Alterations in Corneal Morphology and Thickness Associated with Methylphenidate Treatment in Children with Attention-Deficit/Hyperactivity Disorder
Abstract
1. Introduction
2. Materials and Methods
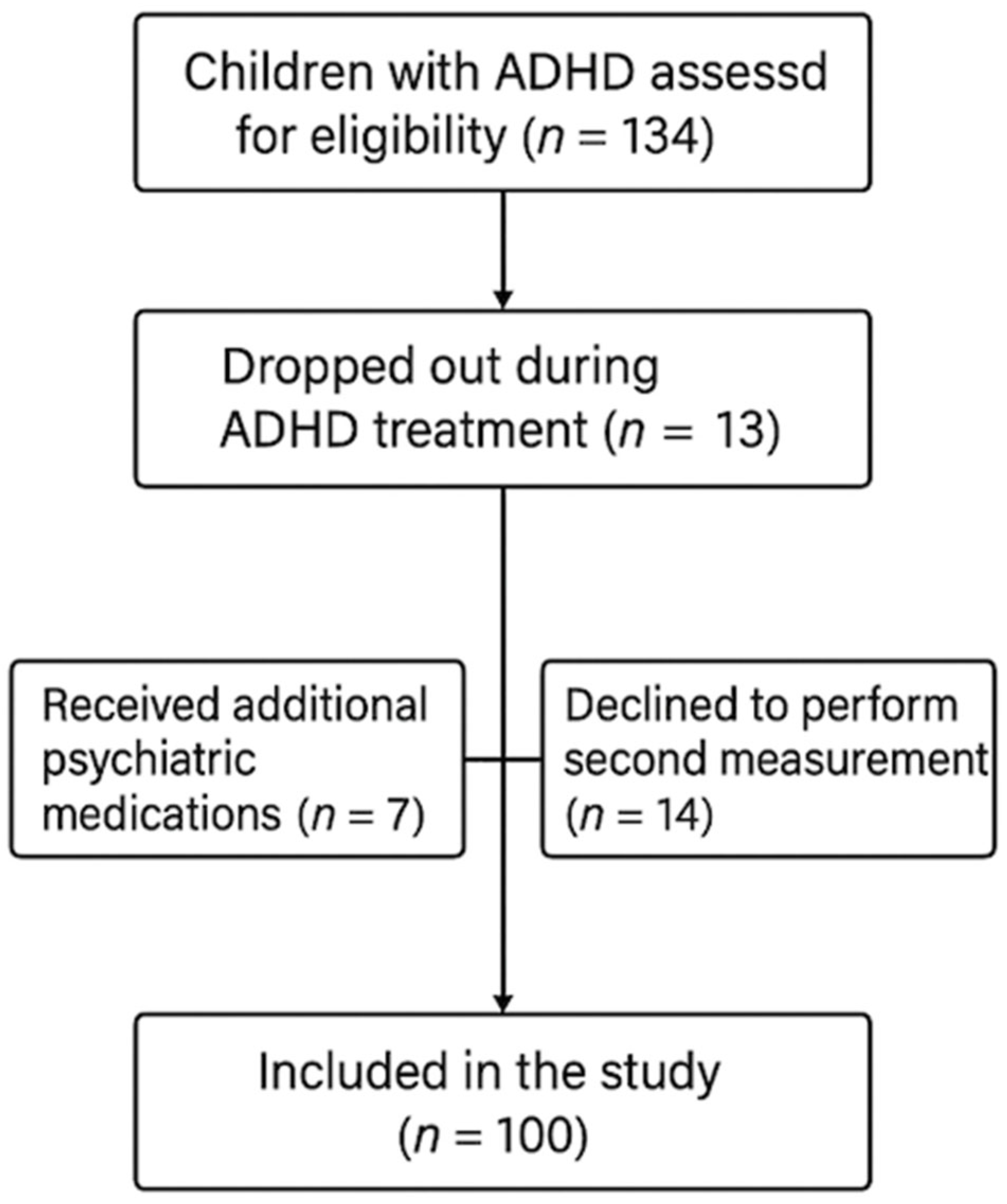
2.1. Study Design and Participants
2.2. Behavioral Assessment Using the Turgay DSM-IV-Based Rating Scale
2.3. Ophthalmological Examination
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Clinical Acceptability and Risk–Benefit Analysis
4.2. Monitoring Recommendations and Clinical Implementation
4.3. Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- First, M.B.; Clarke, D.E.; Yousif, L.; Eng, A.M.; Gogtay, N.; Appelbaum, P.S. DSM-5-TR: Rationale, Process, and Overview of Changes. Psychiatr. Serv. 2023, 74, 869–875. [Google Scholar] [CrossRef]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.S.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Prim. 2015, 1, 15020. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Arthur, E.J.; Gluud, C.; Simonsen, E.; Storebo, O.J. Does Methylphenidate Work in Children and Adolescents with Attention Deficit Hyperactivity Disorder? Pediatr. Rep. 2021, 13, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Grönlund, M.A.; Aring, E.; Landgren, M.; Hellström, A. Visual function and ocular features in children and adolescents with attention deficit hyperactivity disorder, with and without treatment with stimulants. Eye 2007, 21, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Fainberg, G.; Leitner, Y.; Zur, D.; Klein, A.; Mezad-Koursh, D. Short-Term Vision-Related Ocular Side Effects of Treatment with Dexmethylphenidate for Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2022, 32, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Koc, H.; Önal, B.S.; Hoşoğlu, E. Evaluation of corneal endothelial cell morphology off and on treatment by specular microscopy in children and adolescents with attention deficit hyperactivity disorder. Cutan. Ocul. Toxicol. 2024, 43, 120–123. [Google Scholar] [CrossRef]
- Mancera, N.; Wadia, H.P. Corneal Edema Associated With Systemic Dopaminergic Agents. Cornea 2019, 38, 1040–1042. [Google Scholar] [CrossRef]
- Bingöl-Kiziltunç, P.; Yürümez, E.; Atilla, H. Does methylphenidate treatment affect functional and structural ocular parameters in patients with attention deficit hyperactivity disorder?—A prospective, one year follow-up study. Indian J. Ophthalmol. 2022, 70, 1664–1668. [Google Scholar] [CrossRef]
- Soyer, J.; Jean-Louis, J.; Ospina, L.H.; Bélanger, S.A.; Bussières, J.F.; Kleiber, N. Visual disorders with psychostimulants: A paediatric case report. Paediatr. Child Health 2019, 24, 153–155. [Google Scholar] [CrossRef]
- Larrañaga-Fragoso, P.; Noval, S.; Rivero, J.C.; Boto-De-Los-Bueis, A. The effects of methylphenidate on refraction and anterior segment parameters in children with attention deficit hyperactivity disorder. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2015, 19, 322–326. [Google Scholar] [CrossRef]
- Lu, C.K.; Kuang, T.M.; Chou, J.C.K. Methylphenidate (Ritalin)-associated Cataract and Glaucoma. J. Chinese Med. Assoc. 2006, 69, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Uzlu, D.; Bilginer, S.Ç.; Yaşar, Y.; Taşdemir, C.; Erdöl, H.; Günay, M.; Köse, B. An examination of the ocular effects of methylphenidate used in children and adolescents diagnosed with attention-deficit hyperactivity disorder. Int. Ophthalmol. 2025, 45, 104. [Google Scholar] [CrossRef]
- Fabian, I.D.; Kinori, M.; Ancri, O.; Spierer, A.; Tsinman, A.; Ben Simon, G.J. The possible association of attention deficit hyperactivity disorder with undiagnosed refractive errors. J. AAPOS 2013, 17, 507–511. [Google Scholar] [CrossRef]
- Turgay, A. Disruptive Behavior Disorders Child and Adolescent Screening and Rating Scales for Children, Adolescents, Parents and Teachers; Integrative Therapy Institute Publication: West Bloomfield, MI, USA, 1994. [Google Scholar]
- Ercan, E.S.; Amado, S.; Somer, O.; Çıkoğlu, S. An attempt to develop a test battery for attention deficit hyperactivity disorder and disruptive behavioral disorders. J. Child Adolesc. Ment. Health 2001, 8, 132–144. [Google Scholar]
- Chaurasia, S.; Vanathi, M. Specular microscopy in clinical practice. Indian J. Ophthalmol. 2021, 69, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Doughty, M.J.; Wright, L. Reassessment of the corneal endothelial cell organisation in children. Br. J. Ophthalmol. 2000, 84, 692–696. [Google Scholar] [CrossRef]
- Coelho-Santos, V.; Cardoso, F.L.; Leitão, R.A.; Fontes-Ribeiro, C.A.; Silva, A.P. Impact of developmental exposure to methylphenidate on rat brain’s immune privilege and behavior: Control versus ADHD model. Brain. Behav. Immun. 2018, 68, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Engert, V.; Pruessner, J.C. Dopaminergic and Noradrenergic Contributions to Functionality in ADHD: The Role of Methylphenidate. Curr. Neuropharmacol. 2009, 6, 322–328. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, Y.; Zhang, H. Ocular Autonomic Nervous System: An Update from Anatomy to Physiological Functions. Vision 2022, 6, 6. [Google Scholar] [CrossRef]
- Kim, Y.H.; Ahn, D.S.; Kim, M.O.; Joeng, J.H.; Chung, S. Protease-Activated Receptor 2 Activation Inhibits N-Type Ca2+ Currents in Rat Peripheral Sympathetic Neurons. Mol. Cells 2014, 37, 804–811. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Feng, Y.; Li, X.; Lu, Z.; Gu, H.; Li, W.; Hill, L.J.; Ou, S. Evolution of therapeutic strategy based on oxidant-antioxidant balance for fuchs endothelial corneal dystrophy. Ocul. Surf. 2024, 34, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Santos, V.; Cardoso, F.L.; Magalhães, A.; Ferreira-Teixeira, M.; Leitão, R.A.; Gomes, C.; Rito, M.; Barbosa, M.; Fontes-Ribeiro, C.A.; Silva, A.P. Effect of chronic methylphenidate treatment on hippocampal neurovascular unit and memory performance in late adolescent rats. Eur. Neuropsychopharmacol. 2019, 29, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Sanches, E.S.; Leitão, R.A.; Baptista, F.I.; Mota, S.I.; Caldeira, M.V.; Oliveira, P.J.; Ambrósio, A.F.; Fernandes, R.; Silva, A.P. Methylphenidate triggers retinal oxidative stress and mitochondrial dysfunction under physiological conditions but has beneficial effects in inflammatory settings. Neuropharmacology 2025, 279, 110623. [Google Scholar] [CrossRef]
- Kong, N.; Bao, Y.; Zhao, H.; Kang, X.; Tai, X.; Shen, Y. Methylphenidate causes cytotoxicity on photoreceptor cells via autophagy. Hum. Exp. Toxicol. 2021, 40, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Labbé, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef]
- Rossi, G.C.M.; Pasinetti, G.M.; Scudeller, L.; Raimondi, M.; Lanteri, S.; Bianchi, P.E. Risk factors to develop ocular surface disease in treated glaucoma or ocular hypertension patients. Eur. J. Ophthalmol. 2013, 23, 296–302. [Google Scholar] [CrossRef]
- Bourne, W.M. Biology of the corneal endothelium in health and disease. Eye 2003, 17, 912–918. [Google Scholar] [CrossRef]
- Joyce, N.C. Proliferative capacity of corneal endothelial cells. Exp. Eye Res. 2012, 95, 16–23. [Google Scholar] [CrossRef]
- Waring, G.O.; Bourne, W.M.; Edelhauser, H.F.; Kenyon, K.R. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology 1982, 89, 531–590. [Google Scholar] [CrossRef]
- Barkley, R.A.; Fischer, M.; Smallish, L.; Fletcher, K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J. Abnorm. Psychol. 2002, 111, 279–289. [Google Scholar] [CrossRef]
- Jerome, L.; Segal, A.; Habinski, L. What We Know About ADHD and Driving Risk: A Literature Review, Meta-Analysis and Critique. J. Can. Acad. Child Adolesc. Psychiatry 2006, 15, 105. [Google Scholar] [PubMed]
- Faraone, S.V.; Biederman, J.; Mick, E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychol. Med. 2006, 36, 159–165. [Google Scholar] [CrossRef]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- Wallace, D.K.; Morse, C.L.; Melia, M.; Sprunger, D.T.; Repka, M.X.; Lee, K.A.; Christiansen, S.P. Pediatric Eye Evaluations Preferred Practice Pattern®: I. Vision Screening in the Primary Care and Community Setting; II. Comprehensive Ophthalmic Examination. Ophthalmology 2018, 125, P184–P227. [Google Scholar] [CrossRef]
- Wolraich, M.L.; Hagan, J.F.; Allan, C.; Chan, E.; Davison, D.; Earls, M.; Evans, S.W.; Flinn, S.K.; Froehlich, T.; Frost, J.; et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2019, 144, e20192528. [Google Scholar] [CrossRef] [PubMed]
- Granet, D.B.; Gomi, C.F.; Ventura, R.; Miller-Scholte, A. The relationship between convergence insufficiency and ADHD. Strabismus 2005, 13, 163–168. [Google Scholar] [CrossRef]
- Borsting, E.J.; Rouse, M.W.; Mitchell, G.L.; Scheiman, M.; Cotter, S.A.; Cooper, J.; Kulp, M.T.; London, R. Validity and Reliability of the Revised Convergence Insufficiency Symptom Survey in Children Aged 9 to 18 Years. Optom. Vis. Sci. 2003, 80, 832–838. [Google Scholar] [CrossRef]
- Edelhauser, H.F. The balance between corneal transparency and edema: The proctor lecture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1755–1767. [Google Scholar] [CrossRef]
- Maurice, D.M. The location of the fluid pump in the cornea. J. Physiol. 1972, 221, 43–54. [Google Scholar] [CrossRef]
- Scheiman, M.; Cotter, S.; Mitchell, G.L.; Kulp, M.; Rouse, M.; Hertle, R.; Redford, M.; Cooper, J.; Coulter, R.; Gallaway, M.; et al. Randomized clinical trial of treatments for symptomatic convergence insufficiency in children. Arch. Ophthalmol. 2008, 126, 1336–1349. [Google Scholar] [CrossRef]
- Hopkins, S.; Black, A.A.; White, S.L.J.; Wood, J.M. Visual information processing skills are associated with academic performance in Grade 2 school children. Acta Ophthalmol. 2019, 97, e1141–e1148. [Google Scholar] [CrossRef] [PubMed]
| Group | Test Statistics | p | ||
|---|---|---|---|---|
| Control (n = 100) | Patient (n = 100) | |||
| Age | 10.24 ± 2.12 | 10.02 ± 2.4 | 0.681 | 0.496 t |
| Weight-Kg | 43 (21–58) | 39 (21–58) | −1.412 | 0.158 m |
| Number of Siblings | ||||
| 1 | 12 (12) | 7 (7) | 1.779 | 0.613 f |
| 2 | 54 (54) | 54 (54) | ||
| 3 | 32 (32) | 37 (37) | ||
| 4 | 2 (2) | 2 (2) | ||
| Siblings Order | ||||
| 1 | 63 (63) | 67 (67) | 2.510 | 0.311 f |
| 2 | 33 (33) | 25 (25) | ||
| 3 | 4 (4) | 8 (8) | ||
| Mother’s Education Status | ||||
| Primary Education | 23 (23) | 28 (28) | 1.465 | 0.702 f |
| High School | 36 (36) | 29 (29) | ||
| College/University | 32 (32) | 35 (35) | ||
| Graduate/Postgraduate | 9 (9) | 8 (8) | ||
| Father’s Education Status | ||||
| Primary Education | 4 (4) a | 9 (9) a | 15.487 | 0.002 f |
| High School | 52 (52) a | 26 (26) b | ||
| College/University | 36 (36) a | 48 (48) a | ||
| Graduate/Postgraduate | 8 (8) a | 17 (17) a | ||
| Turgay | ||||
| Inattention | 7 (5–9) | 23 (12–32) | −12.322 | <0.001 |
| Hyperactivity/Impulsivity | 6 (0–9) | 12 (0–26) | −8.164 | <0.001 |
| ODD | 5 (0–9) | 8 (0–23) | −5.114 | <0.001 |
| CD | 0 (0–16) | 0 (0–10) | −0.958 | 0.338 |
| Group | Test Statistics | p | Effect Size | ||
|---|---|---|---|---|---|
| Control (n = 100) | Patient (n = 100) | ||||
| IOP | 15 (13–18) | 16 (15–19) | −4.292 | 0.345 m | dc = 2.653 |
| CCT | 537 (507–566) | 538 (523–576) | −5.973 | 0.214 m | dc = 0.932 |
| SE | −0.25 (−13–1.5) | −0.75 (−1.25–0.75) | −2.144 | 0.132 m | dc = 0.307 |
| AL | 23.5 (22.96–24.24) | 23.69 (22.96–24.24) | −1.012 | 0.311 m | dc = 0.143 |
| AD | 3.1 (2.88–3.41) | 3.12 (2.88–3.41) | −0.804 | 0.422 m | dc = 0.114 |
| ACD | 3.43 (2.96–3.89) | 3.25 (2.96–3.89) | −1.812 | 0.070 m | dc = 0.258 |
| LT | 3.56 (3.26–3.94) | 3.56 (3.26–3.94) | −0.042 | 0.967 m | dc = 0.006 |
| WTW | 12.15 (11.52–12.88) | 12.13 (11.52–12.88) | −0.159 | 0.874 m | dc = 0.022 |
| ECD | 2574.5 (1243–2941) | 2595.5 (2461–2760) | −2.304 | 0.061 m | dc = 0.330 |
| NUM | 265.5 (67–302) | 278 (247–313) | −7.973 | 0.255 m | dc = 1.365 |
| AVG | 362 (340–468) | 361.5 (354–368) | −0.594 | 0.555 m | dc = 0.084 |
| SD | 116 (99–196) | 116 (99–136) | −0.108 | 0.914 m | dc = 0.015 |
| CV | 39 (32–47) | 39 (35–47) | −0.009 | 0.993 m | dc = 0.001 |
| CA-MAX | 811.19 ± 60.45 | 808.49 ± 58.42 | 0.321 | 0.748 t | dc = 0.045 |
| CA-MIN | 99 (4–213) | 99.5 (82–128) | −0.377 | 0.707 m | dc = 0.053 |
| 6A | 49 (26–62) | 50 (45–54) | −6.019 | 0.894 m | dc = 1.143 |
| Corneal Volume | 56.71 (51.9–66.3) | 56.57 (53.67–58.94) | −0.623 | 0.533 m | dc = 0.088 |
| K1 | 43.44 (40.36–47.18) | 43.28 (41.99–44.12) | −0.925 | 0.355 m | dc = 0.131 |
| K2 | 42.76 (40.67–46.37) | 42.76 (41.76–44.68) | −0.078 | 0.938 m | dc = 0.011 |
| K AVG | 43.87 ± 1.59 | 43.07 ± 0.46 | 4.773 | <0.001 t | dc = 0.675 |
| K CYL | −0.71 (−3.09–−0.1) | −0.7 (−1.2–−0.06) | −1.548 | 0.122 m | dc = 0.220 |
| HVID | 12.05 (11.1–12.88) | 11.94 (11.37–12.79) | −0.683 | 0.494 m | dc = 0.097 |
| Apex Curvature | 44.69 (43.87–54.23) | 44.68 (43.87–54.23) | −0.363 | 0.717 m | dc = 0.051 |
| Curvature ASYM F | 0.14 (0–0.75) | −1.57 (−1.63–1.61) | −12.009 | <0.001 m | dc = 3.216 |
| Curvature ASYM B | 0.04 (0–1.06) | −0.24 (−0.28–−0.21) | −12.305 | <0.001 m | dc = 3.531 |
| Iridocorneal Angle | 47 (35–55) | 49 (42–57) | −2.859 | 0.213 m | dc = 1.109 |
| First Measurement (n = 100) | Measurement After 6 Months (n = 100) | Test ist. | p | R2 | Cohen’s d | |
|---|---|---|---|---|---|---|
| CCT | 538 (523–576) | 548 (523–586) | −8.678 | <0.001 w | 0.825 | 3.519 |
| IOP | 15 (13–18) | 18 (15–21) | −11.292 | <0.001 w | 0.786 | 3.127 |
| SE | −0.75 (−1.25–0.75) | −1.0 (−1.5–−0.75) | −8.619 | <0.001 w | 0.575 | 3.340 |
| AL | 23.69 (22.96–24.24) | 23.8 (22.91–24.44) | −2.007 | 0.065 w | 0.969 | 0.410 |
| AD | 3.12 (2.88–3.41) | 3.11 (2.87–3.28) | −2.601 | 0.017 w | 0.892 | 0.539 |
| ACD | 3.25 (2.96–3.89) | 3.26 (3.01–3.89) | −1.257 | 0.209 w | 0.995 | 0.253 |
| LT | 3.56 (3.26–3.94) | 3.57 (3.25–3.96) | −2.991 | 0.023 w | 0.986 | 0.627 |
| WTW | 12.13 (11.52–12.88) | 12.12 (11.55–12.86) | −0.52 | 0.603 w | 0.997 | 0.124 |
| ECD | 2595.5 (2461–2760) | 2348 (2199–2590) | −8.682 | <0.001 w | 0.779 | 3.499 |
| NUM | 278 (247–313) | 278 (247–313) | −0.528 | 0.598 w | −0.058 | 0.106 |
| AVG | 361.5 (354–368) | 371 (359–378) | −8.704 | <0.001 w | 0.844 | 3.536 |
| SD | 116 (99–136) | 120.96 ± 9.39 | −12.065 | <0.001 e | 0.920 | 1.229 |
| CV | 39 (35–47) | 42 (37–49) | −9.069 | <0.001 w | 0.979 | 4.305 |
| CA-MAX | 808.49 ± 58.42 | 834.81 ± 60.61 | −20.838 | <0.001 e | 0.978 | 2.148 |
| CA-MIN | 99.5 (82–128) | 114 (94–136) | −8.688 | <0.001 w | 0.880 | 3.509 |
| 6A | 50 (45–54) | 48 (43–52) | −8.981 | <0.001 w | 0.941 | 4.084 |
| Corneal Volume | 56.57 (53.67–58.94) | 56.52 (53.72–58.99) | −2.168 | 0.130 w | 0.997 | 0.444 |
| K1 | 43.28 (41.99–44.12) | 43.32 (41.87–44.16) | −1.256 | 0.209 w | 0.937 | 0.353 |
| K2 | 42.26 (41.66–45.34) | 42.76 (41.78–44.64) | −2.067 | 0.139 w | 0.982 | 0.423 |
| K AVG | 43.07 (42.23–45.12) | 43.05 (42.07–44.41) | −2.363 | 0.018 w | 0.963 | 0.486 |
| K CYL | −0.7 (−1.2–−0.06) | −0.65 ± 0.33 | −1.174 | 0.243 e | 0.579 | 0.117 |
| HVID | 11.94 (11.37–12.79) | 11.96 (11.34–12.77) | −1.061 | 0.289 w | 0.921 | 0.213 |
| Apex Curvature | 44.68 (43.87–54.23) | 44.67 (43.71–54.34) | −0.891 | 0.373 w | 0.995 | 0.179 |
| Curvature Asym-F | −1.57 (−1.63–1.61) | −1.56 (−1.65–−1.46) | −0.202 | 0.840 w | 0.059 | 0.040 |
| Curvature Asym-B | −0.24 (−0.28–−0.21) | −0.24 (−0.29–−0.19) | −3.212 | 0.021 w | 0.549 | 0.678 |
| Irıdocorneal Angle | 49 (42–57) | 48.5 (42–58) | −0.943 | 0.346 w | 0.855 | 0.189 |
| Difference * | Weight-Kg | A-Turgay–Carelessness | Turgay-Hyperactivity | Turgay-Kokgb | Turgay-Db | |
|---|---|---|---|---|---|---|
| IOP | r | 0.097 | 0.049 | −0.057 | 0.143 | 0.122 |
| p | 0.335 | 0.626 | 0.576 | 0.156 | 0.228 | |
| SE | r | 0.025 | 0.213 | 0.073 | 0.095 | 0.022 |
| p | 0.804 | 0.033 | 0.472 | 0.345 | 0.828 | |
| AL | r | 0.002 | 0.062 | 0.018 | −0.241 | −0.073 |
| p | 0.984 | 0.541 | 0.860 | 0.016 | 0.472 | |
| CCT | r | 0.101 | −0.156 | −0.123 | −0.131 | −0.241 |
| p | 0.318 | 0.121 | 0.224 | 0.194 | 0.016 | |
| AD | r | −0.018 | 0.092 | 0.112 | −0.053 | 0.054 |
| p | 0.856 | 0.360 | 0.269 | 0.600 | 0.593 | |
| ACD | r | 0.069 | −0.108 | −0.181 | −0.086 | −0.078 |
| p | 0.495 | 0.287 | 0.072 | 0.396 | 0.441 | |
| LT | r | −0.068 | 0.107 | 0.127 | −0.027 | −0.173 |
| p | 0.499 | 0.290 | 0.209 | 0.788 | 0.084 | |
| WTW | r | 0.010 | −0.167 | −0.046 | 0.023 | 0.025 |
| p | 0.923 | 0.096 | 0.652 | 0.817 | 0.806 | |
| ECD | r | −0.040 | 0.222 | −0.034 | −0.036 | −0.086 |
| p | 0.695 | 0.026 | 0.741 | 0.723 | 0.398 | |
| NUM | r | 0.005 | 0.241 | 0.012 | 0.059 | 0.111 |
| p | 0.958 | 0.016 | 0.908 | 0.559 | 0.271 | |
| AVG | r | −0.146 | −0.005 | −0.091 | −0.037 | 0.039 |
| p | 0.147 | 0.957 | 0.366 | 0.715 | 0.701 | |
| SD | r | 0.092 | 0.079 | −0.063 | 0.059 | −0.031 |
| p | 0.365 | 0.433 | 0.533 | 0.561 | 0.757 | |
| CV | r | 0.021 | 0.111 | −0.043 | 0.061 | 0.136 |
| p | 0.839 | 0.274 | 0.671 | 0.546 | 0.178 | |
| CA-MAX | r | 0.099 | 0.050 | 0.056 | 0.074 | −0.020 |
| p | 0.325 | 0.623 | 0.578 | 0.464 | 0.841 | |
| CA-MIN | r | −0.013 | −0.124 | −0.123 | −0.079 | −0.065 |
| p | 0.895 | 0.221 | 0.223 | 0.437 | 0.521 | |
| 6A | r | 0.113 | −0.109 | 0.191 | −0.030 | 0.045 |
| p | 0.264 | 0.280 | 0.057 | 0.768 | 0.660 | |
| Corneal Volume | r | −0.083 | −0.248 | 0.061 | −0.062 | 0.072 |
| p | 0.413 | 0.013 | 0.546 | 0.540 | 0.474 | |
| K1 | r | 0.100 | 0.146 | 0.096 | −0.041 | 0.117 |
| p | 0.320 | 0.149 | 0.340 | 0.688 | 0.245 | |
| K2 | r | 0.073 | 0.025 | 0.041 | −0.199 | −0.033 |
| p | 0.470 | 0.802 | 0.685 | 0.048 | 0.747 | |
| K AVG | r | 0.140 | 0.205 | 0.174 | −0.068 | 0.160 |
| p | 0.164 | 0.041 | 0.083 | 0.500 | 0.112 | |
| K CYL | r | −0.011 | −0.296 | −0.146 | 0.061 | −0.189 |
| p | 0.915 | 0.003 | 0.148 | 0.549 | 0.059 | |
| HVID | r | 0.075 | 0.085 | 0.096 | −0.040 | 0.041 |
| p | 0.460 | 0.402 | 0.344 | 0.691 | 0.689 | |
| Apex Curvature | r | −0.084 | 0.002 | 0.019 | −0.044 | −0.049 |
| p | 0.408 | 0.981 | 0.852 | 0.661 | 0.630 | |
| Curvature Asym-F | r | 0.055 | −0.016 | 0.028 | 0.221 | 0.021 |
| p | 0.586 | 0.873 | 0.781 | 0.027 | 0.835 | |
| Curvature Asym-B | r | −0.025 | −0.001 | −0.033 | −0.005 | −0.072 |
| p | 0.808 | 0.995 | 0.747 | 0.958 | 0.474 | |
| Irıdocorneal Angle | r | −0.088 | 0.035 | 0.038 | −0.079 | 0.082 |
| p | 0.386 | 0.727 | 0.710 | 0.435 | 0.420 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumer, F.; Yazici, M. Alterations in Corneal Morphology and Thickness Associated with Methylphenidate Treatment in Children with Attention-Deficit/Hyperactivity Disorder. Diagnostics 2025, 15, 2368. https://doi.org/10.3390/diagnostics15182368
Sumer F, Yazici M. Alterations in Corneal Morphology and Thickness Associated with Methylphenidate Treatment in Children with Attention-Deficit/Hyperactivity Disorder. Diagnostics. 2025; 15(18):2368. https://doi.org/10.3390/diagnostics15182368
Chicago/Turabian StyleSumer, Fatma, and Merve Yazici. 2025. "Alterations in Corneal Morphology and Thickness Associated with Methylphenidate Treatment in Children with Attention-Deficit/Hyperactivity Disorder" Diagnostics 15, no. 18: 2368. https://doi.org/10.3390/diagnostics15182368
APA StyleSumer, F., & Yazici, M. (2025). Alterations in Corneal Morphology and Thickness Associated with Methylphenidate Treatment in Children with Attention-Deficit/Hyperactivity Disorder. Diagnostics, 15(18), 2368. https://doi.org/10.3390/diagnostics15182368







