Heterotopic Cesarean Scar Pregnancy: A Systematic Review of Diagnosis, Management and Prognosis
Abstract
1. Introduction
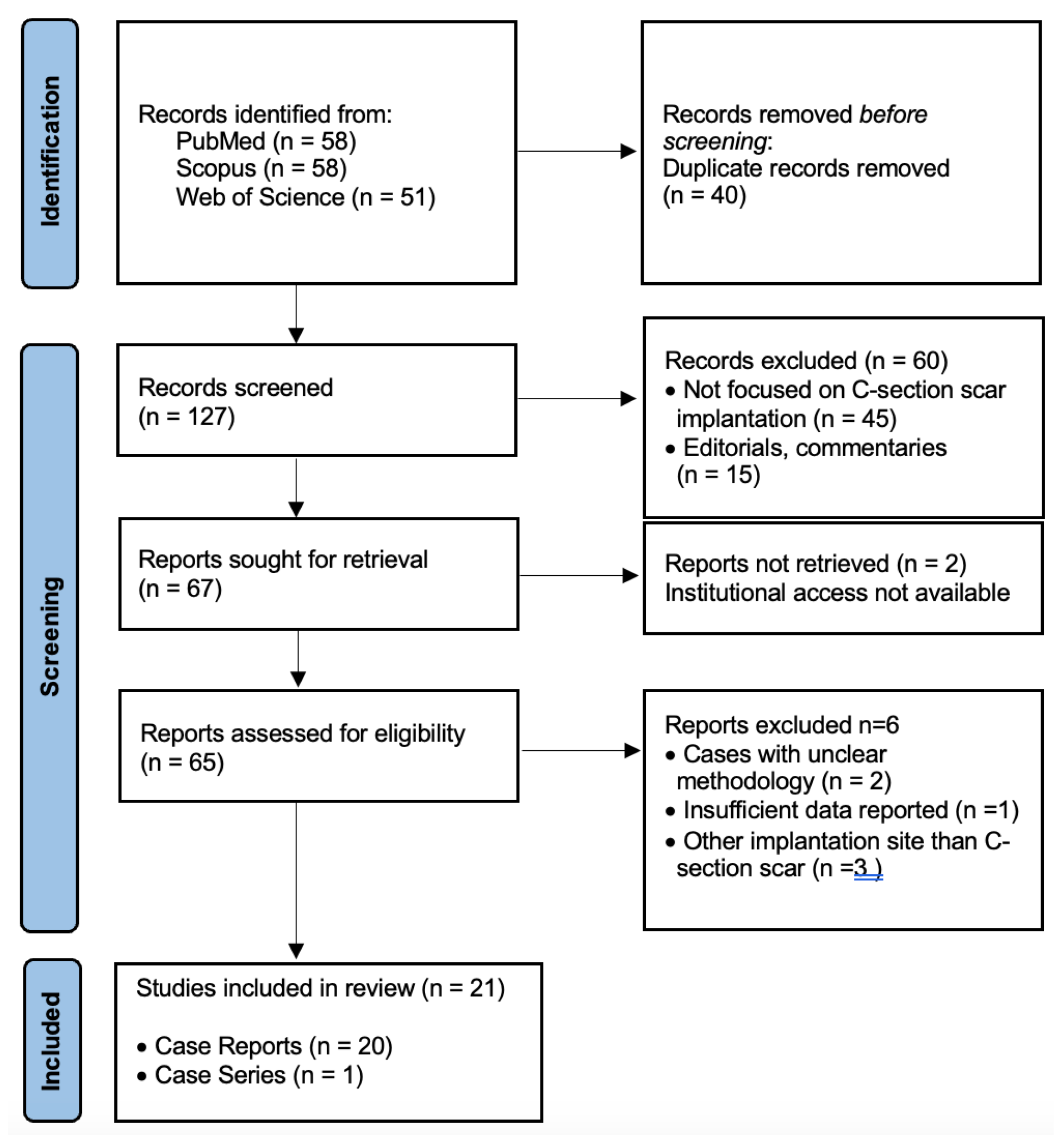
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Eligibility Criteria
- Reported confirmed cases of HCSP;
- Used a case report, case series, retrospective, or observational cohort design;
- Provided sufficient clinical information regarding diagnosis, management, and maternal–fetal outcomes.
- Exclusion criteria were:
- Isolated cesarean scar pregnancies without a concomitant intrauterine gestation;
- Heterotopic pregnancies not involving the cesarean scar;
- Abstracts, conference proceedings, or editorials without full-text availability;
- Non-English publications.
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Ethical Considerations
2.8. Data Availability
3. Results
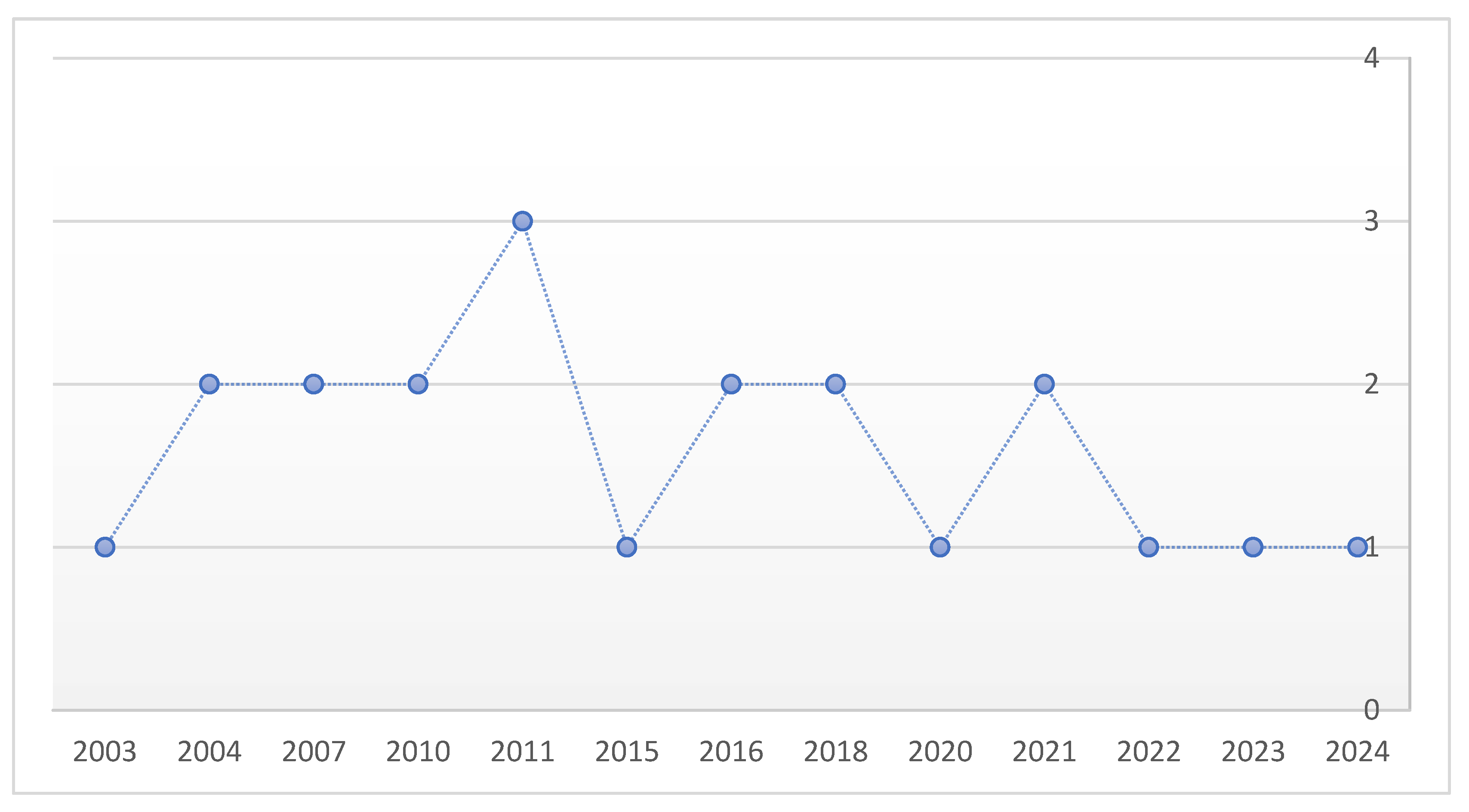
3.1. Study Characteristics
3.2. Patient Characteristics
- ●
- Maternal age was reported in most cases, ranging from 23 to 40 years, with a median of 34 years.
- ●
- Mode of conception:
- ○
- Assisted reproductive technologies (ART/IVF): 29 cases (72.5%).
- ○
- Spontaneous conception: 10 cases (25%).
- ○
- Not specified: 1 case (2.5%).
3.3. Gestational Age and Diagnosis
- The gestational age at diagnosis ranged from 4 to 13 weeks.
- The majority of cases were diagnosed in the first trimester (<12 weeks).
- Transvaginal ultrasonography (TVUS) was the primary diagnostic tool across all studies, while MRI was occasionally employed to clarify equivocal findings or assess placental invasion.
- Diagnostic delay was frequently related to the coexistence of a viable intrauterine pregnancy, which masked suspicion of an ectopic component.
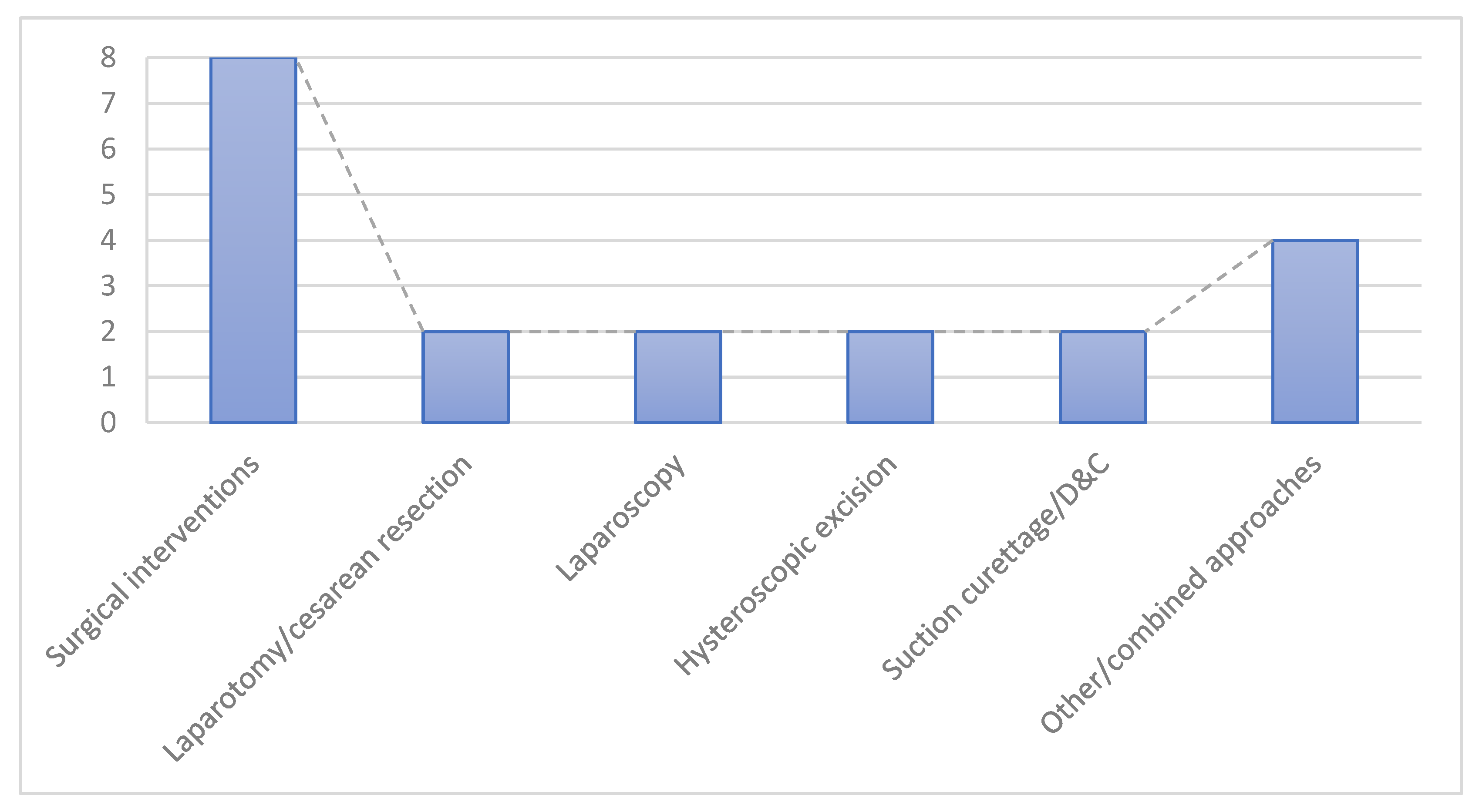
3.4. Management Approaches
- ●
- Expectant/conservative management: 14 cases (35%)—selected in clinically stable patients, especially when the ectopic sac showed spontaneous regression.
- ●
- Medical management: 14 cases (35%)—predominantly ultrasound-guided KCl injection (n = 13, including selective embryo reduction), plus one case managed with systemic methotrexate.
- ●
- Surgical interventions: 8 cases (20%), including:
- ○
- Laparotomy/cesarean resection: 2 cases.
- ○
- Laparoscopy: 2 cases.
- ○
- Hysteroscopic excision: 2 cases.
- ○
- Suction curettage/D&C: 2 cases.
- ●
- Other/combined approaches: 4 cases (10%)—e.g., uterine artery embolization (UAE) with hysterectomy, high-intensity focused ultrasound (HIFU), and mixed procedures (D&C associated with the UAE).
3.5. Maternal and Fetal Outcomes
- ●
- Maternal outcomes
- ○
- No maternal deaths were reported.
- ○
- Hemorrhage was the most frequent complication, occasionally requiring blood transfusion.
- ○
- ●
- Intrauterine pregnancy outcomes (n = 40):
- ○
- 20 cases (50%) resulted in live births, most at term, with reported neonatal weights ranging from 1300 g to 3900 g.
- ○
- 7 cases (17.5%) ended in miscarriage or elective termination, usually associated with severe maternal complications (e.g., hemorrhage, uterine rupture) or poor fetal prognosis (e.g., trisomy 13).
- ○
- 13 cases (32.5%) had unclear or unreported outcomes.
3.6. Summary Table
| First Author | Year | Study Design | Maternal Age (years) | Conception Method | Gestational Age at Diagnosis | Management Strategy | Pregnancy Outcome |
|---|---|---|---|---|---|---|---|
| Litwicka K et al. [13] | 2011 | CASE REPORT | 31 | ART/IVF | 7W/TVUS | Selective embryo reduction by ultrasound-guide KCl directed injection. | At 36 weeks, massive hemorrhage with placental detachment required emergency Cesarean section, delivering a 1900 g male. |
| Z. Laing-Aiken et al. [22] | 2020 | CASE REPORT | 38 | Spontaneous | 9W/TVUS | Ultrasound-guided suction curettage with Foley catheter tamponade failed to control bleeding, necessitating laparotomy and bilateral uterine ART/IVFery ligation, which successfully reduced hemorrhage. | At 28 + 1 weeks, emergency Cesarean was performed after preterm membrane rupture, delivering a 1200 g male who died on day 3 from extreme prematurity, RDS, and severe intraventricular hemorrhage. |
| Wang Chin-Jung et al. [23] | 2010 | CASE REPORT | 31 | ART/IVF | 7W/TVUS | Hysteroscopic management | A healthy male baby, weighed 3250 g, was delivered by cesarean section. |
| Olga Vikhareva et al. [24] | 2018 | CASE REPORT | 27 | Spontaneous | 13W/TVUS | Expectant management | A healthy male neonate weighing 2985 g was delivered, at 37 week |
| WANG CN et al. [11] | 2007 | CASE REPORT | 38 | ART/IVF | 7W/TVUS | Selective embryo reduction by ultrasound-guide KCl directed injection. | 35–36 WEEKS 1820 g, by cesarean section due to preterm labor |
| WANG F et al. [17] | 2023 | CASE REPORT | 35 | Spontaneous | 10W/TVUS | Uterine artery embolization AND HYSTERECTOMY | The patient recovered well and was discharged on postoperative day 3 |
| H. F. Yazicioglu et al. [25] | 2004 | CASE REPORT | 23 | Spontaneous | 6W/TVUS | Selective embryo reduction by ultrasound-guide KCl directed injection. | 30–31 weeks baby weighing 1530 g |
| Yu H et al. [2] | 2016 | CASE REPORT | 33 | ART/IVF | 12W/TVUS | Selective embryo reduction by ultrasound-guide KCl directed injection. | At 37 + 6 weeks of gestation the baby was delivered by elective cesarean section. A healthy male baby weighing 2890 gm was delivered |
| Aldrich et al. [26] | 2024 | CASE REPORT | 30 | Spontaneous | 6W/TVUS | Suction dilation and curettage with concurrent laparoscopic bilateral salpingectomy without complications. | she recovered well and was discharged home in stable condition the day of surgery. |
| Salomon et al. [27] | 2003 | CASE REPORT | NA | ART/IVF | 9W/TVUS | Selective embryo reduction by ultrasound-guide KCl directed injection. | Cesarean section at 36 GW, live female, 2800 g, |
| Debra Paoletti et al. [1] | 2011 | CASE REPORT | 32 | Spontaneous | 5W/TVUS | Selective embryo reduction by ultrasound-guide KCl directed injection. | NA |
| R. Armbrust et al. [28] | 2015 | CASE REPORT | 36 | ART/IVF | 7W/TVUS | The scar pregnancy was surgically excised via laparotomy. | At 37 + 0 weeks, an uncomplicated repeat Cesarean delivered a 2895 g infant. |
| DEMIREL LC et al. [9] | 2007 | CASE REPORT | 34 | Spontaneous | 6W/TVUS | Laparoscopic removal of heterotopic cesarean scar pregnancy. | Live birth by cesarean delivery at 38 weeks’ gestation. |
| Chen ZY et al. [29] | 2021 | CASE REPORT | 34 | Spontaneous | 8W/TVUS | Selective embryo aspiration followed by vacuum suction and curettage to terminate the ectopic pregnancy | Cesarean section was performed at 34 + 6 wk of gestation because of preterm membrane rupture. A healthy male baby weighing 2750 g was delivered. |
| Piotr Czuczwar et al. [5] | 2016 | CASE REPORT | 33 | Spontaneous | 6W/TVUS | Selective embryo termination was performed by ultrasound-guided KCl | The patient delivered a 3060 g healthy male infant by elective Cesarean section at 37 weeks of gestation. |
| Hsieh BC et al. [30] | 2004 | CASE REPORT | 38 | ART/IVF | 6W/TVUS | Selective embryo reduction by ultrasound-guide KCl directed injection. | Cesarean section at 32 GW |
| Dueñas-Garcia et al. [31] | 2011 | CASE REPORT | NA | Spontaneous | 5W/TVUS, MRI | MTX + leucovorin (used for abortion) | NA |
| Gupta et al. [32] | 2010 | CASE REPORT | 37 | ART/IVF | 6W/TVUS | Selective embryo reduction by ultrasound-guide KCl directed injection. | Termination at 12 GW due to trisomy 13 |
| Tymon-Rosario J. et al. [33] | 2018 | CASE REPORT | 40 | NA | 12 W/TVUS | Selective embryo reduction by ultrasound-guide KCl directed injection. | Complicated by septic abortion and hysterectomy. She was discharged home with a two-week office follow-up. |
| Kim H et al. [34] | 2022 | CASE REPORT | 36 | ART/IVF | 6 W/TVUS | Selective embryo reduction by ultrasound-guide KCl directed injection. | Cesarean section at 37 + 6 GW |
| Ouyang Y et al. [21] | 2021 | CASE 1 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Abortion (D&C) | NA |
| Ouyang Y et al. [21] | 2021 | CASE 2 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Selective embryo reduction by ultrasound-guide KCl directed injection. | IUP miscarriage at 14 GW |
| Ouyang Y et al. [21] | 2021 | CASE 3 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Hysteroscopic excision of the CSP due to placenta accreta at 8 GW | NA |
| Ouyang Y et al. [21] | 2021 | CASE 4 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | HIFU/7 | Miscarriage of IUP at 7 GW |
| Ouyang Y et al. [21] | 2021 | CASE 5 (from a series of 20 case reports) | NA | ART/IVF | 5W/TVUS | Abortion (D&C and UAE at 13 GW) | Abortion (D&C and UAE at 13 GW) |
| Ouyang Y et al. [21] | 2021 | CASE 6 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Expectant management. CSP disappeared at 20 GW | Cesarean section at 29 GW, live female, 1300 g |
| Ouyang Y et al. [21] | 2021 | CASE 7 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Expectant management | Cesarean section at 40 GW, live two females, 2900 g and 2200 g |
| Ouyang Y et al. [21] | 2021 | CASE 8 (from a series of 20 case reports) | NA | ART/IVF | 5W/TVUS | Expectant management | IUP miscarriage at 20 GW. Cesarean section at 36 GW, live female (CSP), 3000 g |
| Ouyang Y et al. [21] | 2021 | CASE 9 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Expectant management | Induced abortion at 22 GW |
| Ouyang Y et al. [21] | 2021 | CASE 10 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Expectant management | CSP miscarriage at 10 GW Cesarean section at 37 GW, live male, 2600 g |
| Ouyang Y et al. [21] | 2021 | CASE 11 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Expectant management | CSP Disappeared at 22 GW. Cesarean section at 36 GW, live female, 2900 g |
| Ouyang Y et al. [21] | 2021 | CASE 12 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Expectant management | Cesarean section at 39 GW, live female 3900 g |
| Ouyang Y et al. [21] | 2021 | CASE 13 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Expectant management | Cesarean section at 24 GW |
| Ouyang Y et al. [21] | 2021 | CASE 14 (from a series of 20 case reports) | NA | ART/IVF | 8W/TVUS | Expectant management | Cesarean section at 39 GW, live singleton, 2900 g |
| Ouyang Y et al. [21] | 2021 | CASE 15 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Expectant management | Emergency Cesarean section at 35 GW, live male, 2600 g |
| Ouyang Y et al. [21] | 2021 | CASE 16 (from a series of 20 case reports) | NA | ART/IVF | 7W/TVUS | Expectant management | Induced abortion at 24 GW |
| Ouyang Y et al. [21] | 2021 | CASE 17 (from a series of 20 case reports) | NA | ART/IVF | 6W/TVUS | Expectant management | Cesarean section at 39 GW, live male, 3150 g |
| Ouyang Y et al. [21] | 2021 | CASE 18 (from a series of 20 case reports) | NA | ART/IVF | 5W/TVUS | Abortion (D&C and UAE at 7 GW) | NA |
| Ouyang Y et al. [21] | 2021 | CASE 19 (from a series of 20 case reports) | NA | ART/IVF | 4W/TVUS | Expectant management | IUP miscarriage at 13 GW |
| Ouyang Y et al. [21] | 2021 | CASE 20 (from a series of 20 case reports) | NA | ART/IVF | 11W/TVUS | Expectant management | Uterine rupture at 12 GW |
4. Discussion and Review of the Literature
4.1. Defining Terms and Incidence
4.2. Causes and Etiopathogenesis
- Anatomical Factors
- 2.
- Procedural and Iatrogenic Factors
- 3.
- Molecular and microenvironmental factors
4.3. Clinical and Paraclinical Diagnosis
- Grade I: gestational sac embedded in less than half of the myometrial thickness;
- Grade II: sac occupies more than half of the myometrial depth;
- Grade III: sac protrudes beyond the myometrium and serosa;
4.4. Treatment
4.5. Complications
4.6. Follow-Up and Monitoring
4.7. Long Term Outcomes and Implications for Clinicians
4.8. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| HCSP | Heterotopic Cesarean Scar Pregnancy |
| HP | Heterotopic Pregnancy |
| CSP | Cesarean Scar Pregnancy |
| ART | Assisted Reproductive Technologies |
| IVF | In Vitro Fertilization |
| TVUS | Transvaginal Ultrasonography |
| MRI | Magnetic Resonance Imaging |
| β-hCG | Beta-Human Chorionic Gonadotropin |
| CDFI | Color Doppler Flow Imaging |
| HIFU | High-Intensity Focused Ultrasound |
| MTX | Methotrexate |
| KCl | Potassium Chloride |
| UAE | Uterine Artery Embolization |
| PAS | Placenta Accreta Spectrum |
| IUP | Intrauterine Pregnancy |
References
- Paoletti, D. A heterotopic pregnancy involving a caesarean section scar. Australas. J. Ultrasound Med. 2011, 14, 34–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Yu, H.; Luo, H.; Zhao, F.; Liu, X.; Wang, X. Successful selective reduction of a heterotopic cesarean scar pregnancy in the second trimester: A case report and review of the literature. BMC Pregnancy Childbirth 2016, 16, 380. [Google Scholar] [CrossRef]
- Uysal, F.; Uysal, A. Spontaneous heterotopic cesarean scar pregnancy: Conservative management by transvaginal sonographic guidance and successful pregnancy outcome. J. Ultrasound Med. 2013, 32, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Na, E.D.; Jung, I.; Choi, D.H.; Kwon, H.; Heo, S.J.; Kim, H.C.; Kang, S.H.; Cho, H. The risk factors of miscarriage and obstetrical outcomes of intrauterine normal pregnancy following heterotopic pregnancy management. Medicine 2018, 97, e12233. [Google Scholar] [CrossRef]
- Czuczwar, P.; Stępniak, A.; Woźniak, A.; Woźniak, S.; Paszkowski, T. Successful treatment of spontaneous heterotopic caesarean scar pregnancy by local potassium chloride injection with preservation of the intrauterine pregnancy. Ginekol. Pol. 2016, 87, 727. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bignardi, T.; Condous, G. Transrectal ultrasound-guided surgical evacuation of Cesarean scar ectopic pregnancy. Ultrasound Obstet. Gynecol. 2010, 35, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, X.; Yi, Y.; Gong, F.; Lin, G.; Lu, G. First-trimester diagnosis and management of Cesarean scar pregnancies after in vitro fertilization-embryo transfer: A retrospective clinical analysis of 12 cases. Reprod. Biol. Endocrinol. 2015, 13, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Einenkel, J.; Stumpp, P.; Kösling, S.; Horn, L.C.; Höckel, M. A misdiagnosed case of caesarean scar pregnancy. Arch. Gynecol. Obstet. 2005, 271, 178–181. [Google Scholar] [CrossRef]
- Demirel, L.C.; Bodur, H.; Selam, B.; Lembet, A.; Ergin, T. Laparoscopic management of heterotopic cesarean scar pregnancy with preservation of intrauterine gestation and delivery at term: Case report. Fertil. Steril. 2009, 91, 1293.e5–1293.e7. [Google Scholar] [CrossRef] [PubMed]
- Kaelin Agten, A.; Jurkovic, D.; Timor-Tritsch, I.; Jones, N.; Johnson, S.; Monteagudo, A.; Huirne, J.; Fleisher, J.; Maymon, R.; Herrera, T.; et al. CSP Collaborative Network. First-trimester cesarean scar pregnancy: A comparative analysis of treatment options from the international registry. Am. J. Obstet. Gynecol. 2024, 230, 669.e1–669.e19. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.N.; Chen, C.K.; Wang, H.S.; Chiueh, H.Y.; Soong, Y.K. Successful management of heterotopic cesarean scar pregnancy combined with intrauterine pregnancy after in vitro fertilization-embryo transfer. Fertil. Steril. 2007, 88, 706.e13–706.e16. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Leveno, K.J.; Dashe, J.S.; Hoffman, B.L.; Spong, C.Y.; Casey, B.M. (Eds.) Ectopic pregnancy. In Williams Obstetrics, 26e; McGrawHill: Columbus, OH, USA, 2022; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2977§ionid=254987327 (accessed on 10 August 2025).
- Litwicka, K.; Greco, E.; Prefumo, F.; Fratelli, N.; Scarselli, F.; Ferrero, S.; Iammarrone, E.; Frusca, T. Successful management of a triplet heterotopic caesarean scar pregnancy after in vitro fertilization-embryo transfer. Fertil. Steril. 2011, 95, 291.e1–291.e3. [Google Scholar] [CrossRef]
- Honda, R.; Matsuura, K.; Okamura, H. Heterotopic cervical pregnancy with preservation of the intrauterine gestation. Reprod. Med. Biol. 2005, 4, 221–223. [Google Scholar] [CrossRef]
- Larsen, J.V.; Solomon, M.H. Pregnancy in a uterine scar sacculus-an unusual cause of postabortal haemorrhage. A case report. S. Afr. Med. J. 1978, 53, 142–143. [Google Scholar] [PubMed]
- Sadeghi, H.; Rutherford, T.; Rackow, B.W.; Campbell, K.H.; Duzyj, C.M.; Guess, M.K.; Kodaman, P.H.; Norwitz, E.R. Cesarean scar ectopic pregnancy: Case series and review of the literature. Am. J. Perinatol. 2010, 27, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Vaught, A.; Rosner, M.; Baschat, A.; Darwin, K.; Halscott, T.; Kush, M.; Miller, J.; Gomez, E. Dichorionic diamniotic heterotopic twin gestation with cesarean section scar implantation and placenta increta. Radiol. Case Rep. 2023, 18, 4006–4011. [Google Scholar] [CrossRef]
- Maleki, A.; Khalid, N.; Rajesh Patel, C.; El-Mahdi, E. The rising incidence of heterotopic pregnancy: Current perspectives and associations with in-vitro fertilization. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 266, 138–144. [Google Scholar] [CrossRef]
- Silva, B.; Viana Pinto, P.; Costa, M.A. Cesarean Scar Pregnancy: A systematic review on expectant management. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 288, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Authreya, A.J.; Agrawal, P.; Makam, A. Ultrasound-guided procedures in the management of heterotopic caesarean scar pregnancy—A review of case reports and case series. Australas. J. Ultrasound Med. 2021, 24, 70–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ouyang, Y.; Chen, H.; Lin, G.; Xiang, S.; Qin, J.; Gong, F.; Li, X. Heterotopic Cesarean Scar Pregnancy: An Analysis of 20 Cases Following in vitro Fertilization-Embryo Transfer. J. Ultrasound Med. 2021, 40, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Laing-Aiken, Z.; Robson, D.; Wu, J. Surgical management of first-trimester bleeding in a heterotopic caesarean scar pregnancy: A case report and review of literature. Case Rep. Womens Health 2020, 27, e00209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.J.; Tsai, F.; Chen, C.; Chao, A. Hysteroscopic management of heterotopic cesarean scar pregnancy. Fertil. Steril. 2010, 94, 1529.e15–1529.e18. [Google Scholar] [CrossRef]
- Vikhareva, O.; Nedopekina, E.; Herbst, A. Normal vaginal delivery at term after expectant management of heterotopic caesarean scar pregnancy: A case report. J. Med. Case Rep. 2018, 12, 179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yazicioglu, H.F.; Turgut, S.; Madazli, R.; Aygün, M.; Cebi, Z.; Sönmez, S. An unusual case of heterotopic twin pregnancy managed successfully with selective feticide. Ultrasound Obstet. Gynecol. 2004, 23, 626–627. [Google Scholar] [CrossRef]
- Aldrich, Z.F.; Ow, R.; Modii, K.; O’Leary, T. Spontaneous Heterotopic Cesarean Scar Triplet Gestation Following Uterine Ablation. AJP Rep. 2024, 14, e91–e95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salomon, L.J.; Fernandez, H.; Chauveaud, A.; Doumerc, S.; Frydman, R. Successful management of a heterotopic Caesarean scar pregnancy: Potassium chloride injection with preservation of the intrauterine gestation: Case report. Hum. Reprod. 2003, 18, 189–191. [Google Scholar] [CrossRef]
- Armbrust, R.; Krätschell, R.; Henrich, W.; David, M. Operative Therapy for Heterotopic Scar Pregnancy and Successful Birth of the Intrauterine Foetus—Case Report and Review of the Literature. Geburtshilfe Frauenheilkd. 2015, 75, 384–388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Z.Y.; Zhou, Y.; Qian, Y.; Luo, J.M.; Huang, X.F.; Zhang, X.M. Management of heterotopic cesarean scar pregnancy with preservation of intrauterine pregnancy: A case report. World J. Clin. Cases 2021, 9, 6428–6434. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, B.C.; Hwang, J.L.; Pan, H.S.; Huang, S.C.; Chen, C.Y.; Chen, P.H. Heterotopic Caesarean scar pregnancy combined with intrauterine pregnancy successfully treated with embryo aspiration for selective embryo reduction: Case report. Hum. Reprod. 2004, 19, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-Garcia, O.F.; Young, C. Heterotopic cesarean scar pregnancy associated with a levonorgestrel-releasing intrauterine device. Int. J. Gynaecol. Obstet. 2011, 114, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Vaikousi, E.; Whitlow, B. Heterotopic caesarean section scar pregnancy. J. Obstet. Gynaecol. 2010, 30, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Tymon-Rosario, J.; Chuang, M. Selective Reduction of a Heterotopic Cesarean Scar Pregnancy Complicated by Septic Abortion. Case Rep. Obstet. Gynecol. 2018, 2018, 6478589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.; Koh, J.H.; Lee, J.; Sim, Y.; Lee, S.H.; Lee, S.J.; Ahn, J.W.; Roh, H.J.; Kim, J.S. Successful Full-Term Delivery via Selective Ectopic Embryo Reduction Accompanied by Uterine Cerclage in a Heterotopic Cesarean Scar Pregnancy: A Case Report and Literature Review. Diagnostics 2022, 12, 762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krispin, E.; Belfort, M.A.; Shamshirsaz, A.A. Surgical management of first-trimester heterotopic dichorionic diamniotic Cesarean scar pregnancy. Ultrasound Obstet. Gynecol. 2022, 59, 823–824. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yue, Y.; Hou, X.; Han, H.; Wang, W.; Lin, X. Combined hysteroscopic Bigatti shaver (IBS) and resectoscope removal of a heterotopic cesarean scar pregnancy in the first trimester. Fertil. Steril. 2024, 122, 546–548. [Google Scholar] [CrossRef]
- Tariq Ahmed Alabsi, M.; Sunder, A.; AlSada, A. Heterotopic Cesarean Scar Pregnancy: A Case Report. Cureus 2024, 16, e55943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, P.; Li, X.; Li, W.; Li, Y.; Zhang, Y.; Yang, Y. The trend of the distribution of ectopic pregnancy sites and the clinical characteristics of caesarean scar pregnancy. Reprod. Health 2022, 19, 182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharami, S.H.; Arzpeyma, S.F.; Montazeri, S.; Ghadim-Limudahi, Z.H.; Eslami-Kenarsari, H.; Attari, S.M.; Kamakoli, H.T. Clinical and historical features of cesarean scar pregnancies in a tertiary hospital with a high rate of cesarean section: A case-control survey. Health Sci. Rep. 2024, 7, e1823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teshima, D.R.K.; Pereira, P.P.; Francisco, R.P.V.; De Oliveira, M.A.; Schulz, R.; Antonângelo, L.; Cabar, F.R. Tissue concentration of vascular endothelial growth factor is not related to the depth of trophoblastic invasion in ampullary pregnancies-A pilot study. Front. Pharmacol. 2022, 13, 989031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hewlett, K.; Howell, C.M. Heterotopic pregnancy: Simultaneous viable and nonviable pregnancies. JAAPA 2020, 33, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Lincenberg, K.R.; Behrman, E.R.; Bembry, J.S.; Kovac, C.M. Uterine Rupture with Cesarean Scar Heterotopic Pregnancy with Survival of the Intrauterine Twin. Case Rep. Obstet. Gynecol. 2016, 2016, 6832094. [Google Scholar] [CrossRef]
- Qian, Z.D.; Weng, Y.; Wang, C.F.; Huang, L.L.; Zhu, X.M. Research on the expression of integrin β3 and leukaemia inhibitory factor in the decidua of women with cesarean scar pregnancy. BMC Pregnancy Childbirth 2017, 17, 84. [Google Scholar] [CrossRef]
- Qian, Z.D.; Guo, Q.Y.; Huang, L.L. Identifying risk factors for recurrent cesarean scar pregnancy: A case-control study. Fertil. Steril. 2014, 102, 129–134.e1. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Kumar, S.; Rani, A.; Patil, S.; Voorkara, U.; SKamath, V. Interventional Challenges in Non-Tubal Ectopic Pregnancy. J. Fam. Reprod Health 2022, 16, 78–85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stabile, G.; Zinicola, G.; Romano, F.; Buonomo, F.; Mangino, F.P.; Ricci, G. Management of Non-Tubal Ectopic Pregnancies: A Single Center Experience. Diagnostics 2020, 10, 652. [Google Scholar] [CrossRef]
- Chong, Y.; Wang, W.; Zhang, A.; Zhao, Y. Ultrasound for monitoring twin cesarean scar pregnancy following feticide. J. Int. Med. Res. 2022, 50, 3000605221095683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiao, L.; Lu, J.; Zhang, B.; Liu, A.; Wang, X.; Wang, M.; Li, J.; Meng, J. Accuracy of multimodal vaginal ultrasound in the detection and assessment of scar healing after caesarean section: A correlational meta-analysis. Ann. Med. 2025, 57, 2523558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, S.Y.; Hsieh, C.J.; Tu, Y.A.; Li, Y.P.; Lee, C.N.; Hsu, W.W.; Shih, J.C. New ultrasound grading system for cesarean scar pregnancy and its implications for management strategies: An observational cohort study. PLoS ONE 2018, 13, e0202020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tarafdari, A.; Eshraghi, N.; Shbeeb, H.; Poorabdoli, M.; Ghaemi, M.; Parsaei, M. Spontaneous Heterotopic Triplet Pregnancies with Single Intrauterine and Twin Tubal Pregnancies Post-Levonorgestrel Use: Case Report and Literature Review. Clin. Case Rep. 2025, 13, e70080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Houser, M.; Kandalaft, N.; Khati, N.J. Ectopic pregnancy: A resident’s guide to imaging findings and diagnostic pitfalls. Emerg. Radiol. 2022, 29, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Bohiltea, R.; Ducu, I.; Mihai, B.; Iordache, A.M.; Dorobat, B.; Vladareanu, E.M.; Iordache, S.M.; Bohiltea, A.T.; Bacalbasa, N.; Grigorescu, C.E.A.; et al. Uterine Artery Embolization Combined with Subsequent Suction Evacuation as Low-Risk Treatment for Cesarean Scar Pregnancy. Diagnostics 2021, 11, 2350. [Google Scholar] [CrossRef]
- Leziak, M.; Żak, K.; Frankowska, K.; Ziółkiewicz, A.; Perczyńska, W.; Abramiuk, M.; Tarkowski, R.; Kułak, K. Future Perspectives of Ectopic Pregnancy Treatment-Review of Possible Pharmacological Methods. Int. J. Environ. Res. Public Health 2022, 19, 14230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mao, Y.; Peng, Y.; Zheng, M.; Xiao, J.; Gong, F.; Li, X.; Ouyang, Y. First-trimester ultrasound diagnosis and risk factor analysis of cesarean scar pregnancy after in vitro fertilization-embryo transfer. Quant. Imaging Med. Surg. 2024, 14, 5028–5039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohapatra, I.; Samantray, S.R. Scar Ectopic Pregnancy—An Emerging Challenge. Cureus 2021, 13, e16673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karbasi, M.; Aletaha, R.; Ahangar-Sirous, R.; Alamdari, A.H.; Gharepapagh, E.; Rezaei, S. A rare case report of heterotopic cesarean scar pregnancy in the 8th week of gestation that was managed successfully by exploratory laparotomy with dilation and curettage. Clin. Case Rep. 2024, 12, e9025. [Google Scholar] [CrossRef]
- Timor-Tritsch, I.E.; Cali, G.; Monteagudo, A.; Khatib, N.; Berg, R.E.; Forlani, F.; Avizova, E. Foley balloon catheter to prevent or manage bleeding during treatment for cervical and Cesarean scar pregnancy. Ultrasound Obstet. Gynecol. 2015, 46, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Luo, Y.; Huang, J. Cesarean scar pregnancy with expectant management. J. Obstet. Gynaecol. Res. 2022, 48, 1683–1690. [Google Scholar] [CrossRef]
- OuYang, Z.; Yin, Q.; Xu, Y.; Ma, Y.; Zhang, Q.; Yu, Y. Heterotopic cesarean scar pregnancy: Diagnosis, treatment, and prognosis. J. Ultrasound Med. 2014, 33, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Guraslan, H.; Akgul, O.K.; Aydin, D.E.; Kovalak, E.E.; Aksoy, N.K.; Aydin, T.O. Laparoscopic removal of heterotopic cesarean scar pregnancy. Fertil. Steril. 2024, 122, 543–545. [Google Scholar] [CrossRef]
- Ge, F.; Ding, W.; Zhao, K.; Qu, P. Management of heterotopic pregnancy: Clinical analysis of sixty-five cases from a single institution. Front. Med. 2023, 10, 1166446. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Săndulescu, M.S.; Veliscu Carp, A.; Vrabie, S.C.; Anișoara, S.; Vulcănescu, A.; Mihaela, M.; Dominic, I.; Pătrașcu, Ș.; Dijmărescu, L.; Manolea, M.M. Heterotopic Cesarean Scar Pregnancy: A Systematic Review of Diagnosis, Management and Prognosis. Diagnostics 2025, 15, 2373. https://doi.org/10.3390/diagnostics15182373
Săndulescu MS, Veliscu Carp A, Vrabie SC, Anișoara S, Vulcănescu A, Mihaela M, Dominic I, Pătrașcu Ș, Dijmărescu L, Manolea MM. Heterotopic Cesarean Scar Pregnancy: A Systematic Review of Diagnosis, Management and Prognosis. Diagnostics. 2025; 15(18):2373. https://doi.org/10.3390/diagnostics15182373
Chicago/Turabian StyleSăndulescu, Maria Sidonia, Andreea Veliscu Carp, Sidonia Cătălina Vrabie, Siminel Anișoara, Anca Vulcănescu, Marin Mihaela, Iliescu Dominic, Ștefan Pătrașcu, Lorena Dijmărescu, and Maria Magdalena Manolea. 2025. "Heterotopic Cesarean Scar Pregnancy: A Systematic Review of Diagnosis, Management and Prognosis" Diagnostics 15, no. 18: 2373. https://doi.org/10.3390/diagnostics15182373
APA StyleSăndulescu, M. S., Veliscu Carp, A., Vrabie, S. C., Anișoara, S., Vulcănescu, A., Mihaela, M., Dominic, I., Pătrașcu, Ș., Dijmărescu, L., & Manolea, M. M. (2025). Heterotopic Cesarean Scar Pregnancy: A Systematic Review of Diagnosis, Management and Prognosis. Diagnostics, 15(18), 2373. https://doi.org/10.3390/diagnostics15182373








