Kinetics of High-Sensitive Cardiac Troponin I in Patients with ST-Segment Elevation Myocardial Infarction and Non-ST Segment Elevation Myocardial Infarction
Abstract
1. Introduction
2. Methods
2.1. Study Objectives
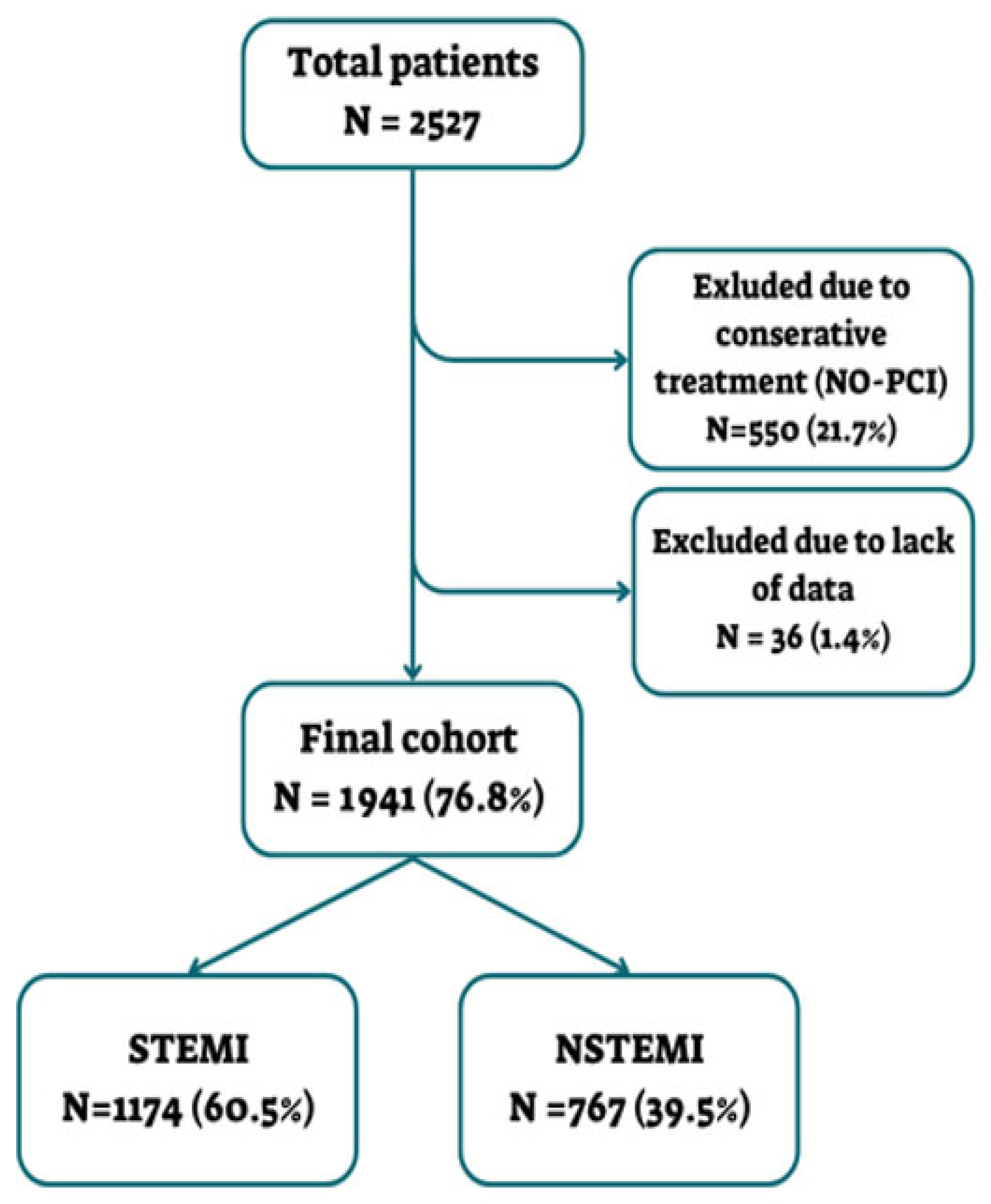
2.2. Study Population
2.3. Data Collection
2.4. Measurement of Hs-cTnI
2.5. Coronary Angiography and PCI
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Patient Characteristics
3.3. Complications and Outcomes
3.4. Kinetics of Hs-cTnI
3.5. Time to Peak and Level of the Hs-cTnI Peak
3.6. Association Between Number of Hs-cTnI Peaks and Patient Baseline Characteristics
3.7. Association Between Number of Hs-cTnI Peaks and Clinical Complications
4. Discussion
4.1. Clinical Implications
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babuin, L.; Jaffe, A.S. Troponin: The biomarker of choice for the detection of cardiac injury. Can. Med. Assoc. J. 2005, 173, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth universal definition of myocardial infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.S.; MacDonald, D.C. Appropriate roles of cardiac troponins in evaluating patients with chest pain. J. Am. Board Fam. Pract. 1999, 12, 214–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Apple, F.S. A new season for cardiac troponin assays: It’s time to keep a scorecard. Clin. Chem. 2009, 55, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, A.; Gibson, C.M. Narrative review: Alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann. Intern. Med. 2005, 142, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Solecki, K.; Dupuy, A.M.; Kuster, N.; Leclercq, F.; Gervasoni, R.; Macia, J.C.; Cung, T.T.; Lattuca, B.; Cransac, F.; Cade, S.; et al. Kinetics of high-sensitivity cardiac troponin T or troponin I compared to creatine kinase in patients with revascularized acute myocardial infarction. Clin. Chem. Lab. Med. 2015, 53, 707–714. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, W.P.T.M.; Vroemen, W.H.M.; Smulders, M.W.; van Suijlen, J.D.; van Cauteren, Y.J.M.; Bekkers, S.C.A.M.; Bekers, O.; Meex, S.J.R. High-sensitivity cardiac troponin I and T kinetics after non-ST-segment elevation myocardial infarction. J. Appl. Lab. Med. 2020, 5, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Laugaudin, G.; Kuster, N.; Petiton, A.; Leclercq, F.; Gervasoni, R.; Macia, J.C.; Cung, T.T.; Dupuy, A.M.; Solecki, K.; Lattuca, B.; et al. Kinetics of high-sensitivity cardiac troponin T and I differ in patients with ST-segment elevation myocardial infarction treated by primary coronary intervention. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Healthcare Improvement Scotland. Scottish Intercollegiate Guidelines Network (SIGN) 148: Acute Coronary Syndrome: A National Clinical Guideline; SIGN: Edinburgh, UK, 2016; Available online: https://www.sign.ac.uk/assets/sign148.pdf (accessed on 13 July 2013).
- Hamm, C.W.; Bassand, J.P.; Agewall, S.; Bax, J.; Boersma, E.; Bueno, H.; Caso, P.; Dudek, D.; Gielen, S.; Huber, K.; et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 2999–3054. [Google Scholar] [CrossRef] [PubMed]
- Koerbin, G.; Abhayaratna, W.P.; Potter, J.M.; Apple, F.S.; Jaffe, A.S.; Ravalico, T.H.; Hickman, P.E. Effect of population selection on 99th percentile values for high-sensitivity cardiac troponin I and T assays. Clin. Biochem. 2013, 46, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, T.S.; Goßling, A.; Sörensen, N.A.; Lehmacher, J.; Neumann, J.T.; Blankenberg, S.; Westermann, D. Prognostic Implications of a Second Peak of High-Sensitivity Troponin T After Myocardial Infarction. Front. Cardiovasc. Med. 2022, 8, 780198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Loutati, R.; Bruoha, S.; Taha, L.; Karmi, M.; Perel, N.; Maller, T.; Sabouret, P.; Galli, M.; Zoccai, G.B.; De Rosa, S.; et al. Association between peak troponin level and prognosis among patients admitted to intensive cardiovascular care unit. Int. J. Cardiol. 2024, 417, 132556. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Bergamaschi, L.; Paolisso, P.; Belmonte, M.; Angeli, F.; Sansonetti, A.; Stefanizzi, A.; Bertolini, D.; Bodega, F.; Amicone, S.; et al. Prognostic Relevance of Type 4a Myocardial Infarction and Periprocedural Myocardial Injury in Patients with Non–ST-Segment–Elevation Myocardial Infarction. Circulation 2025, 151, 11. [Google Scholar] [CrossRef] [PubMed]
- Katus, H.A.; Remppis, A.; Scheffold, T.; Diederich, K.W.; Kuebler, W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am. J. Cardiol. 1991, 67, 1360–1367. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Overall (N = 1941) | NSTEMI (N = 767) | STEMI (N = 1174) | p-Value |
|---|---|---|---|---|
| Age, mean (IQR) | 65 (57–77) | 67 (58–79) | 63 (56–75) | <0.001 |
| BMI, mean (kg/m2) | 28 | 28 | 28 | 0.876 |
| Male gender, % | 83.1% | 82.1% | 83.7% | 0.353 |
| EF, median (IQR) | 47.22% (41–52) | 48.89% (44–54) | 45.81% (39–49) | <0.001 |
| Hospital length of stay (LOS), median (IQR) | 2.0 days (1–3) | 2 days (1–3) | 3 days (1–4) | 0.02 |
| Hypertension | 57.2% | 66.1% | 51.4% | <0.001 |
| Dyslipidemia | 57.1% | 62.5% | 53.4% | <0.001 |
| Diabetes mellitus | 37.4% | 42.0% | 34.3% | 0.001 |
| CAD | 30.2% | 40.9% | 23.3% | <0.001 |
| Prior CABG | 5.3% | 9.5% | 2.5% | <0.001 |
| PAD | 4.1% | 7.2% | 2.1% | <0.001 |
| Heart failure | 5.3% | 7.4% | 3.8% | 0.001 |
| Pulmonary hypertension | 0.9% | 1.7% | 0.3% | 0.002 |
| Smoking | 40.9% | 34.7% | 44.5% | <0.001 |
| Family history of CAD | 11.3% | 9.4% | 12.5% | 0.033 |
| CVA/TIA | 5.2% | 6.0% | 4.6% | 0.173 |
| COPD/asthma | 5.5% | 6.0% | 5.2% | 0.449 |
| Pulmonary embolism | 0.6% | 0.9% | 0.4% | 0.238 |
| Overall (N = 1941) | NSTEMI (N = 767) | STEMI (N = 1174) | p-Value | |
|---|---|---|---|---|
| Cardiogenic Shock | 4.3% | 3.8% | 4.7% | 0.339 |
| Heart Failure | 4.4% | 3.1% | 5.3% | 0.024 |
| Mechanical Complications (VSR/Rupture) | 0.6% | 0.3% | 0.8% | 0.218 |
| LV Thrombus | 1.7% | 0.7% | 2.4% | 0.004 |
| Stroke/TIA | 1.6% | 1.3% | 1.9% | 0.335 |
| Reinfarction | 0.5% | 0.7% | 0.4% | 0.529 |
| Stent Thrombosis | 0.9% | 1.4% | 0.5% | 0.033 |
| Major Bleeding | 2.9% | 3.0% | 2.9% | 0.896 |
| In-Hospital Mortality | 2.3% | 1.2% | 3.0% | 0.009 |
| STEMI | NSTEMI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | β | SE | OR | 95% CI | p value | Predictor | β | SE | OR | 95% CI | p value |
| Hours to Peak Troponin Level | 0.019 | 0.001 | 1.019 | [1.017, 1.021] | <0.001 | Hours to Peak Troponin Level | 0.008 | 0.001 | 1.008 | [1.006, 1.010] | <0.001 |
| Cardiogenic Shock | 0.615 | 0.122 | 1.85 | [1.460, 2.344] | <0.001 | Cardiogenic Shock | 1.966 | 0.153 | 7.144 | [5.326, 9.577] | <0.001 |
| Peak Troponin Level | <0.001 | <0.001 | ~1.000 | [1.000, 1.000] | 0.013 | Peak Troponin Level | <0.001 | <0.001 | ~1.000 | [0.999, 1.000] | 0.028 |
| EF | −0.015 | 0.004 | 0.985 | [0.977, 0.993] | <0.001 | IHD | 0.388 | 0.125 | 1.474 | [1.149, 1.891] | 0.002 |
| CHF/CMP/MVR | 0.214 | 0.097 | 1.239 | [1.024, 1.499] | 0.028 | ||||||
| Constant | 0.171 | <0.001 | Constant | 0.033 | <0.001 | ||||||
| Clinical Complication | Number of hs-cTnI Peaks in NSTEMI (Complication vs. No Complication) | p-Value NSTEMI | Number of hs-cTnI Peaks in STEMI (Complication vs. No Complication) | p-Value STEMI |
|---|---|---|---|---|
| Cardiogenic Shock | 2.10 vs. 1.27 | 0.001 | 2.55 vs. 1.25 | <0.001 |
| Post-Procedural HF | 2.33 vs. 1.27 | 0.001 | 2.76 vs. 1.23 | <0.001 |
| Post-Procedural Stroke/TIA | 3.40 vs. 1.27 | 0.003 | 2.91 vs. 1.28 | <0.001 |
| Stent Thrombosis | 2.36 vs. 1.29 | 0.032 | 3.33 vs. 1.30 | 0.009 |
| Significant Bleeding | 2.13 vs. 1.28 | 0.048 | 2.62 vs. 1.27 | <0.001 |
| Re-Infarction | 3.40 vs. 1.29 | <0.001 | 2.80 vs. 1.30 | 0.003 |
| LV Thrombus | 1.20 vs. 1.30 | 0.896 | 2.61 vs. 1.28 | 0.001 |
| Blood Transfusion | 1.71 vs. 1.29 | 0.128 | 2.33 vs. 1.30 | <0.001 |
| VSR/Septal Rupture | 1.00 vs. 1.30 | 0.457 | 1.00 vs. 1.31 | 0.177 |
| In-Hospital Mortality | 3 vs. 1.28 | 0.033 | 1.43 vs. 1.31 | 0.274 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haizler, A.; Loutati, R.; Taha, L.; Karmi, M.; Deeb, D.; Manassra, M.; Fink, N.; Sabouret, P.; S. Rana, J.; Mamas, M.A.; et al. Kinetics of High-Sensitive Cardiac Troponin I in Patients with ST-Segment Elevation Myocardial Infarction and Non-ST Segment Elevation Myocardial Infarction. Diagnostics 2025, 15, 2390. https://doi.org/10.3390/diagnostics15182390
Haizler A, Loutati R, Taha L, Karmi M, Deeb D, Manassra M, Fink N, Sabouret P, S. Rana J, Mamas MA, et al. Kinetics of High-Sensitive Cardiac Troponin I in Patients with ST-Segment Elevation Myocardial Infarction and Non-ST Segment Elevation Myocardial Infarction. Diagnostics. 2025; 15(18):2390. https://doi.org/10.3390/diagnostics15182390
Chicago/Turabian StyleHaizler, Adi, Ranel Loutati, Louay Taha, Mohammad Karmi, Dana Deeb, Mohammed Manassra, Noam Fink, Pierre Sabouret, Jamal S. Rana, Mamas A. Mamas, and et al. 2025. "Kinetics of High-Sensitive Cardiac Troponin I in Patients with ST-Segment Elevation Myocardial Infarction and Non-ST Segment Elevation Myocardial Infarction" Diagnostics 15, no. 18: 2390. https://doi.org/10.3390/diagnostics15182390
APA StyleHaizler, A., Loutati, R., Taha, L., Karmi, M., Deeb, D., Manassra, M., Fink, N., Sabouret, P., S. Rana, J., Mamas, M. A., Rabi, O., Brin, A., Moatz, A., Shrem, M., Qadan, A., Levi, N., Glikson, M., Asher, E., & on behalf of the Jerusalem Platelets Thrombosis and Intervention in Cardiology (JUPITER-26) Study Group. (2025). Kinetics of High-Sensitive Cardiac Troponin I in Patients with ST-Segment Elevation Myocardial Infarction and Non-ST Segment Elevation Myocardial Infarction. Diagnostics, 15(18), 2390. https://doi.org/10.3390/diagnostics15182390







