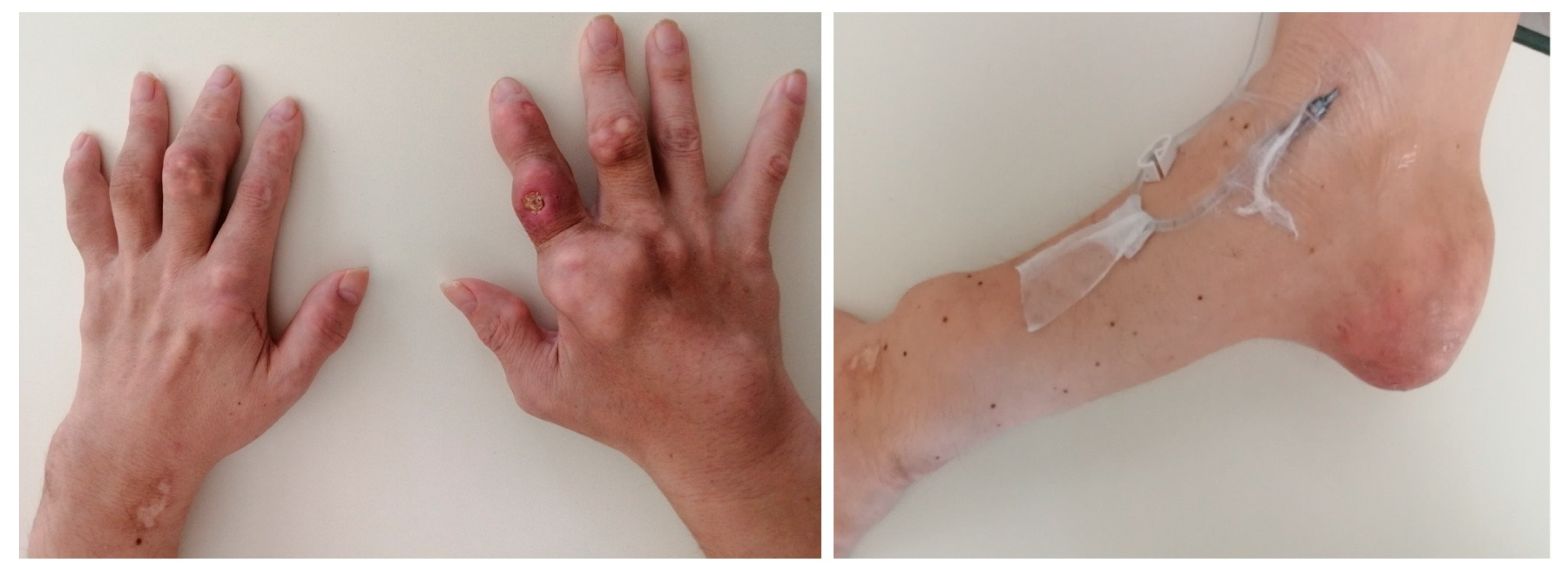
Gouty Tophi in Developed Countries: Uncovering Underlying Brain Diseases
Abstract

Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| MSU | Monosodium urate |
| SWI | Susceptibility-weighted imaging |
| CAA | Cerebral amyloid angiopathy |
References
- Forbess, L.J.; Fields, T.R. The broad spectrum of urate crystal deposition: Unusual presentations of gouty tophi. Semin. Arthritis Rheum. 2012, 42, 146–154. [Google Scholar] [CrossRef]
- Chhana, A.; Dalbeth, N. The gouty tophus: A review. Curr. Rheumatol. Rep. 2015, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef]
- Khanna, P.P.; Nuki, G.; Bardin, T.; Tausche, A.-K.; Forsythe, A.; Goren, A.; Vietri, J.; Khanna, D. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: Results from a cross-sectional survey. Health Qual. Life Outcomes 2012, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Dalbeth, N.; Otter, S.; Gow, P.; Kumar, S.; Rome, K. Clinically-evident tophi are associated with reduced muscle force in the foot and ankle in people with gout: A cross-sectional study. J. Foot Ankle Res. 2017, 10, 25. [Google Scholar] [CrossRef]
- Vincent, Z.L.; Gamble, G.; House, M.; Knight, J.; Horne, A.; Taylor, W.J.; Dalbeth, N. Predictors of Mortality in People with Recent-onset Gout: A Prospective Observational Study. J. Rheumatol. 2017, 44, 368–373. [Google Scholar] [CrossRef]
- Jiang, H.; Su, Y.; Liu, R.; Xu, X.; Xu, Q.; Yang, J.; Lin, Y. Hyperuricemia and the risk of stroke incidence and mortality: A systematic review and meta-analysis. Arch. Rheumatol. 2025, 40, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yu, X.; Wang, L.; Jiang, J.; Dai, Q.; Kang, Y.; Li, J.; Chen, X. Can hyperuricemia predict the progression risk of cerebral small vessel disease? Neurol. Res. 2022, 44, 910–917. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, K.; Sato, M.; Nakaya, Y.; Hayashi, M.; Miura, T.; Matsuda, H.; Kobayashi, Y. Gouty Tophi in Developed Countries: Uncovering Underlying Brain Diseases. Diagnostics 2025, 15, 2424. https://doi.org/10.3390/diagnostics15192424
Hayashi K, Sato M, Nakaya Y, Hayashi M, Miura T, Matsuda H, Kobayashi Y. Gouty Tophi in Developed Countries: Uncovering Underlying Brain Diseases. Diagnostics. 2025; 15(19):2424. https://doi.org/10.3390/diagnostics15192424
Chicago/Turabian StyleHayashi, Koji, Mamiko Sato, Yuka Nakaya, Maho Hayashi, Toyoaki Miura, Hidetaka Matsuda, and Yasutaka Kobayashi. 2025. "Gouty Tophi in Developed Countries: Uncovering Underlying Brain Diseases" Diagnostics 15, no. 19: 2424. https://doi.org/10.3390/diagnostics15192424
APA StyleHayashi, K., Sato, M., Nakaya, Y., Hayashi, M., Miura, T., Matsuda, H., & Kobayashi, Y. (2025). Gouty Tophi in Developed Countries: Uncovering Underlying Brain Diseases. Diagnostics, 15(19), 2424. https://doi.org/10.3390/diagnostics15192424





