Diagnostic Power of the Fibrinogen-to-Albumin Ratio for Estimating Malignancy in Patients with Adnexal Masses: A Methodological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Data and Variables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montes de Oca, M.K.; Dotters-Katz, S.K.; Kuller, J.A.; Previs, R.A. Adnexal masses in pregnancy. Obstet. Gynecol. Surv. 2021, 76, 437–450. [Google Scholar] [CrossRef]
- Hermans, A.J.; Kluivers, K.B.; Janssen, L.M.; Siebers, A.G.; Wijnen, M.; Bulten, J.; Massuger, L.; Coppus, S. Adnexal masses in children, adolescents and women of reproductive age in the netherlands: A nationwide population-based cohort study. Gynecol. Oncol. 2016, 143, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.R.; Shah, A.C. Diagnosis and management of adnexal masses. Int. J. Reprod. Contracept. Obstet. Gynecol. 2024, 13, 1735–1739. [Google Scholar] [CrossRef]
- Kang, G.G.; So, K.A.; Hwang, J.Y.; Kim, N.R.; Yang, E.J.; Shim, S.H.; Lee, S.J.; Kim, T.J. Ultrasonographic diagnosis and surgical outcomes of adnexal masses in children and adolescents. Sci. Rep. 2022, 12, 3949. [Google Scholar] [CrossRef] [PubMed]
- Xac, M.C.; Jetelina, K.K.; Jarin, J.; Wilson, E. Benign, borderline, and malignant pediatric adnexal masses: A 10-year review. J. Pediatr. Adolesc. Gynecol. 2021, 34, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Melamed, A.; Miller, D.T. Management of ovarian masses in the older woman. In Handbook of Gynecology; Shoupe, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–10. [Google Scholar]
- Terzic, M.; Rapisarda, A.M.C.; Della Corte, L.; Manchanda, R.; Aimagambetova, G.; Norton, M.; Garzon, S.; Riemma, G.; King, C.R.; Chiofalo, B.; et al. Diagnostic work-up in paediatric and adolescent patients with adnexal masses: An evidence-based approach. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2021, 41, 503–515. [Google Scholar] [CrossRef]
- Fischerova, D.; Zikan, M.; Dundr, P.; Cibula, D. Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist 2012, 17, 1515–1533. [Google Scholar] [CrossRef]
- Huchon, C.; Bourdel, N.; Abdel Wahab, C.; Azaïs, H.; Bendifallah, S.; Bolze, P.A.; Brun, J.L.; Canlorbe, G.; Chauvet, P.; Chereau, E.; et al. Borderline ovarian tumors: French guidelines from the cngof. Part 1. Epidemiology, biopathology, imaging and biomarkers. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101965. [Google Scholar] [CrossRef]
- Timmerman, D.; Planchamp, F.; Bourne, T.; Landolfo, C.; du Bois, A.; Chiva, L.; Cibula, D.; Concin, N.; Fischerova, D.; Froyman, W.; et al. Esgo/isuog/iota/esge consensus statement on preoperative diagnosis of ovarian tumors. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2021, 58, 148–168. [Google Scholar] [CrossRef]
- Biggs, W.S.; Marks, S.T. Diagnosis and management of adnexal masses. Am. Fam. Physician 2016, 93, 676–681. [Google Scholar] [PubMed]
- Stein, E.B.; Roseland, M.E.; Shampain, K.L.; Wasnik, A.P.; Maturen, K.E. Contemporary guidelines for adnexal mass imaging: A 2020 update. Abdom. Radiol. 2021, 46, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, R.F.; Timmerman, D.; Strachowski, L.M.; Froyman, W.; Benacerraf, B.R.; Bennett, G.L.; Bourne, T.; Brown, D.L.; Coleman, B.G.; Frates, M.C.; et al. O-rads us risk stratification and management system: A consensus guideline from the acr ovarian-adnexal reporting and data system committee. Radiology 2020, 294, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Van Calster, B.; Testa, A.; Savelli, L.; Fischerova, D.; Froyman, W.; Wynants, L.; Van Holsbeke, C.; Epstein, E.; Franchi, D.; et al. Predicting the risk of malignancy in adnexal masses based on the simple rules from the international ovarian tumor analysis group. Am. J. Obstet. Gynecol. 2016, 214, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, S.; Valentin, L.; Ceusters, J.; Testa, A.C.; Landolfo, C.; Sladkevicius, P.; Van Holsbeke, C.; Domali, E.; Fruscio, R.; Epstein, E.; et al. External validation of the ovarian-adnexal reporting and data system (o-rads) lexicon and the international ovarian tumor analysis 2-step strategy to stratify ovarian tumors into o-rads risk groups. JAMA Oncol. 2023, 9, 225–233. [Google Scholar] [CrossRef]
- Watrowski, R.; Obermayr, E.; Wallisch, C.; Aust, S.; Concin, N.; Braicu, E.I.; Van Gorp, T.; Hasenburg, A.; Sehouli, J.; Vergote, I.; et al. Biomarker-based models for preoperative assessment of adnexal mass: A multicenter validation study. Cancers 2022, 14, 1780. [Google Scholar] [CrossRef]
- Matsas, A.; Stefanoudakis, D.; Troupis, T.; Kontzoglou, K.; Eleftheriades, M.; Christopoulos, P.; Panoskaltsis, T.; Stamoula, E.; Iliopoulos, D.C. Tumor markers and their diagnostic significance in ovarian cancer. Life 2023, 13, 1689. [Google Scholar] [CrossRef]
- Sahin, F.; Aktürk, E.; Günkaya, O.S.; Özdemir, S.; Konal, M.; Genç, S.; Yurci, A.; Akbayir, O. Borderline ovarian tumors: Twenty years of experience at a tertiary center. Anatol. Curr. Med. J. 2023, 5, 196–200. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, S.; Xiang, Y.; Yang, J.; Jia, C.; Leng, J. Factors associated with misdiagnosis of frozen section of mucinous borderline ovarian tumor. J. Int. Med. Res. 2019, 47, 96–104. [Google Scholar] [CrossRef]
- Baykuş, Y.; Deniz, R.; Çelik Kavak, E. Factors affecting compliance of intraoperative frozen and final histopathology in borderline ovarian tumors: Retrospective cohort study. J. Surg. Med. 2019, 3, 316–319. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, M.J.; Gao, B. Hepatocytes: A key cell type for innate immunity. Cell. Mol. Immunol. 2016, 13, 301–315. [Google Scholar] [CrossRef]
- Powanda, M.C.; Moyer, E.D. A brief, highly selective history of acute phase proteins as indicators of infection, inflammation and injury. Inflammopharmacology 2021, 29, 897–901. [Google Scholar] [CrossRef] [PubMed]
- May, J.E.; Wolberg, A.S.; Lim, M.Y. Disorders of fibrinogen and fibrinolysis. Hematol. Oncol. Clin. N. Am. 2021, 35, 1197–1217. [Google Scholar] [CrossRef]
- Bode, J.G.; Albrecht, U.; Häussinger, D.; Heinrich, P.C.; Schaper, F. Hepatic acute phase proteins—Regulation by il-6- and il-1-type cytokines involving stat3 and its crosstalk with nf-κb-dependent signaling. Eur. J. Cell Biol. 2012, 91, 496–505. [Google Scholar] [CrossRef]
- Schmidt-Arras, D.; Rose-John, S. Il-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.S.; Gelinas, D.; Dreesen, E.; Van Santbergen, J.; Andersen, J.T.; Silvestri, N.J.; Kiss, J.E.; Sleep, D.; Rader, D.J.; Kastelein, J.J.P.; et al. Clinical significance of serum albumin and implications of fcrn inhibitor treatment in igg-mediated autoimmune disorders. Front. Immunol. 2022, 13, 892534. [Google Scholar] [CrossRef] [PubMed]
- Makkar, K.; Sharma, Y.P.; Batta, A.; Hatwal, J.; Panda, P.K. Role of fibrinogen, albumin and fibrinogen to albumin ratio in determining angiographic severity and outcomes in acute coronary syndrome. World J. Cardiol. 2023, 15, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhu, C.; Zhang, Q.; Hu, Z.; Ji, K.; Qian, L. Association between fibrinogen-to-albumin ratio and prognosis of patients with heart failure. Eur. J. Clin. Investig. 2023, 53, e14049. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Deng, H.; Lei, B.; Chen, L.; Zhang, X.; Sha, D. The prognostic value of fibrinogen to albumin ratio in malignant tumor patients: A meta-analysis. Front. Oncol. 2022, 12, 985377. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Yan, F.H.; Liu, C.; Chen, J.; Wang, D.; Zhang, C.H.; Lou, C.J.; Lian, J.; Yao, Y.; Wang, B.J.; et al. Systemic inflammatory biomarkers, especially fibrinogen to albumin ratio, predict prognosis in patients with pancreatic cancer. Cancer Res. Treat. 2021, 53, 131–139. [Google Scholar] [CrossRef]
- Xu, W.Y.; Zhang, H.H.; Xiong, J.P.; Yang, X.B.; Bai, Y.; Lin, J.Z.; Long, J.Y.; Zheng, Y.C.; Zhao, H.T.; Sang, X.T. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J. Gastroenterol. 2018, 24, 3281–3292. [Google Scholar] [CrossRef]
- Wang, K.; Xu, W.; Zha, B.; Shi, J.; Wu, G.; Ding, H. Fibrinogen to albumin ratio as an independent risk factor for type 2 diabetic kidney disease. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 4557–4567. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.W.; Lyu, G.R.; Kang, Z.; Li, L.Y.; Zhang, Y.; Huang, Y.J. Comparison of o-rads, gi-rads, and adnex for diagnosis of adnexal masses: An external validation study conducted by junior sonologists. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2022, 41, 1497–1507. [Google Scholar] [CrossRef]
- Pan, R.K.; Zhang, S.Q.; Zhang, X.Y.; Xu, T.; Cui, X.W.; Li, R.; Yu, M.; Zhang, B. Clinical value of acr o-rads combined with ca125 in the risk stratification of adnexal masses. Front. Oncol. 2024, 14, 1369900. [Google Scholar] [CrossRef]
- Barreñada, L.; Ledger, A.; Dhiman, P.; Collins, G.; Wynants, L.; Verbakel, J.Y.; Timmerman, D.; Valentin, L.; Van Calster, B. Adnex risk prediction model for diagnosis of ovarian cancer: Systematic review and meta-analysis of external validation studies. BMJ Med. 2024, 3, e000817. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Su, H.; Lyu, G. Comparison of the value of the gi-rads and adnex models in the diagnosis of adnexal tumors by junior physicians. Front. Oncol. 2024, 14, 1435636. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J. Prognostic role of fibrinogen-to-albumin ratio in patients with gynecological cancers: A meta-analysis. Front. Oncol. 2025, 15, 1580940. [Google Scholar] [CrossRef]
- Yu, W.; Ye, Z.; Fang, X.; Jiang, X.; Jiang, Y. Preoperative albumin-to-fibrinogen ratio predicts chemotherapy resistance and prognosis in patients with advanced epithelial ovarian cancer. J. Ovarian Res. 2019, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Qi, J.; Zhao, J.; Bai, S.; Wu, Q.; Xu, R. Diagnostic value of ca125, he4, and systemic immune-inflammation index in the preoperative investigation of ovarian masses. Medicine 2023, 102, e35240. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, T.; Ouyang, L.; Li, X.; He, W.; Zhang, Z.; Shen, H.; You, Z.; Yang, G.; Lai, H. A novel diagnostic nomogram based on serological and ultrasound findings for preoperative prediction of malignancy in patients with ovarian masses. Gynecol. Oncol. 2021, 160, 704–712. [Google Scholar] [CrossRef]
- Liu, J.; Berchuck, A.; Backes, F.J.; Cohen, J.; Grisham, R.; Leath, C.A.; Martin, L.; Matei, D.; Miller, D.S.; Robertson, S.; et al. Nccn guidelines® insights: Ovarian cancer/fallopian tube cancer/primary peritoneal cancer, version 3.2024. J. Natl. Compr. Cancer Netw. JNCCN 2024, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Mabuchi, S.; Kawahara, N.; Kawaguchi, R. Prognostic significance of tumor laterality in advanced ovarian cancer. Obstet. Gynecol. Sci. 2021, 64, 524–531. [Google Scholar] [CrossRef]
- Adhikari, L.; Hassell, L. Who Classification. Available online: https://www.pathologyoutlines.com/topic/ovarytumorwhoclassif.html (accessed on 19 May 2025).
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Pelayo, M.; Sancho-Sauco, J.; Sanchez-Zurdo, J.; Abarca-Martinez, L.; Borrero-Gonzalez, C.; Sainz-Bueno, J.A.; Alcazar, J.L.; Pelayo-Delgado, I. Ultrasound features and ultrasound scores in the differentiation between benign and malignant adnexal masses. Diagnostics 2023, 13, 2152. [Google Scholar] [CrossRef]
- Meys, E.M.J.; Jeelof, L.S.; Achten, N.M.J.; Slangen, B.F.M.; Lambrechts, S.; Kruitwagen, R.; Van Gorp, T. Estimating risk of malignancy in adnexal masses: External validation of the adnex model and comparison with other frequently used ultrasound methods. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2017, 49, 784–792. [Google Scholar] [CrossRef]
- Bozkurt, M.; Yumru, A.E.; Aral, I. Evaluation of the importance of the serum levels of ca-125, ca15-3, ca-19-9, carcinoembryonic antigen and alpha fetoprotein for distinguishing benign and malignant adnexal masses and contribution of different test combinations to diagnostic accuracy. Eur. J. Gynaecol. Oncol. 2013, 34, 540–544. [Google Scholar] [PubMed]
- Bacanakgil, B.H.; Unal, F.; Aliyeva, S.; Oz, I.S.; Karimova, R. Serum tumor markers for preoperative discrimination of benign and malignant adnexal masses. Cell. Mol. Med. Res. 2017, 1, 27–30. [Google Scholar] [CrossRef]
- Skates, S.J.; Mai, P.; Horick, N.K.; Piedmonte, M.; Drescher, C.W.; Isaacs, C.; Armstrong, D.K.; Buys, S.S.; Rodriguez, G.C.; Horowitz, I.R.; et al. Large prospective study of ovarian cancer screening in high-risk women: Ca125 cut-point defined by menopausal status. Cancer Prev. Res. 2011, 4, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Van Calster, B.; Valentin, L.; Van Holsbeke, C.; Zhang, J.; Jurkovic, D.; Lissoni, A.A.; Testa, A.C.; Czekierdowski, A.; Fischerová, D.; Domali, E.; et al. A novel approach to predict the likelihood of specific ovarian tumor pathology based on serum ca-125: A multicenter observational study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Sagi-Dain, L.; Lavie, O.; Auslander, R.; Sagi, S. Clinical use and optimal cutoff value of ca15-3 in evaluation of adnexal mass: Retrospective cohort study and review of the literature. Am. J. Clin. Oncol. 2018, 41, 838–844. [Google Scholar] [PubMed]
- Ruma, S.A.; Vinod, R.; Jain, S.; Huhtinen, K.; Hynninen, J.; Leivo, J.; Pettersson, K.; Sundfeldt, K.; Gidwani, K. Muc1 and glycan probing of ca19-9 captured biomarkers from cyst fluids and serum provides enhanced recognition of ovarian cancer. Sci. Rep. 2025, 15, 3171. [Google Scholar] [CrossRef] [PubMed]
- Sagi-Dain, L.; Lavie, O.; Auslander, R.; Sagi, S. Ca 19-9 in evaluation of adnexal mass: Retrospective cohort analysis and review of the literature. Int. J. Biol. Markers 2015, 30, e333–e340. [Google Scholar] [CrossRef]
- Sagi-Dain, L.; Lavie, O.; Auslander, R.; Sagi, S. Cea in evaluation of adnexal mass: Retrospective cohort analysis and review of the literature. Int. J. Biol. Markers 2015, 30, e394–e400. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, G. Prognostic significance of the ratio of fibrinogen and albumin in human malignancies: A meta-analysis. Cancer Manag. Res. 2019, 11, 3381–3393. [Google Scholar] [CrossRef]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the interface of human health and disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef]
- Suri, A.; Perumal, V.; Ammalli, P.; Suryan, V.; Bansal, S.K. Diagnostic measures comparison for ovarian malignancy risk in epithelial ovarian cancer patients: A meta-analysis. Sci. Rep. 2021, 11, 17308. [Google Scholar] [CrossRef]
- Shittu, K.A.; Rabiu, K.A.; Akinola, O.I.; Ahmed, S.B.; Adewunmi, A.A. Comparison of the diagnostic accuracy of he4 with ca125 and validation of the roma index in differentiating malignant and benign epithelial ovarian tumours among patients in lagos, nigeria. Ecancermedicalscience 2023, 17, 1568. [Google Scholar] [CrossRef]
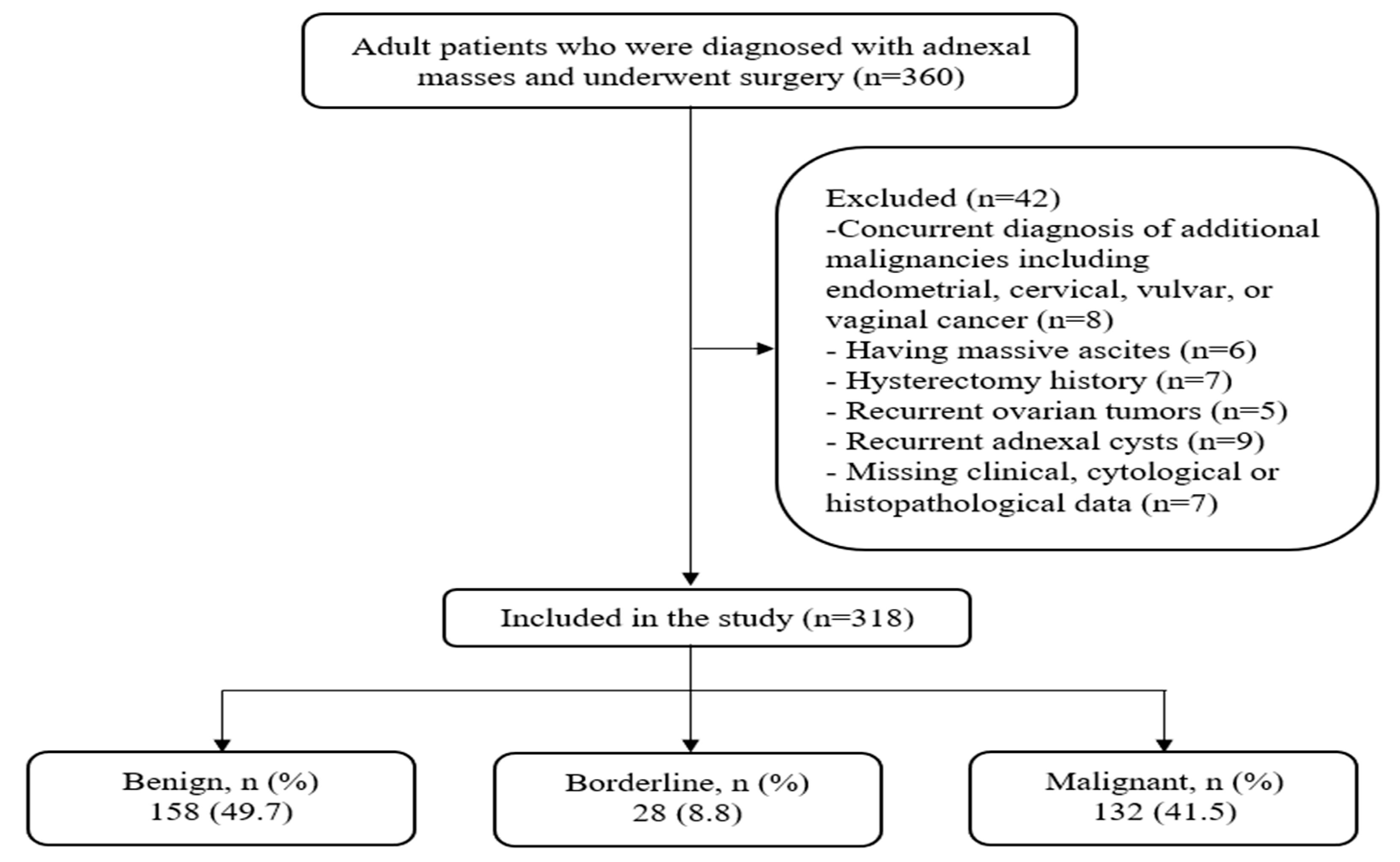

| Variables | All Patients (n = 318) | Patients with Benign Masses (n = 158) | Patients with Borderline Tumors (n = 28) | Patients with Malignant Tumors (n = 132) | p |
|---|---|---|---|---|---|
| Age (year), Median (IQR) | 51.0 (44.0–60.0) | 49.0 (41.0–57.0) | 51.5 (42.0–60.5) | 57.0 (49.0–65.0) | <0.001 a |
| Postmenopausal, n (%) | 209 (65.7) | 87 (55.1) | 18 (64.3) | 104 (78.8) | <0.001 b |
| Fibrinogen (g/L), Median (IQR) | 3.80 (3.24–4.60) | 3.48 (3.07–4.08) | 3.38 (3.09–4.48) | 4.42 (3.61–5.66) | <0.001 a |
| Albumin (g/L), Median (IQR) | 43.0 (38.0–46.0) | 45.0 (40.7–47.0) | 44.0 (38.7–45.0) | 40.0 (32.0–44.0) | <0.001 a |
| FAR, Median (IQR) | 8.97 (7.23–12.30) | 7.85 (6.71–9.60) | 8.56 (6.59–11.01) | 11.72 (8.46–15.17) | <0.001 a |
| CA125 | 32.80 (14.30–189.00) | 17.45 (10.50–34.35) | 28.50 (19.97–154.75) | 185.50 (51.15–746.75) | <0.001 a |
| CA19-9 | 13.90 (6.13–31.72) | 10.60 (5.05–24.12) | 19.60 (6.58–51.47) | 14.80 (7.79–41.12) | 0.006 a |
| CA15-3 | 20.50 (14.85–33.10) | 16.65 (12.00–22.42) | 19.15 (15.72–23.22) | 36.55 (21.55–99.95) | <0.001 a |
| CEA | 1.66 (1.01–2.66) | 1.41 (0.90–2.32) | 1.70 (1.24–2.55) | 1.92 (1.13–3.00) | 0.004 a |
| AFP | 2.20 (1.47–3.32) | 2.13 (1.48–3.05) | 2.76 (1.65–3.92) | 2.22 (1.31–3.28) | 0.167 a |
| LDH | 225.00 (181.75–147.05) | 211.00 (175.75–262.50) | 211.00 (180.50–232.75) | 239.00 (193.50–320.25) | 0.002 a |
| Procalcitonin | 0.29 (0.11–0.87) | 0.22 (0.10–0.52) | 0.49 (0.21–1.39) | 0.37 (0.11–1.43) | 0.005 a |
| Tumor Size (cm), Median (IQR) | 9.00 (5.00–15.00) | 7.50 (4.50–12.00) | 14.50 (8.00–20.75) | 10.25 (5.00–16.00) | <0.001 a |
| Bilaterality, n (%) | 126 (39.6) | 47 (29.7) | 4 (14.3) | 75 (56.8) | <0.001 b |
| Histological Types (n = 318) | n (%) | |
|---|---|---|
| Epithelial | 258 (81.1) | |
| Serous | 148 (46.5) | |
| Mucinous | 64 (20.1) | |
| Endometrioid | 31 (9.7) | |
| Clear cell | 10 (3.1) | |
| Brenner | 1 (0.3) | |
| Mixed epithelial | 4 (1.3) | |
| Mesenchymal | 1 (0.3) | |
| Sex-cord stromal | 15 (4.7) | |
| Germ cell | 19 (6.0) | |
| Tumor-like lesions | 24 (7.5) | |
| Mixed epithelial–germ cell | 1 (0.3) |
| Variables | Patients with Borderline Tumors (n = 28), n (%) | Patients with Malignant Tumors (n = 132), n (%) |
|---|---|---|
| Stage | ||
| I | 27 (96.4) | 37 (28.0) |
| II | 1 (3.6) | 23 (17.4) |
| III | 0 (0.0) | 43 (32.6) |
| IV | 0 (0.0) | 29 (22.0) |
| PPLNI | - | 56 (42.4) |
| Distant metastasis | - | 45 (34.1) |
| Markers (n = 318) | Borderline/Malignant | Malignant | ||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | p a | Cut-Off | AUC (95% CI) | p a | Cut-Off | |
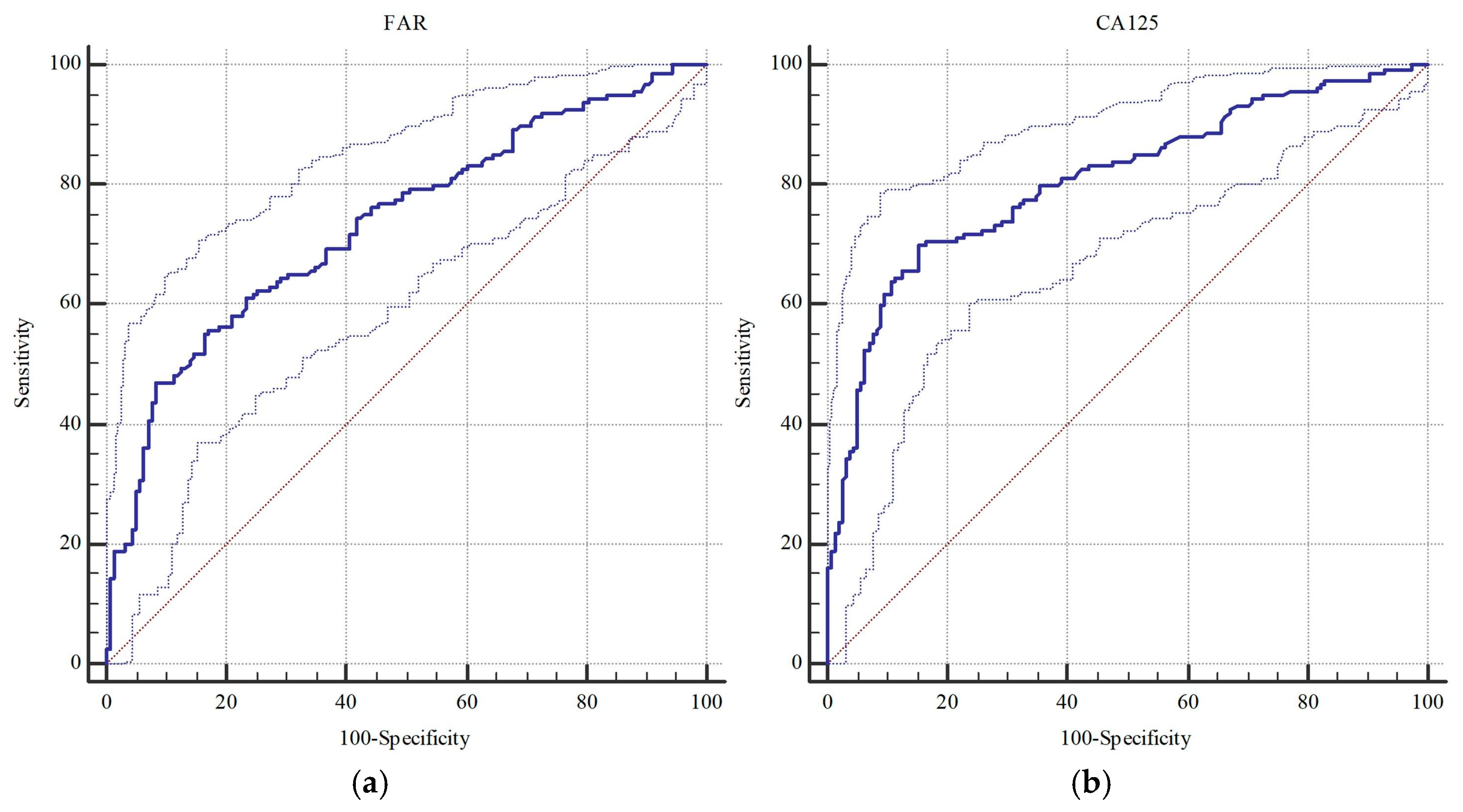
| FAR | 0.735 (0.683–0.783) | <0.001 | >11.51 | 0.767 (0.717–0.812) | <0.001 | >11.51 |
| CA 125 | 0.808 (0.761–0.850) | <0.001 | >49.9 | 0.813 (0.766–0.855) | <0.001 | >49.9 |
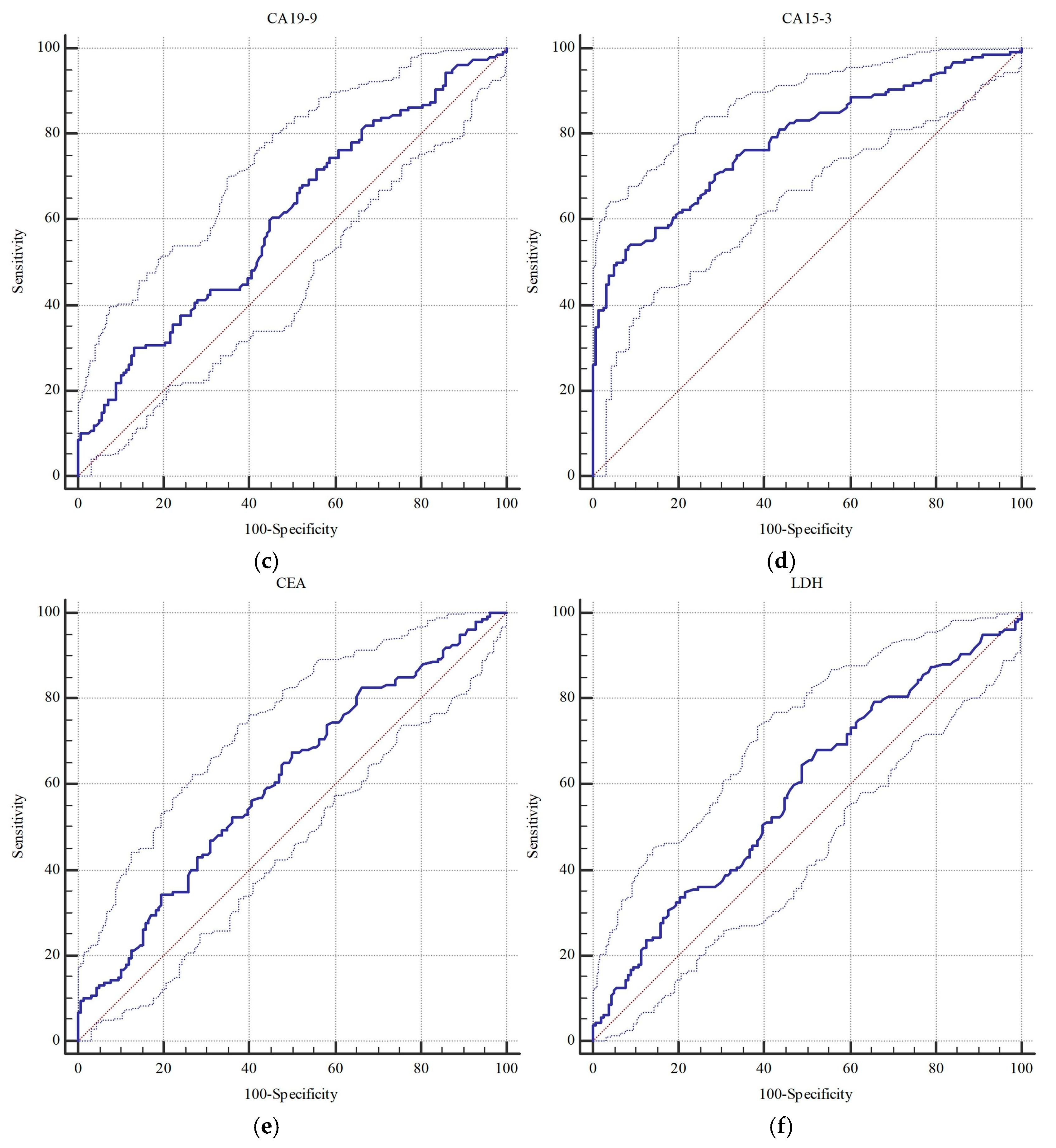
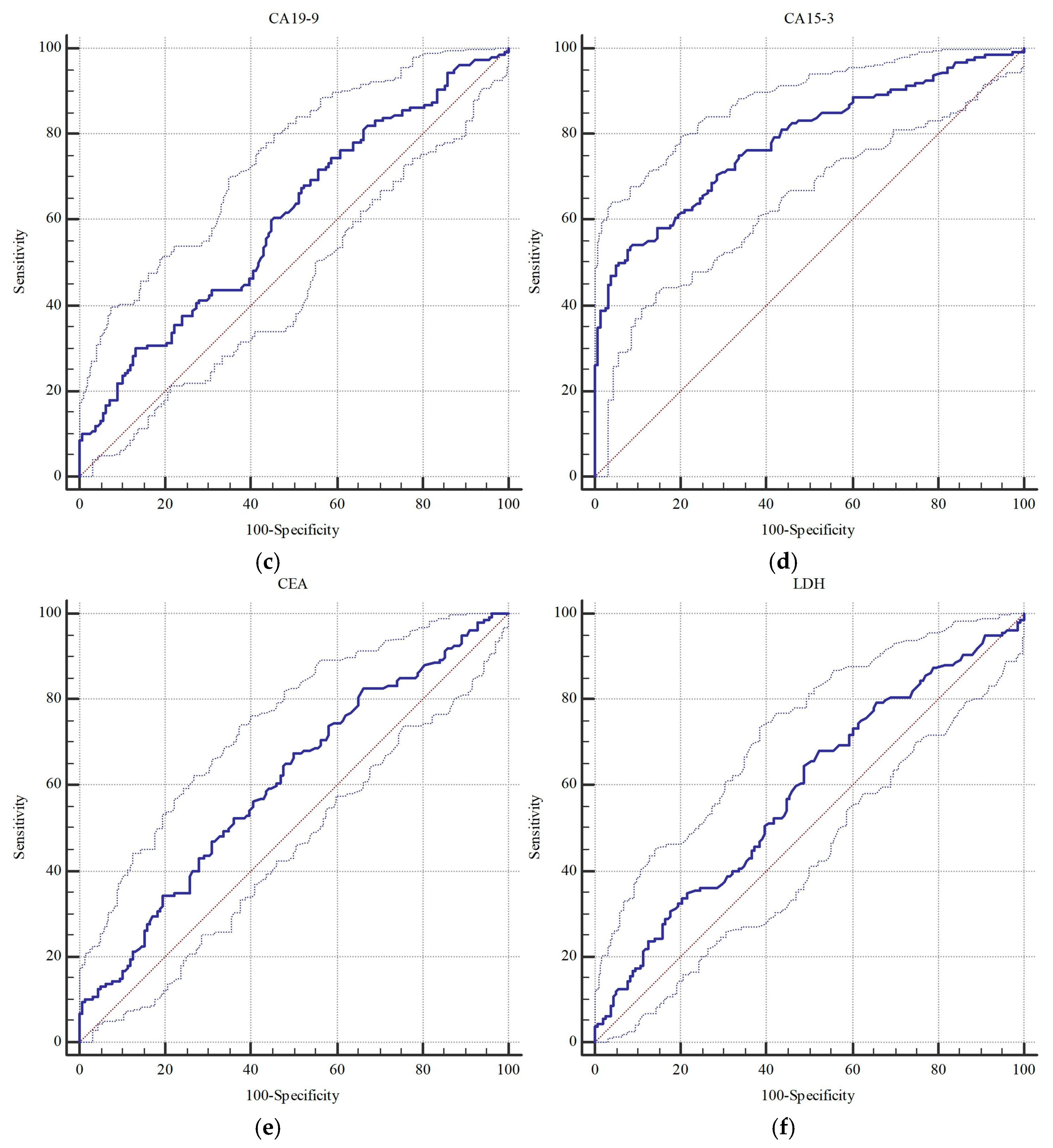
| CA 19-9 | 0.603 (0.546–0.657) | 0.001 | >34.8 | 0.577 (0.521–0.632) | 0.018 | >8.4 |
| CA 15-3 | 0.785 (0.736–0.829) | <0.001 | >29.8 | 0.820 (0.773–0.861) | <0.001 | >29.5 |
| CEA | 0.606 (0.550–0.660) | <0.001 | >1.4 | 0.600 (0.544–0.654) | 0.002 | >2.1 |
| LDH | 0.584 (0.528–0.639) | 0.008 | >211.0 | 0.616 (0.560–0.669) | <0.001 | >211.0 |
| Parameters (n = 318) | Histopathological Findings | Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | ||
|---|---|---|---|---|---|---|
| Benign | Borderline/Malignant | |||||
| Cytology | Benign | 158 (49.7) | 86 (27.0) | 46.2 (38.3–54.3) | 100.0 (97.7–100.0) | 73.0 (67.7–77.8) |
| Malignant | 0 (0.0) | 74 (23.3) | ||||
| FAR | ≤11.51 | 145 (45.6) | 85 (26.7) | 46.9 (39.0–54.9) | 91.8 (86.3–95.5) | 69.2 (63.8–74.2) |
| >11.51 | 13 (4.1) | 75 (23.6) | ||||
| CA 125 | ≤49.9 | 134 (42.1) | 48 (15.1) | 70.0 (62.3–77.0) | 84.8 (78.2–90.0) | 77.4 (72.4–81.8) |
| >49.9 | 24 (7.5) | 112 (35.2) | ||||
| CA 19-9 | ≤34.8 | 137 (43.1) | 112 (35.2) | 30.0 (23.0–37.7) | 86.7 (80.4–91.6) | 58.2 (52.5–63.7) |
| >34.8 | 21 (6.6) | 48 (15.1) | ||||
| CA 15-3 | ≤29.8 | 146 (45.9) | 75 (23.6) | 53.1 (45.1–61.0) | 92.4 (87.1–96.0) | 72.6 (67.4–77.5) |
| >29.8 | 12 (3.8) | 85 (26.7) | ||||
| CEA | ≤1.4 | 79 (24.8) | 52 (16.4) | 67.5 (59.7–74.7) | 50.0 (42.0–58.0) | 58.8 (53.2–64.3) |
| >1.4 | 79 (24.8) | 108 (34.0) | ||||
| LDH | ≤211.0 | 81 (25.5) | 57 (17.9) | 64.4 (56.4–71.8) | 51.3 (43.2–59.3) | 57.9 (52.2–63.3) |
| >211.0 | 77 (24.2) | 103 (32.4) | ||||
| Parameters (n = 318) | Histopathological Findings | Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | ||
|---|---|---|---|---|---|---|
| Benign /Borderline | Malignant | |||||
| Cytology | Benign | 185 (58.2) | 59 (18.6) | 55.3 (46.4–64.0) | 99.5 (97.0–100.0) | 81.1 (76.4–85.3) |
| Malignant | 1 (0.3) | 73 (23.0) | ||||
| FAR | ≤11.51 | 168 (52.8) | 62 (19.5) | 53.0 (44.2–61.8) | 90.3 (85.1–94.2) | 74.8 (69.7–79.5) |
| >11.51 | 18 (5.7) | 70 (22.0) | ||||
| CA 125 | ≤49.9 | 151 (47.5) | 31 (9.7) | 76.5 (68.4–83.5) | 81.2 (74.8–86.5) | 79.3 (74.4–83.6) |
| >49.9 | 35 (11.0) | 101 (31.8) | ||||
| CA 19-9 | ≤8.4 | 80 (25.2) | 36 (11.3) | 72.7 (64.3–80.1) | 43.0 (35.8–50.5) | 55.4 (49.7–60.9) |
| >8.4 | 106 (33.3) | 96 (30.2) | ||||
| CA 15-3 | ≤29.5 | 170 (53.5) | 49 (15.4) | 62.9 (54.0–71.1) | 91.4 (86.4–95.0) | 79.6 (74.7–83.9) |
| >29.5 | 16 (5.0) | 83 (26.1) | ||||
| CEA | ≤2.1 | 133 (41.8) | 76 (23.9) | 42.4 (33.9–51.3) | 71.5 (64.4–77.9) | 59.4 (53.8–64.9) |
| >2.1 | 53 (16.7) | 56 (17.6) | ||||
| LDH | ≤211.0 | 96 (30.2) | 42 (13.2) | 68.2 (59.5–76.0) | 51.6 (44.2–59.0) | 58.5 (52.9–94.0) |
| >211.0 | 90 (28.3) | 90 (28.3) | ||||
| Variables a | Borderline/Malignant | Malignant | |||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| FAR | 1.140 (1.053–1.234) | 0.001 | 1.165 (1.077–1.260) | <0.001 | |
| CA125 | 1.003 (1.001–1.005) | 0.003 | 1.003 (1.001–1.005) | 0.005 | |
| CA15-3 | 1.048 (1.021–1.075) | <0.001 | 1.060 (1.021–1.089) | <0.001 | |
| Menopausal status | Pre/Perimenopausal | R | R | ||
| Postmenopausal | 2.262 (1.225–4.174) | 0.009 | 2.815 (1.375–5.761) | 0.005 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahin, G.; HazırBulan, A.; Atalmış, H.A.; Yüksel, İ.T.; Sözen, I.; Koçbıyık, A.; Kocadal, N.Ç.; Alkış, İ. Diagnostic Power of the Fibrinogen-to-Albumin Ratio for Estimating Malignancy in Patients with Adnexal Masses: A Methodological Study. Diagnostics 2025, 15, 2372. https://doi.org/10.3390/diagnostics15182372
Şahin G, HazırBulan A, Atalmış HA, Yüksel İT, Sözen I, Koçbıyık A, Kocadal NÇ, Alkış İ. Diagnostic Power of the Fibrinogen-to-Albumin Ratio for Estimating Malignancy in Patients with Adnexal Masses: A Methodological Study. Diagnostics. 2025; 15(18):2372. https://doi.org/10.3390/diagnostics15182372
Chicago/Turabian StyleŞahin, Gözde, Ayşe HazırBulan, Hatice Argun Atalmış, İlkbal Temel Yüksel, Işık Sözen, Alper Koçbıyık, Nilüfer Çetinkaya Kocadal, and İsmet Alkış. 2025. "Diagnostic Power of the Fibrinogen-to-Albumin Ratio for Estimating Malignancy in Patients with Adnexal Masses: A Methodological Study" Diagnostics 15, no. 18: 2372. https://doi.org/10.3390/diagnostics15182372
APA StyleŞahin, G., HazırBulan, A., Atalmış, H. A., Yüksel, İ. T., Sözen, I., Koçbıyık, A., Kocadal, N. Ç., & Alkış, İ. (2025). Diagnostic Power of the Fibrinogen-to-Albumin Ratio for Estimating Malignancy in Patients with Adnexal Masses: A Methodological Study. Diagnostics, 15(18), 2372. https://doi.org/10.3390/diagnostics15182372






