Colorectal Cancer—One Disease, Two Fires: Distinct Inflammatory Landscapes in Colon and Rectal Cancer
Abstract
1. Introduction
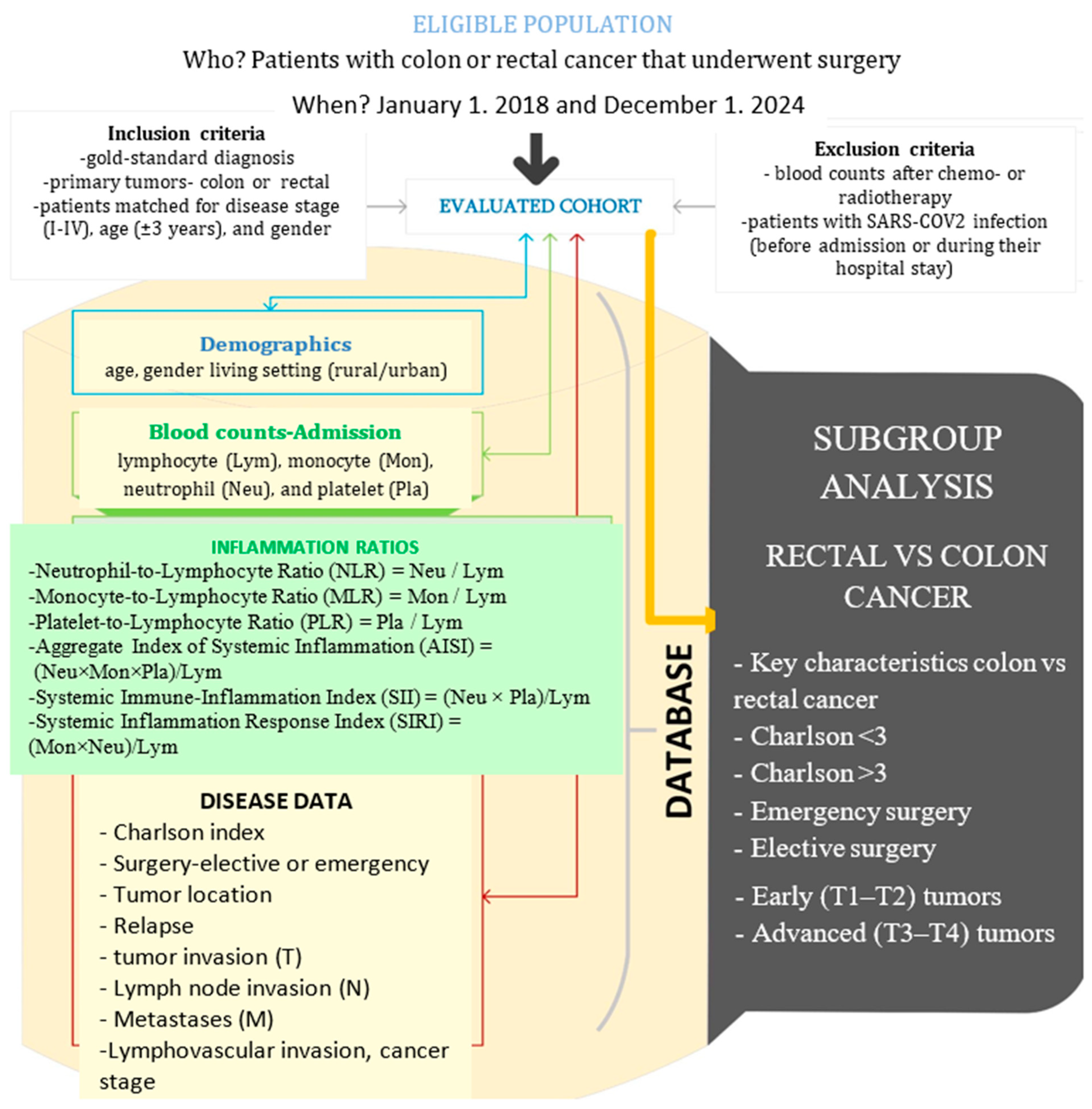
2. Materials and Methods
Statistical Analyses
3. Results
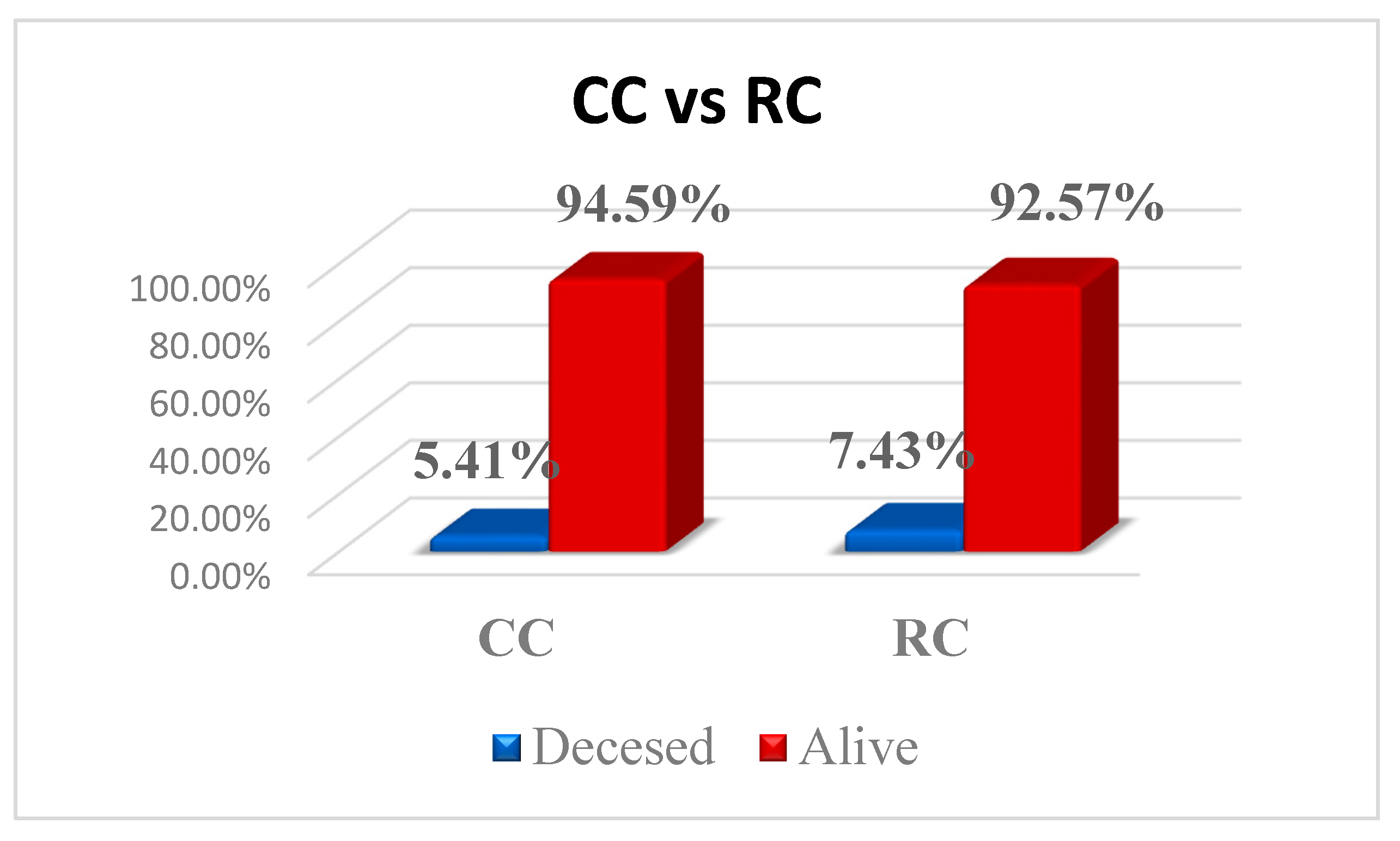
3.1. Key Information
3.2. Impact of Comorbidities
3.3. Emergency vs. Elective Surgery
- Urban residence (22 (53.7%) vs. 12 (32.4%), p = 0.048)
- T stage (p = 0.022):
- ○
- T2 (2 (5.9%) vs. 0)
- ○
- T3 (16 (47.1%) vs. 33 (75%))
- ○
- T4 (16 (47.1%) vs. 11 (25%))
- Urban residence (78 (68.4%) vs. 54 (51.9%), p = 0.009)
- T stage p = 0.004:
- ○
- T1 (4 (3.5%) vs. 2 (1.9%))
- ○
- T2 (10 (8.8%) vs. 20 (19.2%))
- ○
- T3 (64 (56.1%) vs. 68 (65.4%))
- ○
- T4 (36 (31.6%) vs. 11 (13.5%))
3.4. Evaluating Early (T1–T2) vs. Advanced (T3–T4) Tumors
4. Discussion
4.1. Impact of Comorbidities
4.2. Elective vs. Emergency Surgery
4.3. Early (T1–T2) vs. Advanced (T3–T4) Tumors
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abreu Lopez, B.A.; Pinto-Colmenarez, R.; Caliwag, F.M.C.; Ponce-Lujan, L.; Fermin, M.D.; Cortés, A.V.G.; Martínez, A.G.M.; Martinez, I.G.Z.; León, F.G. Colorectal Cancer Screening and Management in Low- and Middle-Income Countries and High-Income Countries: A Narrative Review. Cureus 2024, 16, e70933. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Data Version: Globocan 2022, Version 1.1; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdf (accessed on 17 July 2024).
- Ola, I.; Cardoso, R.; Hoffmeister, M.; Brenner, H. Utilization of colorectal cancer screening tests: A systematic review and time trend analysis of nationally representative data. eClinicalMedicine 2024, 75, 102783. [Google Scholar] [CrossRef]
- Ola, I.; Cardoso, R.; Hoffmeister, M.; Brenner, H. Recent trends in self-reported utilization of colonoscopy and fecal occult blood test in Europe: Analysis of the European Health Interview Surveys 2013–2015 and 2018–2020. Eur. J. Epidemiol. 2025, 40, 767–778. [Google Scholar] [CrossRef]
- Wang, C.C.; Sung, W.W.; Yan, P.Y.; Ko, P.Y.; Tsai, M.C. Favorable colorectal cancer mortality-to-incidence ratios in countries with high expenditures on health and development index: A study based on GLOBOCAN database. Medicine 2021, 100, e27414. [Google Scholar] [CrossRef]
- Barna, R.; Dema, A.; Jurescu, A.; Văduva, A.O.; Lăzureanu, D.-C.; Vița, O.; Natarâș, B.; Hurmuz, I.; Vidac, A.; Tăban, S.; et al. The Relevance of Sex and Age as Non-Modifiable Risk Factors in Relation to Clinical-Pathological Parameters in Colorectal Cancer. Life 2025, 15, 156. [Google Scholar] [CrossRef]
- Leijssen, L.G.J.; Dinaux, A.M.; Kunitake, H.; Bordeianou, L.G.; Berger, D.L. Pathologic factors are more important than tumor location in long-term survival in colon cancer. Int. J. Color. Dis. 2018, 33, 709–717. [Google Scholar] [CrossRef]
- Gheju, A.; Jurescu, A.; Tăban, S.; Al-Jobory, D.; Lazăr, F.; Dema, A. Different disease characteristics in young patients with colorectal cancer: A large retrospective study in a city in Romania. J. Int. Med. Res. 2021, 49, 3000605211016630. [Google Scholar] [CrossRef] [PubMed]
- Alinia, S.; Ahmadi, S.; Mohammadi, Z.; Shirvandeh, F.R.; Asghari-Jafarabadi, M.; Mahmoudi, L.; Safari, M.; Roshanaei, G. Exploring the impact of stage and tumor site on colorectal cancer survival: Bayesian survival modeling. Sci. Rep. 2024, 14, 4270. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, N.P.M.; Bos, A.C.R.K.; Lemmens, V.E.P.P.; Tanis, P.J.; Hugen, N.; Nagtegaal, I.D.; de Wilt, J.H.; Verhoeven, R.H. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int. J. Cancer 2018, 143, 2758–2766. [Google Scholar] [CrossRef]
- Kim, M.; Son, I.T.; Oh, B.Y. Inflammatory Response Markers as Predictors of Colorectal Cancer Prognosis. Ewha Med. J. 2023, 46 (Suppl. 1), e24. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fukuda, M.; Okuchi, Y.; Oshimo, Y.; Nishikawa, Y.; Hisano, K.; Kawai, T.; Iguchi, K.; Okuda, Y.; Kamimura, R.; et al. Clinical impact of lymphocyte/C-reactive protein ratio on postoperative outcomes in patients with rectal cancer who underwent curative resection. Sci. Rep. 2022, 12, 17136. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Jiang, W.; Zhang, D.; Cheng, C.; Liu, C.; Zhao, Z.; Wang, H. Predictive value of the systemic inflammation grade for overall survival in patients with colorectal cancer after surgery: Outperforming NLR and mGPS. Front. Oncol. 2025, 15, 1529670. [Google Scholar] [CrossRef]
- Ma, L.; Yang, F.; Guo, W.; Tang, S.; Ling, Y. Prognostic role of platelet-to-lymphocyte ratio in patients with rectal cancer undergoing resection: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1415443. [Google Scholar] [CrossRef]
- Moise, G.V.; Feier, C.V.I.; Gaborean, V.; Faur, A.M.; Rus, V.I.; Muntean, C. From Blood to Outcome: Inflammatory Biomarkers in Rectal Cancer Surgery at a Romanian Tertiary Hospital. Diseases 2025, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Ciocan, A.; Ciocan, R.A.; Al Hajjar, N.; Gherman, C.D.; Bolboacă, S.D. Abilities of Pre-Treatment Inflammation Ratios as Classification or Prediction Models for Patients with Colorectal Cancer. Diagnostics 2021, 11, 566. [Google Scholar] [CrossRef]
- Menyhart, O.; Fekete, J.T.; Győrffy, B. Inflammation and Colorectal Cancer: A Meta-Analysis of the Prognostic Significance of the Systemic Immune-Inflammation Index (SII) and the Systemic Inflammation Response Index (SIRI). Int. J. Mol. Sci. 2024, 25, 8441. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, Z.; Ye, Y.; Wu, H.; Sun, W.; Wang, N. Association of aggregate index of systemic inflammation with increased all-cause and cardiovascular mortality in female cancer patients. Front. Oncol. 2025, 15, 1552341. [Google Scholar] [CrossRef]
- Șerban, R.E.; Popescu, D.M.; Boldeanu, M.V.; Florescu, D.N.; Șerbănescu, M.-S.; Șandru, V.; Panaitescu-Damian, A.; Forțofoiu, D.; Șerban, R.-C.; Gherghina, F.-L.; et al. The Diagnostic and Prognostic Role of Inflammatory Markers, Including the New Cumulative Inflammatory Index (IIC) and Mean Corpuscular Volume/Lymphocyte (MCVL), in Colorectal Adenocarcinoma. Cancers 2025, 17, 990. [Google Scholar] [CrossRef]
- Ose, J.; Gigic, B.; Hardikar, S.; Lin, T.; Himbert, C.; Warby, C.A.; Peoples, A.R.; Lindley, C.L.; Boehm, J.; Schrotz-King, P.; et al. Presurgery Adhesion Molecules and Angiogenesis Biomarkers Are Differently Associated with Outcomes in Colon and Rectal Cancer: Results from the ColoCare Study. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Lino-Silva, L.S.; Salcedo-Hernández, R.A.; Ruiz-García, E.B.; García-Pérez, L.; Herrera-Gómez, Á. Pre-operative Neutrophils/Lymphocyte Ratio in Rectal Cancer Patients with Preoperative Chemoradiotherapy. Med. Arch. 2016, 70, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Absenger, G.; Szkandera, J.; Stotz, M.; Postlmayr, U.; Pichler, M.; Ress, A.L.; Schaberl-Moser, R.; Loibner, H.; Samonigg, H.; Gerger, A. Preoperative neutrophil-to-lymphocyte ratio predicts clinical outcome in patients with stage II and III colon cancer. Anticancer Res. 2013, 33, 4591–4594. [Google Scholar]
- Andor, M.; Man, D.E.; Nistor, D.C.; Buda, V.; Dragan, S. The Influence of COVID-19 in Glycemic Control: Predictive Value of Inflammation and Metabolic Parameters. Biomedicines 2024, 12, 2642. [Google Scholar] [CrossRef]
- Daliu, P.; Bogdan, I.; Rosca, O.; Licker, M.; Stanga, L.C.; Hogea, E.; Vaduva, D.B.; Muntean, D. Fungal Pulmonary Coinfections in COVID-19: Microbiological Assessment, Inflammatory Profiles, and Clinical Outcomes. Biomedicines 2025, 13, 864. [Google Scholar] [CrossRef]
- Fericean, R.M.; Rosca, O.; Citu, C.; Manolescu, D.; Bloanca, V.; Toma, A.-O.; Boeriu, E.; Dumitru, C.; Ravulapalli, M.; Barbos, V.; et al. COVID-19 Clinical Features and Outcomes in Elderly Patients during Six Pandemic Waves. J. Clin. Med. 2022, 11, 6803. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Xu, M.; Chen, K.; Li, S.; Guan, G. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci. Rep. 2020, 10, 8017. [Google Scholar] [CrossRef]
- Bouvier, A.M.; Jooste, V.; Lillini, R.; Marcos-Gragera, R.; Katalinic, A.; Rossi, P.G.; Launoy, G.; Bouvier, V.; Guevara, M.; Ardanaz, E.; et al. Differences in survival and recurrence of colorectal cancer by stage across population-based European registries. Int. J. Cancer 2024, 155, 807–815. [Google Scholar] [CrossRef]
- Sepassi, A.; Li, M.; Zell, J.A.; Chan, A.; Saunders, I.M.; Mukamel, D.B. Rural-Urban Disparities in Colorectal Cancer Screening, Diagnosis, Treatment, and Survivorship Care: A Systematic Review and Meta-Analysis. Oncologist 2024, 29, e431–e446. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.L.; Wang, M.; Liang, M.; Kameni, A.; Stoltzfus, K.C.; Onega, T. County-level characteristics associated with incidence, late-stage incidence, and mortality from screenable cancers. Cancer Epidemiol. 2021, 75, 102033. [Google Scholar] [CrossRef]
- Morishima, T.; Matsumoto, Y.; Koeda, N.; Shimada, H.; Maruhama, T.; Matsuki, D.; Nakata, K.; Ito, Y.; Tabuchi, T.; Miyashiro, I. Impact of Comorbidities on Survival in Gastric, Colorectal, and Lung Cancer Patients. J. Epidemiol. 2019, 29, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Ostenfeld, E.B.; Nørgaard, M.; Thomsen, R.W.; Iversen, L.H.; Jacobsen, J.B.; Søgaard, M. Comorbidity and survival of Danish patients with colon and rectal cancer from 2000–2011: A population-based cohort study. Clin. Epidemiol. 2013, 5 (Suppl. 1), 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, A.; Komori, K.; Kinoshita, T.; Oshiro, T.; Kunitomo, A.; Ito, S.; Abe, T.; Shimizu, Y. Possibilities for and limits of upfront surgical strategy with lateral pelvic node dissection for low rectal cancer. Jpn. J. Clin. Oncol. 2021, 51, 713–721. [Google Scholar] [CrossRef]
- Basile, D.; Garattini, S.K.; Corvaja, C.; Montico, M.; Cortiula, F.; Pelizzari, G.; Gerratana, L.; Audisio, M.; Lisanti, C.; Fanotto, V.; et al. The MIMIC Study: Prognostic Role and Cutoff Definition of Monocyte-to-Lymphocyte Ratio and Lactate Dehydrogenase Levels in Metastatic Colorectal Cancer. Oncologist 2020, 25, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Chen, J.H.; Zhai, E.T.; Yuan, Y.J.; Wu, K.-M.; Xu, J.-B.; Peng, J.-J.; Chen, C.-Q.; He, Y.-L.; Cai, S.-R. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol. 2017, 23, 6261–6272. [Google Scholar] [CrossRef]
- Xie, Q.K.; Chen, P.; Hu, W.M.; Sun, P.; He, W.-Z.; Jiang, C.; Kong, P.-F.; Liu, S.-S.; Chen, H.-T.; Yang, Y.-Z.; et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J. Transl. Med. 2018, 16, 273. [Google Scholar] [CrossRef]
- Moro-Valdezate, D.; Martín-Arévalo, J.; Cózar-Lozano, C.; García-Botello, S.; Pérez-Santiago, L.; Casado-Rodrigo, D.; Martínez-Ciarpaglini, C.; Tarazona, N.; Pla-Martí, V. Prognostic value of routine blood biomarkers in 3-year survival of resectable colorectal cancer patients: A prognostic nomogram for clinical practice. Int. J. Color. Dis. 2025, 40, 58. [Google Scholar] [CrossRef]
- Tominaga, T.; Nonaka, T.; Oyama, S.; Takamura, Y.; Hashimoto, S.; Shiraishi, T.; Sawai, T.; Nagayasu, T. Efficacy of Neutrophil-to-Lymphocyte Ratio for Cancer-Specific Survival in Elderly Patients with Localized Colon Cancer: A Single Center Propensity Score-Matched Analysis. Clin. Exp. Gastroenterol. 2023, 16, 1–9. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Maby, P.; Angelova, M.; Tougeron, D.; Church, S.E.; Lafontaine, L.; Fischer, M.; Fredriksen, T.; et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity 2016, 44, 698–711. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Hernandez-Ainsa, M.; Velamazan, R.; Lanas, A.; Carrera-Lasfuentes, P.; Piazuelo, E. Blood-Cell-Based Inflammatory Markers as a Useful Tool for Early Diagnosis in Colorectal Cancer. Front. Med. 2022, 9, 843074. [Google Scholar] [CrossRef]
- An, S.; Shim, H.; Kim, K.; Kim, B.; Bang, H.-J.; Do, H.; Lee, H.-R.; Kim, Y. Pretreatment inflammatory markers predicting treatment outcomes in colorectal cancer. Ann. Coloproctol. 2022, 38, 97–108. [Google Scholar] [CrossRef]
- Dolan, R.D.; McSorley, S.T.; Park, J.H.; Watt, D.G.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: Comparison of composite ratios and cumulative scores. Br. J. Cancer 2018, 119, 40–51. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Chen, T.; Li, Q.; Wu, J.; Wu, Y.; Peng, W.; Li, H.; Wang, J.; Tang, X.; Peng, Y.; Fu, X. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol. Immunother. 2018, 67, 1635–1646. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef]
- Galasso, L.; Termite, F.; Mignini, I.; Esposto, G.; Borriello, R.; Vitale, F.; Nicoletti, A.; Paratore, M.; Ainora, M.E.; Gasbarrini, A.; et al. Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights. Cancers 2025, 17, 368. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Sarandria, N. A Literature Review in Immuno-Oncology: Pathophysiological and Clinical Features of Colorectal Cancer and the Role of the Doctor-Patient Interaction. J. Cancer Ther. 2022, 13, 654–684. [Google Scholar] [CrossRef]
| Characteristic | All, n = 296 | Colon Cancer, n = 148 | Rectal Cancer, n = 148 | p |
|---|---|---|---|---|
| Age (M ± SD), years | 62.43 ± 8.79 | 63.07 ± 8.38 | 61.80 ± 9.20 | 0.445 |
| Gender, men | 190 (64.2%) | 94 (63.5%) | 96 (64.9%) | 0.904 |
| Rural | 123 (41.6%) | 48 (32.4%) | 75 (50.7%) | 0.002 |
| CHARLSON > 3 | 162 (54.9%) | 70 (47.3%) | 92 (62.6%) | 0.010 |
| Emergency | 78 (26.4%) | 34 (23%) | 44 (29.7%) | 0.235 |
| Relapse | 29 (9.8%) | 6 (4.1%) | 23 (15.5%) | 0.001 |
| Stage | 1 | |||
| I | 28 (9.5%) | 14 (9.5%) | 14 (9.5%) | |
| II | 84 (28.4%) | 42 (28.4%) | 42 (28.4%) | |
| III | 152 (51.4%) | 76 (51.4%) | 76 (51.4%) | |
| IV | 32 (10.8%) | 16 (10.8%) | 16 (10.8%) | |
| Lymphatic invasion | 123 (42.1%) | 82 (55.4%) | 41 (27.7%) | <0.001 |
| pT | 0.002 | |||
| 1 | 6 (2%) | 4 (2.7%) | 2 (1.4%) | |
| 2 | 32 (10.8%) | 12 (8.1%) | 20 (13.5%) | |
| 3 | 181 (61.1%) | 80 (54.1%) | 101 (68.2%) | |
| 4 | 77 (26%) | 52 (35.1%) | 25 (16.9%) | |
| pN | 0.489 | |||
| 0 | 116 (39.2%) | 56 (37.8%) | 60 (40.5%) | |
| 1 | 114 (38.5%) | 58 (39.2%) | 56 (37.8%) | |
| 2 | 66 (22.3%) | 34 (23%) | 32 (21.7) | |
| pM | 32 (10.8%) | 16 (10.8%) | 16 (10.8%) | 1 |
| Marker | All, n = 296 | Colon Cancer, n = 148 | Rectal Cancer, n = 148 | p |
|---|---|---|---|---|
| Lymphocytes | 1850 ± 768 | 1697 ± 681 | 2048 ± 830 | <0.001 |
| Monocytes | 651 ± 215 | 655 ± 124 | 647 ± 252 | 0.950 |
| Platelets | 313,100 ± 126,912 | 331,581 ± 137,239 | 289,242 ± 108,195 | 0.007 |
| Neutrophils | 5670 ± 2717 | 6094 ± 3161 | 5285 ± 2185 | 0.034 |
| NLR | 3.39 ± 2.19 | 3.99 ± 2.2 | 2.84 ± 1.53 | <0.001 |
| MLR | 0.4 ± 0.23 | 0.43 ± 0.18 | 0.35 ± 0.19 | 0.245 |
| PLR | 195.15 ± 115.22 | 219.84 ± 129.27 | 163.28 ± 84.47 | <0.001 |
| AISI | 1126.37 ± 907.76 | 1714.68 ± 1108.26 | 593.55 ± 458.9 | 0.009 |
| SIRI | 2.76 ± 2.18 | 3.7 ± 3.76 | 1.91 ± 1.4 | 0.004 |
| SII | 1173.85 ± 1284.8 | 1533.83 ± 1284.9 | 847.83 ± 629.31 | <0.001 |
| Marker | CC, n = 78 | RC, n = 55 | p |
|---|---|---|---|
| Lymphocytes | 1665 ± 711 | 2055 ± 892 | 0.022 |
| Monocytes | 756 ± 170 | 647 ± 185 | 0.590 |
| Neutrophils | 5944 ± 3596 | 5554 ± 2349 | 0.555 |
| Platelets | 332,997 ± 151,989 | 338,589 ± 116,530 | 0.828 |
| NLR | 4.08 ± 3.06 | 3.2 ± 2.12 | 0.127 |
| PLR | 230.99 ± 141.66 | 196.68 ± 108.94 | 0.159 |
| MLR | 0.53 ± 0.45 | 0.38 ± 0.25 | 0.310 |
| AISI | 1370.98 ± 785.54 | 769.17 ± 685.54 | 0.068 |
| SII | 1405.54 ± 1084.45 | 1084.54 ± 845.45 | 0.128 |
| SIRI | 3.87 ± 1.93 | 2.21 ± 1.97 | 0.068 |
| Marker | CC, n = 71 | RC, n = 92 | p |
|---|---|---|---|
| Lymphocytes | 1730 ± 652 | 2045 ± 806 | 0.012 |
| Monocytes | 547 ± 323 | 646 ± 285 | 0.058 |
| Neutrophils | 6232 ± 2731 | 5004 ± 1771 | 0.007 |
| Platelets | 330,041 ± 120,263 | 262,151 ± 94,242 | <0.001 |
| NLR | 3.91 ± 2.17 | 2.58 ± 0.92 | <0.001 |
| PLR | 208.37 ± 115.06 | 145.14 ± 61.27 | <0.001 |
| MLR | 0.34 ± 0.23 | 0.33 ± 0.15 | 0.797 |
| AISI | 1100.89 ± 773.14 | 487.38 ± 348.89 | 0.001 |
| SII | 1468.16 ± 1099.75 | 703.87 ± 410.67 | <0.001 |
| SIRI | 2.89 ± 2.26 | 1.7 ± 0.88 | 0.001 |
| Marker | CC, n = 34 | RC, n = 44 | p |
|---|---|---|---|
| Lymphocytes | 1650± 684 | 1628 ± 780 | 0.907 |
| Monocytes | 476 ± 314 | 620 ± 298 | 0.065 |
| Neutrophils | 6.41 ± 3.94 | 5.80 ± 3.41 | 0.303 |
| Platelets | 320,625 ± 150,987 | 304,000 ± 103,749 | 0.610 |
| NLR | 4.50 ± 2.95 | 3.94 ± 2.38 | 0.490 |
| PLR | 211.13 ± 126.59 | 215.48 ± 100.73 | 0.881 |
| AISI | 1166 ± 815 | 917.50 ± 777.59 | 0.315 |
| SII | 1652.06 ± 1013 | 1226 ± 937.82 | 0.246 |
| SIRI | 3.16 ± 2.75 | 2.79 ± 2.30 | 0.624 |
| Marker | CC (M ± SD) n = 114 | RC (M ± SD) n = 104 | p |
|---|---|---|---|
| Lymphocytes | 1710 ± 682 | 2221 ± 791 | <0.001 |
| Monocytes | 705 ± 140 | 658 ± 231 | 0.726 |
| Neutrophils | 5.87 ± 2.91 | 5.1 ± 1.5 | 0.041 |
| Platelets | 334,769 ± 133,548 | 283,187 ± 110,046 | 0.004 |
| NLR | 3.85 ± 2.53 | 2.44 ±0.78 | <0.001 |
| PLR | 222.17 ± 130.44 | 141.86 ± 66.47 | <0.001 |
| MLR | 0.47 ± 0.19 | 0.31 ± 0.08 | 0.077 |
| AISI | 1606.29 ± 527.99 | 577.26 ± 322.55 | 0.013 |
| SII | 1350.09 ± 509.21 | 711.99 ± 401.11 | <0.001 |
| SIRI | 3.84 ± 2.32 | 1.59 ± 0.68 | 0.003 |
| Marker | CC, n = 16 | RC, n = 22 | p |
|---|---|---|---|
| Lymphocytes | 1810 ± 964 | 2467 ± 885 | 0.044 |
| Monocytes | 577 ± 276 | 754 ± 197 | 0.041 |
| Neutrophils | 4352 ± 1634 | 5765 ± 276 | 0.022 |
| Platelets | 245,125 ± 71,687 | 336,600 ± 148,520 | 0.022 |
| NLR | 2.63 ± 1.47 | 2.43 ±1.47 | 0.627 |
| PLR | 189.92 ± 154.41 | 139.94 ± 41.95 | 0.143 |
| MLR | 0.45 ± 0.19 | 0.36 ± 0.05 | 0.296 |
| AISI | 551.19 ± 474.46 | 617.98 ± 349.45 | 0.702 |
| SII | 711.71 ± 520.49 | 815.59 ± 444.47 | 0.536 |
| SIRI | 1.94 ± 1.23 | 1.77 ± 0.33 | 0.720 |
| Marker | CC, n = 132 | RC, n = 126 | p |
|---|---|---|---|
| Lymphocytes | 1683 ± 640 | 1956 ± 792 | 0.006 |
| Monocytes | 664 ± 131 | 623 ± 257 | 0.733 |
| Neutrophils | 6391 ± 3268 | 5176± 2276 | 0.006 |
| Platelets | 342,560 ± 139,823 | 278,718 ± 94,931 | <0.001 |
| NLR | 4.22 ± 2.71 | 2.94 ±1.67 | <0.001 |
| PLR | 222.24 ± 126.25 | 168.46 ± 90.62 | <0.001 |
| MLR | 0.43 ± 0.12 | 0.36 ± 0.21 | 0.349 |
| AISI | 1713.33 ± 741 | 587.87 ± 498.66 | 0.008 |
| SII | 1474.2 ± 763.49 | 855.33 ± 6666.9 | <0.001 |
| SIRI | 4 ± 2.14 | 1.94 ± 1.55 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feier, C.V.I.; Grama, F.; Moise, G.V.; Vonica, R.C.; Gaborean, V.; Faur, A.M.; Rus, V.I.; Muntean, C. Colorectal Cancer—One Disease, Two Fires: Distinct Inflammatory Landscapes in Colon and Rectal Cancer. Diagnostics 2025, 15, 2387. https://doi.org/10.3390/diagnostics15182387
Feier CVI, Grama F, Moise GV, Vonica RC, Gaborean V, Faur AM, Rus VI, Muntean C. Colorectal Cancer—One Disease, Two Fires: Distinct Inflammatory Landscapes in Colon and Rectal Cancer. Diagnostics. 2025; 15(18):2387. https://doi.org/10.3390/diagnostics15182387
Chicago/Turabian StyleFeier, Catalin Vladut Ionut, Florin Grama, Georgiana Viorica Moise, Razvan Constantin Vonica, Vasile Gaborean, Alaviana Monique Faur, Vladut Iosif Rus, and Calin Muntean. 2025. "Colorectal Cancer—One Disease, Two Fires: Distinct Inflammatory Landscapes in Colon and Rectal Cancer" Diagnostics 15, no. 18: 2387. https://doi.org/10.3390/diagnostics15182387
APA StyleFeier, C. V. I., Grama, F., Moise, G. V., Vonica, R. C., Gaborean, V., Faur, A. M., Rus, V. I., & Muntean, C. (2025). Colorectal Cancer—One Disease, Two Fires: Distinct Inflammatory Landscapes in Colon and Rectal Cancer. Diagnostics, 15(18), 2387. https://doi.org/10.3390/diagnostics15182387








