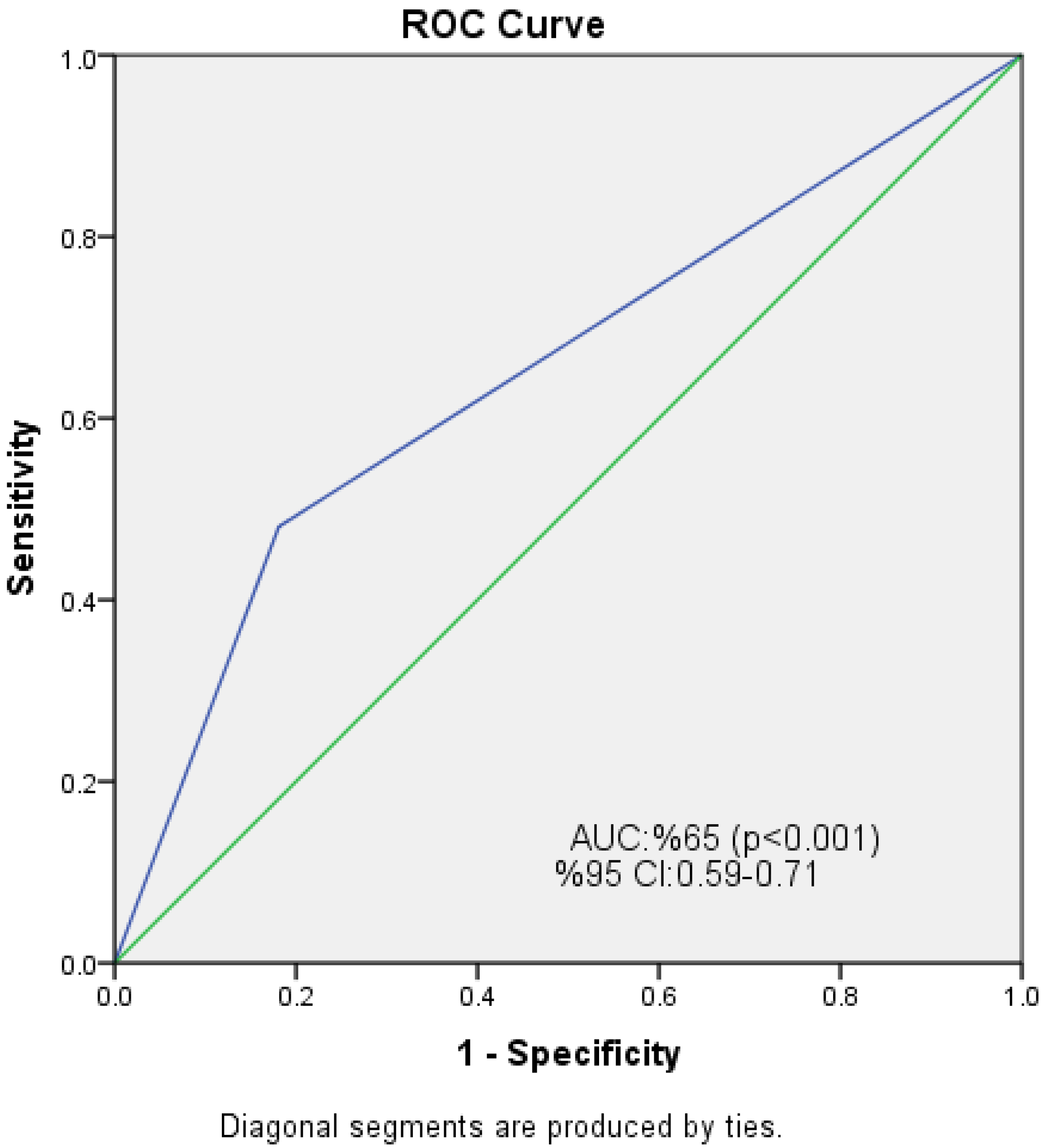
| NPS | 0.239 (0.138–0.412) | <0.001* | 0.226 (0.130–0.393) | <0.001 * |
| Smoking | 0.916 (0.731–1.149) | 0.449 | - | - |
| Hypertension | 0.691 (0.426–1.123) | 0.136 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tunçez, A.; Bütün, S.; Gürses, K.M.; Tezcan, H.; Toprak Su, A.M.; Erdoğan, B.; Kırmızıgül, M.; Yalçın, M.U.; Özen, Y.; Demir, K.; et al. The Association Between Naples Prognostic Score and Coronary Collateral Circulation in Patients with Chronic Coronary Total Occlusion. Diagnostics 2025, 15, 2500. https://doi.org/10.3390/diagnostics15192500
Tunçez A, Bütün S, Gürses KM, Tezcan H, Toprak Su AM, Erdoğan B, Kırmızıgül M, Yalçın MU, Özen Y, Demir K, et al. The Association Between Naples Prognostic Score and Coronary Collateral Circulation in Patients with Chronic Coronary Total Occlusion. Diagnostics. 2025; 15(19):2500. https://doi.org/10.3390/diagnostics15192500
Chicago/Turabian StyleTunçez, Abdullah, Sevil Bütün, Kadri Murat Gürses, Hüseyin Tezcan, Aslıhan Merve Toprak Su, Burak Erdoğan, Mustafa Kırmızıgül, Muhammed Ulvi Yalçın, Yasin Özen, Kenan Demir, and et al. 2025. "The Association Between Naples Prognostic Score and Coronary Collateral Circulation in Patients with Chronic Coronary Total Occlusion" Diagnostics 15, no. 19: 2500. https://doi.org/10.3390/diagnostics15192500
APA StyleTunçez, A., Bütün, S., Gürses, K. M., Tezcan, H., Toprak Su, A. M., Erdoğan, B., Kırmızıgül, M., Yalçın, M. U., Özen, Y., Demir, K., Aygül, N., & Altunkeser, B. B. (2025). The Association Between Naples Prognostic Score and Coronary Collateral Circulation in Patients with Chronic Coronary Total Occlusion. Diagnostics, 15(19), 2500. https://doi.org/10.3390/diagnostics15192500






