Abstract
Corneal sensitivity is an important indicator of corneal health and innervation. Corneal hypoesthesia may be an early indicator of corneal diseases such as neurotrophic keratopathy. Various instruments have been used to measure corneal sensitivity, the first being the Cochet–Bonnet aesthesiometer. Over the years, new devices employing different stimuli have been developed, such as the gas-based Belmonte aesthesiometer, the Swiss liquid-jet aesthesiometer, and the most recently released corneal Brill aesthesiometer. In this review, the progress and advancement of aesthesiometers since their introduction is described. The mechanism, advantages, and disadvantages of these aesthesiometers are discussed and compared. We also report the relationship between corneal sensitivity and corneal innervation in various conditions, including diabetes mellitus, Fuchs’ endothelial dystrophy, dry eye disease, glaucoma, keratoconus, herpes simplex keratitis, post-refractive surgery, and ocular graft-versus-host disease. Through this review, we aim to highlight the importance of the assessment of corneal sensitivity and innervation in the diagnosis, treatment, and monitoring of anterior and posterior segment ocular disorders.
1. Introduction
1.1. The Cornea, Corneal Sensation, and Neurotrophic Keratopathy
The cornea is the most richly and densely innervated part of the body [1], exceeding even that of the skin by 400 times. It is supplied by a complex network of sensory nerves arising from the ophthalmic branch of the trigeminal nerve, with fibres travelling through the nasociliary nerve and long ciliary nerve, before entering the corneal stroma at the periphery and penetrating the Bowman’s and basal epithelial layer. The corneal nerves serve key roles in the maintenance of tear film, the stimulation of blinking, the perception of foreign bodies and noxious stimuli, and the neurotrophic renewal of the corneal epithelium. These protective and neurotrophic functions of the cornea serve to preserve a healthy ocular surface and maintain epithelial integrity [2].
The overlapping sensory fields of the cornea are supplied by various types of nociceptors which transmit mechanical, pain, and thermal stimuli [3]. Approximately 20% are mechanoreceptors which transmit sharp acute pain via thin myelinated, fast-conducting Aδ-type fibres. The most abundant receptors are the polymodal nociceptors which convey a sharp and sustained pain via unmyelinated, slow-conducting C-type fibres, mediated through chemical substances such as prostaglandins and bradykinin, and heat and mechanical irritation. Lastly, the detection of cold air or fluid is sensed by Aδ and C fibre cold receptors [4].
Any disease, whether ocular or systemic, which causes a lesion along the course of the trigeminal nerve, can lead to corneal neuropathy (Table 1). Various corneal diseases are the most common causes of corneal neuropathy. These include viral or bacterial infection such as herpes simplex virus keratitis and herpes zoster keratoconjunctivitis, as well as chemical burns or physical trauma [1]. Surgical procedures involving the cornea such as various forms of keratoplasty or refractive surgery also cause corneal subbasal nerve plexus transection and, thus, neuropathy [5,6]. Other local ocular etiologies include chronic dry eye disease, chronic contact lens users, keratoconus, and corneal dystrophies [7]. Pharmacological agents such as anti-glaucoma medication [8], intravitreal injections [9], and topical non-steroidal anti-inflammatory agents (NSAIDs) [10] have an adverse effect on corneal sensation. Besides ocular causes, intracranial space-occupying lesions such as a meningioma or neuroma may compress onto the nucleus in the pons or the Gasserian ganglion. Systemic conditions may affect the ophthalmic branch of the trigeminal nerve, the long ciliary nerve, or the nasociliary nerve. These causes include acquired and inherited neuropathies such as diabetic corneal neuropathy, chemotherapy, and Charcot–Marie–Tooth disease [11,12,13]. Autoimmune diseases such as rheumatoid arthritis and fibromyalgia can cause corneal neuropathy [14]. Rarer diseases such as leprosy and metabolic causes including Vitamin A or B12 deficiencies [15,16] have also been reported to result in corneal anaesthesia. Studies have shown that peripheral and, subsequently, central corneal sensitivity decrease in a parabolic fashion with aging [17]. However, the results may differ depending on the aesthesiometer used [18]. Other distinctive patient factors such as iris colour and gender may also impact corneal sensitivity and corneal thresholds to mechanical and chemical stimuli [19].

Table 1.
Etiologies of corneal neuropathy.
The loss of protective and neurotrophic sensory innervation leads to corneal epithelial breakdown from repetitive microtraumas, ulceration, and scarring [2]. This condition, known as neurotrophic keratopathy, characterised by repeated epithelial breakdown and poor wound healing, is progressive in nature. The loss of protective blink reflexes and a reduction in tear secretion leads to the impaired perception of noxious stimuli and reduced clearing of the ocular surface, and may eventually lead to the opacification of the cornea and, possibly, blindness [5]. Neurotrophic keratopathy affects 5 or fewer in 10,000 people [20]. It is, therefore, crucial to detect neurotrophic keratopathy early and to monitor disease progression. Using corneal aesthesiometers, early detection can eventually prevent permanent and irreversible corneal damage.
Various aesthesiometers have been used over the years to enable a direct and quantitative assessment of corneal nerve function. In addition to aesthesiometry, a qualitative assessment of corneal nerve morphology, via in vivo confocal microscopy (IVCM), allows for the correlation of corneal sensitivity with corneal innervation [4,21]. In this paper, we aim to discuss the etiologies of corneal neuropathy, the history and types of aesthesiometers, and the advancement of corneal aesthesiometry over the years. We also report the relationship between corneal sensitivity and corneal innervation.
1.2. Search Strategy and Selection Criteria
The authors performed a search on the online database PubMed and Embase for relevant original articles on neurotrophic keratopathy, corneal nerves, and corneal neuropathy. The characteristics of various aesthesiometers, and the relation between corneal sensitivity measured by aesthesiometry, and innervation measured by IVCM were investigated. Articles were included up to July 2024. Two main search strategies were employed, using keywords such as “Corneal sensitivity”, “Corneal sensation”, “Corneal neuropathy”, “Corneal aesthesiometry”, “Cochet-Bonnet aesthesiometer” OR “Swiss Liquid Jet Aesthesiometer” OR “Corneal aesthesiometer Brill”, “In-vivo confocal microscopy”, AND “Corneal aesthesiometry”. Our review only included papers that were written in English as early as 2000 for the earliest original descriptions of corneal structure and neutrotrophic keratopathy. Relevant supplementary articles were extracted from the bibliography of existing articles. Duplicates were removed based on our selection criteria. Out of the original 431 articles derived from the preliminary search strategy, the abstracts were individually screened and further shortlisted. Based on the shortlisted abstracts, the full-text articles were reviewed and 31 were utilised in the final manuscript. The selection process of references is detailed in Figure 1.
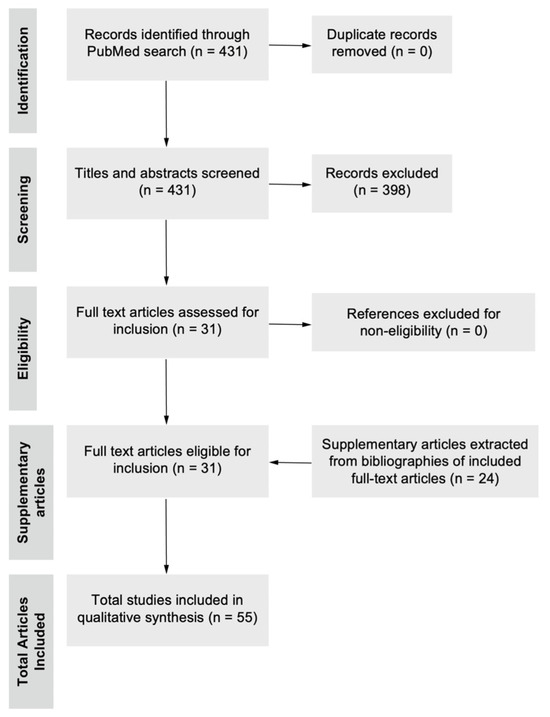
Figure 1.
Flow diagram of literature selection process. A search of the database PubMed was performed from the beginning of the study until 24 August 2024 using the search strategy as stated above. After duplicate removal, 431 titles and abstracts were screened, excluding articles based on the inclusion and exclusion criteria, and 31 full-text articles were eligible for inclusion and were surveyed, subsequently adding 24 relevant supplementary articles from currently included full-text articles. A total of 55 articles were used in the writing of the final manuscript.
2. Overview of the History and Types of Aesthesiometers
Before the invention and adoption of aesthesiometry, the cotton wisp test was first used to grossly test corneal sensitivity. The fibres of a cotton tipped applicator would be twirled into a single strand and used to lightly touch the corneal surface, stimulating the blink reflex mediated by the afferent ophthalmic branch of the trigeminal nerve, and the efferent facial nerve [22]. However, this only allowed for a qualitative assessment of corneal sensitivity.
The first quantitative method to evaluate corneal sensitivity, which was later known as aesthesiometry, was first described by Von Frey in 1894 [22]. He attached horse hairs to glass rods, modifying the lengths of hair to vary the pressure exerted on the corneal surface. However, certain factors, such as the condition and age of the hair, air humidity, room temperature, speed, and angle of contact with the surface, affected the measurement. In 1955, Boberg-Ans (University Eye-Clinic, Rigshospitalet, Copenhagen, Denmark) [22] improved on Von Frey’s version by replacing the horse hairs with fine nylon filaments, with a constant diameter but varying lengths. This enabled the adjustment of the rigidity of the filaments when used as a stimulus. In 1966, the Cochet–Bonnet aesthesiometer was introduced, by altering the thread holder from Boberg-Ans’ instrument [23]. The length is inverse to the pressure produced. The Cochet–Bonnet is currently considered the gold standard of corneal sensitivity assessment, being widely used in clinical practice. In the pre-clinical space, the Cochet–Bonnet aesthesiometer has been the predominant instrument of choice, owing to its ease of use, portability, and widely accepted status as a gold standard for assessing mechanosensation. The Cochet–Bonnet aesthesiometer was used to assess the response to mechanical stimuli in mice after the induction of ciliary nerve constriction for neuropathic corneal pain [24]. After the application of the nylon filament, the response was measured by a blink reflex in the mice. In another study involving mice, aimed to showcase the effect of aging on several parameters including corneal sensitivity [25], the Cochet–Bonnet aesthesiometer was similarly used. In mice with diabetic corneal neuropathy, the Cochet–Bonnet aesthesiometer was utilised to show the improved corneal sensitivity after the use of topical and oral peroxisome-proliferator-activated receptor-α agonist [26].
Over the years, the methods and instruments utilised have evolved and advanced significantly. While traditional methods involve direct mechanical stimulation, recent years have seen the development of non-contact aesthesiometers. In 1999, Carlos Belmonte invented the Belmonte Non-contact Corneal Aesthesiometer (NCCA). Instead of direct mechanical stimulation, the NCCA uses a pneumatic stimulus. A calibrated pulse of air of pre-determined pressure and diameter (0.5 mm) is released at a pre-determined distance away from the eye [23]. The air-pulse produces a momentary change in temperature and surface remodelling of the cornea [27], which is commonly perceived by subjects as a “cold” sensitivity. Besides mechanical and thermal stimulation, chemical stimulation was also possible by varying the concentration of the carbon dioxide and air mixture [28]. The CRCERT–Belmonte aesthesiometer is a modified version of the NCCA, incorporating a temperature-controlling device that allows temperature adjustments to eliminate the thermal stimulus [29]. Besides the air-jet stimulus, a new modified aesthesiometer, employing an isotonic saline solution in the form of a liquid jet, was first presented in 2018 [30]. Based on this prototype, the Swiss liquid-jet aesthesiometer for corneal sensitivity (SLACS) was developed [31]. The latest addition to the myriad of options is the new non-contact Brill aesthesiometer, which utilises air pulses but has the added benefit of being portable and mounted on a slit lamp [32], as opposed to older non-contact corneal aesthesiometers which are not handheld.
2.1. Cochet–Bonnet Aesthesiometry
The Cochet–Bonnet aesthesiometer (Luneau Ophthalmologie; Chartes, France) measures ocular sensitivity by altering the length of the nylon filament, which, in turns, affects the pressure applied to the ocular surface. It is widely considered as the gold standard for aesthesiometry, given its portability and availability via various manufacturers. With two different diameters (0.08 mm and 0.12 mm), the length can be modified from 5 to 60 mm, applying a transmitted pressure of 11 mm/g to 200 mm/g [32]. The thicker filament is used in most studies owing to reduced filament bending and movement, and commercial availability [33]. During the measurement of corneal sensitivity, the longest nylon monofilament is first used. A measurement is counted when the patient feels a mechanical stimulus. If there is no feeling and once a 5° bend is observed [33], the filament length will be gradually decreased until the patient reports a physical sensitivity. A shorter filament will require greater force in order to bend the filament, exerting more pressure on the cornea (Figure 2). The Cochet–Bonnet aesthesiometer is used as the common standard against which new aesthesiometers are compared [27,32], and to evaluate corneal sensitivity in various diseases [34,35,36].

Figure 2.
Illustration of the Cochet–Bonnet aesthesiometer used to assess corneal sensitivity. The filament is fully extended to its maximum length (60 mm) and applied to the cornea until the patient reports feeling the physical stimulus.
Despite being widely considered as the gold standard for aesthesiometry, the Cochet–Bonnet aesthesiometer also has certain drawbacks, which has led to the development and adoption of new tools to measure corneal sensitivity. The most significant disadvantage is the risk of abrasion of the corneal epithelial surface. When patients are unable to feel the stimulus, the length of the nylon filament will be gradually decreased. The corneal epithelium is fragile and the invasive stimulus, especially at shorter lengths and, thus, higher pressures, may cause epithelial injury [23]. The risk of injury is higher given that many patients undergoing sensory evaluation already have pre-existing corneal disease or surgery. The trauma to the epithelium will further worsen corneal neuropathy by lowering the corneal touch threshold [23].
Besides epithelial injury, there are various user-, patient-, environment-, and instrument-dependent factors that may limit the reliability of the Cochet–Bonnet aesthesiometer. Depending on the clinician who uses the tool, there may be difficulties in standardising the location on the cornea, the angle at which the corneal aesthesiometer is held and at which the cornea is approached, and the measurement of the angle of filament bending. Although a 4% flexure or 5° bend was agreed upon as a common standard, it is impractical to suggest that the operator can accurately measure the angle of filament bending. These will all affect corneal threshold values. From the patients’ perspective, as the Cochet–Bonnet aesthesiometer utilises a contact stimulus, the patient may be hesitant and apprehensive about the visible approaching tool, even flinching and further creating subjectivity [27]. Some studies have chosen to assess only the peripheral cornea to reduce the visual awareness of the instrument. This may limit the number of available corneal locations that can be assessed accurately. Furthermore, studies have reported the effect of ambient humidity on the amount of force exerted by the monofilaments. While keeping the diameter and filament length constant, the force decreases when ambient room humidity levels increase, as there is more absorption of moisture which reduces the material rigidity of the monofilament. The monofilament will thus bend more easily even at the same length [33].
The Cochet–Bonnet aesthesiometer has a limited range of stimulus intensities, and has shown to be less effective in detecting low-intensity stimulus as the minimum possible intensity is often suprathreshold for many patients [37]. In a large majority of subjects, the Cochet–Bonnet aesthesiometer may not be able to measure the true corneal sensitivity threshold. This could be attributed to a more pronounced bend of the filament at its longest lengths (around 60 mm). The limited range will lead to the usage of truncated threshold measurements [27]. When compared to newer aesthesiometers such as the Swiss Liquid or the air-jet non-contact corneal aesthesiometer, these newer instruments were able to detect even lower corneal thresholds as there were corneal responses even at lower stimulus intensities. This would be clinically significant for tracking minute changes and losses of corneal sensitivity over time [18]. Lastly, the Cochet–Bonnet aesthesiometer works by providing a direct mechanical stimulus, but is unable to produce a thermal or chemical stimulus [37]. This may possibly have clinical relevance as different etiologies of neurotrophic keratopathy may impact varying types of corneal fibres which transmit different stimuli. Having the ability to measure different types of nerves may improve the diagnosis, understanding, and, eventually, management of neurotrophic keratopathy.
2.2. Gas-Based Non-Contact Corneal Aesthesiometers
The gas-based NCCA operates by generating a controlled jet of air of pre-determined pressure that is directed towards the corneal surface. This air pressure is measured in millibars. The NCCA was developed in an attempt to address some of the drawbacks of the Cochet–Bonnet aesthesiometer. It improves on the sensitivity to lower-intensity stimuli. When the CRCERT–Belmonte (Deriva Global, S.L.; Valencia, Spain), a type of NCCA, and the Cochet–Bonnet aesthesiometer was compared, the corneal sensitivity was measured to be higher with the Belmonte aesthesiometer, as it is able to produce and measure much lower-intensity stimuli and, thus, detect lower corneal sensitivity thresholds, even below the limit of the Cochet–Bonnet aesthesiometer. More than half of the subjects could not be assessed with the standard Cochet–Bonnet aesthesiometer, while, with the Belmonte one, only 11% could not be assessed [38]. This is especially useful at higher sensitivity levels where there may be smaller and more minute changes.
The NCCA is also safe as it is non-invasive and does not possess the risk of epithelial injury. An assessment of the ocular surface after the usage of NCCA showed no evidence of conjunctival hyperemia or epithelial defects on corneal fluorescein staining [39], ensuring the safe assessment of corneal sensitivity recovery post-ocular surgery, or in corneal diseases such as recurrent corneal erosions. The NCCA also measures different stimuli, not only mechanical, but also thermal and chemical, as the mechanism of the air pulse creates a cooling effect when the evaporation of the tear film stimulates the thermoreceptors and, thus, the corneal nerve endings, whereas the carbon dioxide from the gas stimulates chemoreceptors.
However, this creates subjectivity in which stimulus is being measured by the NCCA. While a neural response is undoubtedly recorded, subjects have reported the sensitivity of a general coldness of the eye or a breeze blowing in their eye but were unable to accurately discriminate its location on the cornea [23]. The NCCA may not be measuring the mechanical touch threshold but the threshold to a composite stimulus comprising air pressure and tear evaporation. This would stimulate the Aδ fibres which primarily respond to mechanical stimuli, and the C fibres which respond to thermal stimuli [23]. The inadvertent recruitment of thermal-sensitive nociceptors on top of mechanical receptors may overstimulate and overestimate corneal sensitivity [27]. However, updated versions of the CRCERT–Belmonte aesthesiometer have addressed these limitations by allowing for a controlled temperature to eliminate any thermal component of the stimulus, producing a true mechanical stimulus [31]. Furthermore, the gas jet released by the NCCA is less localised and spread over a wider area as compared to the 0.12 mm filament of the Cochet–Bonnet aesthesiometer. Computational fluid dynamics demonstrates a reduction in the velocity and a pivot in the direction of air flow to a lateral motion upon impact with the central cornea. This exerts pressure on the peripheral region of the cornea one-third that of the apex, limiting the measurement of the corneal sensitivity in specific and precise locations [40].
In terms of portability and convenience, the NCCA loses out to the Cochet–Bonnet aesthesiometer as it has to be mounted onto a slit lamp apparatus which allows for more customisation of the stimulus. The NCCA was briefly commercially available, and would mostly be suitable for use in a dedicated ophthalmology practice and in the academic research setting.
2.3. Swiss Liquid-Jet Aesthesiometer for Corneal Sensitivity
In order to overcome some of the deficiencies of the gas-jet aesthesiometer such as the cooling effect and the dispersion of the gas-jet stimulus over a wider area of the cornea, a new prototype was created [30]. This prototype utilised droplets of isotonic saline solution, released from a microvalve of 0.1 mm diameter mounted on a slip lamp. It included a coil for heating and temperature sensor which allows for the precise control of the stimulus temperature to match the temperature of the ocular surface. The temperature regulator eliminates the thermal stimulus as it does not cause secondary evaporative cooling, thus producing a more accurate mechanical stimulus [31]. The stimulus intensity is determined by whether the microvalve is on or off (at a frequency of up to 4 kHz) and the duration (minimally 0.15 ms), while the pressure is kept fixed at 300 mbar [31]. Thus, the stimulus intensity is controlled by the pulse ratio (the ratio of the time when the microvalve is on vs. off). A new modified liquid-jet aesthesiometer, the SLACS (University of Applied Sciences FHNW, Windisch, Switzerland), alters the stimulus intensity by varying the pressure levels from 100 to 1500 mbar with a fixed stimulus duration of 40 ms, providing pulsed stimuli rather than a continuous jet. Compared to the carbon dioxide gas used in the Belmonte aesthesiometer, the pH of the liquid jet may be neutral to eliminate the chemical stimulus, or varied accordingly to create a chemical stimulus. The SLACS can modify pressure levels by increments of 1 mbar, thereby producing a very precise control of the stimulus intensity, and is thus able to detect even subtler changes in corneal sensitivity. A high-speed recording of the liquid jet making contact with the ocular surface showed the stimulus reaches a precise and localised region of the cornea, with little vertical and horizontal displacement [31].
Despite the improvements in the precise localisation of the stimulus, there still exists some uncertainty in the exact pressure exerted on the cornea, as this is affected by the speed and force of the release jet. Although it improves on the NCCA, the measurements may not be as precise as those of the Cochet–Bonnet aesthesiometer. The psychophysical stimulus makes it difficult to determine exactly which stimulus is being measured—the mechanical, thermal, or chemical. Being a relatively newer device like the gas-based NCCA, the SLACS is not widely available commercially and mainly used within research settings, not sharing the convenience, portability, and accessibility of the Cochet–Bonnet aesthesiometer.
2.4. Non-Contact Brill Aesthesiometer
The new corneal Brill aesthesiometer (Brill Engines, S.L; Barcelona, Spain) was introduced in 2023 and is a non-contact, gas-based aesthesiometer like the Belmonte one. The device uses ambient air instead of gas emitted from cylinders. The air is released from an outlet nozzle placed 4 mm from the corneal surface. At this distance, two light-emitting diode (LED) lights converge on the subject’s corneal surface. A screen and camera allow the operator to adjust and pinpoint the exact area for stimulation at a correct distance [32]. It can deliver pulses of air at five different levels of intensities and a pressure range of 2–10 mbar [41]. The Brill aesthesiometer can be used as a handheld device or mounted on a slit lamp (Figure 3), matching the Cochet–Bonnet aesthesiometer in portability and accessibility. It is non-invasive and does not risk causing epithelial injury and trauma. Although the Brill aesthesiometer is a relatively newer aesthesiometer and not many studies have been performed on it, current results show that the Brill was effective in evaluating corneal sensitivity in both healthy individuals and patients with ocular diseases such as dry eye disease and ocular graft-versus-host disease, demonstrating good repeatability [32,42,43]. Corneal sensitivity obtained using the Brill aesthesiometer showed a significant positive correlation and a good agreement with that obtained from the Cochet–Bonnet aesthesiometer [32,42,43]. The most recent and largest study thus far examining the repeatability and reproducibility of the Brill aesthesiometer by Ruiz-Lozano [42] showed excellent intra- and inter-observed repeatability (ICC > 0.9). However, the values from the Brill and Cochet–Bonnet aesthesiometers are not interchangeable, with a significant difference between the mean corneal sensitivity thresholds from both devices: 0.052 ± 0.021 mN for Brill vs. 0.046 ± 0.005 mN, p = 0.001 for Cochet.
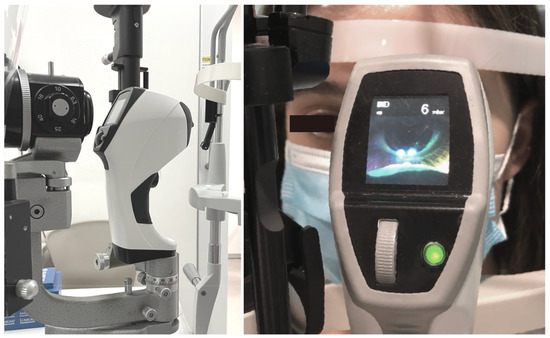
Figure 3.
Setup of the Brill aesthesiometer used as a slip lamp attachment. The Brill aesthesiometer features a camera and small screen to accurately target the area for corneal stimulation, using the alignment of two converging LED lights projected onto the cornea to ensure precise stimulation at the appropriate distance.
However, the Brill aesthesiometer shares similar limitations to the Belmonte one, being an air-based aesthesiometer. The gas jet produces a mechanical, thermal, and chemical stimulus that all have a shared mechanism in stimulating the corneal surface, due to evaporative cooling and tear film depletion. The gas jet is dispersed across a wider area on the ocular surface, spreading over a diameter of 0.5 mm, which may compromise the precise localisation of the stimulus on the cornea. As there are only five intensity levels, with the pressure of the air stimulus ranging from 2.0 mbar to 10.0 mbar, the Brill aesthesiometer may not be able to detect smaller changes in corneal sensitivity. In a recent study, there were subjects that could not feel the stimulus at the maximum pressure (10 mbar) and, hence, the measurement had to be taken as 11 mbar, one above the maximum value [41]. More extensive study on the reliability and accuracy of the Brill aesthesiometer will be helpful for guiding its future use for the management of patients in clinical settings and in research.
Table 2 summarises the main characteristics of different types of aesthesiometers.

Table 2.
Characteristics of different aesthesiometers.
3. Correlation Between Corneal Sensitivity and Innervation
The quantitative relationship between corneal sensitivity and innervation has been explored and studied. As stated in the previous section, studies ranging across different diseases show a correlation between various parameters, including the subbasal nerve density, number, length, branching and tortuosity, with decreased corneal sensitivity. These diseases include dry eye disease [44], glaucoma [45], keratoconus [46], herpes keratitis [47], post-refractive surgery [48], diabetes [49], and Fuchs’ corneal dystrophy [50]. In type 1 diabetes, corneal sensitivity was significantly and positively correlated with the number of long nerve fibre bundles (r = 0.42; p = 0.048) [49]. IVCM shows a loss of nerve fibre bundles which precedes sensitivity impairment, with mild–moderate neuropathy showing decreased corneal nerve innervation but only severe neuropathy presenting with reduced sensitivity [49]. Similar findings were reported by Takavoli et al., who described a significant reduction in corneal sensitivity assessed using the Cochet–Bonnet aesthesiometer in mild, moderate, and severe diabetic peripheral neuropathy, while NCCA measurements showed a significant reduction in moderate and severe cases (all p < 0.05), with neuropathy severity evaluated using the Neuropathy Deficit Score [51].
For patients with Fuchs’ endothelial dystrophy, Aggarwal et al. reported that corneal sensitivity assessed by the Cochet-Bonnet aesthesiometer was significantly correlated with the number (r = 0.47; p = 0.010) and length (r = 0.32; p = 0.044) of subbasal nerves [50]. Another study on Fuchs’ endothelial dystrophy by Dikmetas et al. demonstrated Cochet–Bonnet measurements were significantly correlated with corneal subbasal nerve plexus densities (r = 0.46, p = 0.025) and nerve tortuosity (r = 0.44, p = 0.031) [52].
With regard to dry eye disease, Labbé et al. found a significant positive correlation between corneal sensitivity, as measured by the Cochet–Bonnet aesthesiometer, and both the density (r = 0.64; p = 0.045) and number of subbasal nerves (r = 0.65; p = 0.043) [53]. Benítez-del-Castillo et al. found that subbasal nerve density correlated significantly with mechanical, chemical, and thermal sensitivity thresholds assessed by NCCA (r = −0.79, r = −0.80, and r = −0.63, respectively; all p < 0.001) [44].
When looking at the correlation between corneal sensitivity and corneal nerve tortuosity, two studies which performed analyses on glaucoma patients revealed an inverse relationship between Cochet–Bonnet measurements and subbasal nerve tortuosity (r = −0.54 and r = −0.62, both p < 0.05) [45,53]. This inverse relationship indicates a worse corneal nerve morphology correlating with corneal sensitivity.
In keratoconus, the corneal sensitivity assessed by NCCA significantly correlated to subbasal nerve density (r = −0.31, p = 0.001) [46]. Additionally, a study on herpes simplex keratitis found that Cochet–Bonnet measurements were significantly correlated to subbasal nerve density (r = 0.65, p < 0.0001) and main nerve trunks (r = 0.62, p < 0.0002) [47]. In patients who have undergone refractive surgery, the corneal sensitivity assessed by the Cochet–Bonnet aesthesiometer was significantly correlated with subbasal corneal nerve fibre bundle density in both post-laser-assisted in situ keratomileusis (LASIK) (r = 0.93, p < 0.001) and post-laser epithelial keratomileusis (LASEK) eyes (r = 0.68, p < 0.001) [54].
Recently, Surico et al. reported a significant negative correlation between the corneal sensitivity assessed by the Brill aesthesiometer and the total (r = −0.83; p < 0.001), main trunk (r = −0.62; p < 0.001), and branch (r = −0.72; p < 0.001) nerve densities in patients with ocular graft-versus-host disease [43]. These results suggest that corneal nerves’ quantity and quality can be directly correlated with corneal sensitivity assessment using aesthesiometry. Table 3 summarises the key findings of the literature on the correlation between corneal sensitivity and subbasal nerve plexus parameters.

Table 3.
Corneal sensitivity correlated with subbasal nerve plexus parameters measured on IVCM in various ocular conditions.
However, there are some studies which have shown no apparent correlation between corneal sensitivity and corneal nerve metrics. This can be explained as follows:
Firstly, at an early stage of the disease with low severity, there may not have been a drop in corneal nerve metrics and innervation. In the study on diabetic peripheral neuropathy by Rosenberg et al., the early stages of the disease with mild–moderate peripheral neuropathy reveals decreased corneal innervation, but not reduced corneal sensitivity. Only patients with severe peripheral neuropathy presents with corneal neuropathy [49,51]. In Fuchs’ endothelial dystrophy, there may be certain parts of the subbasal nerve plexus which undergo alteration and are rendered dysfunctional, for example, the central cornea, before finally leading to the loss of the subbasal nerve plexus in the entire cornea [52,55].
Secondly, the current instruments and methods available for both the measurement of corneal sensitivity in aesthesiometry and corneal morphology on the IVCM evaluation may not be adequately sensitive enough to detect minute and early changes. For IVCM, improvements can be made in increasing the field of view and automatic quantification [53,56]. The small field of view in IVCM (0.16 mm2) limits the comprehensive overview of the entire cornea. Improvements in the field of view and reconstruction will enhance the corneal morphology assessment [57]. In addition, there have been new software developed which enable the precise calculation and measurement of subbasal nerve metrics [58]. For corneal aesthesiometry, one drawback is that contact aesthesiometry merely evaluates mechanoreceptors while missing out on chemoreceptors and thermoreceptors, thus not being an absolute and all-encompassing measure of corneal sensitivity [52]. Furthermore, many of the studies examining the correlation between corneal sensitivity and corneal innervation use contact aesthesiometry (Cochet–Bonnet) [47,49,50,53], while other studies used non-contact aesthesiometry [44,46]. As these readings are not interchangeable, standardising the use of aesthesiometers may help to elucidate a clearer correlation between corneal sensitivity and innervation.
Thirdly, as of now, IVCM most reliably and sensitively detects and images subbasal nerve bundles. However, epithelial and stromal nerves, due to their far lower density, are sporadically missed out by IVCM [59]. Hence, functional neural loss that occurs in the epithelial or stromal nerves, or even in the synaptic or nuclear level, may not be noticeable on IVCM [60].
4. Conclusions and Future Directions
The ideal next-generation aesthesiometer should integrate multiple types of stimuli, such as mechanical, thermal, and chemical stimuli, in a controlled and reproducible manner, while also balancing precision, accuracy, accessibility, and ease of use in clinical settings. A gas-based system remains promising due to its non-contact nature and safety profile. Besides the diagnosis of neuropathic corneas, aesthesiometers also plays a role in monitoring the disease progression and the status of corneal reinnervation after treatment. Thus, the assessment of the structure and function of the corneal nerves continues to be a highly essential and beneficial clinical priority.
Author Contributions
E.E.: literature search, drafting the manuscript, and reviewing and editing. M.Y.: drafting the manuscript, and reviewing and editing. C.L.: investigation. I.X.Y.L.: data curation. R.K.T.W.: investigation. J.H.F.W.: investigation. Y.-C.L.: conceptualisation, funding acquisition, supervision, and reviewing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Clinician Scientist Awards (MOH-CSAINV21jun-0001 and CSAINV24jul-0005), and Clinician Scientist individual research grant (CIRG24jul-0010), from the Singapore National Medical Research Council.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article material; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NSAIDs | Non-steroidal anti-inflammatory agents |
| IVCM | In vivo confocal microscopy |
| NCCA | Non-contact corneal aesthesiometer |
| SLACS | Swiss liquid-jet aesthesiometer for corneal sensitivity |
| LED | Light-emitting diode |
| LASIK | Laser-assisted in situ keratomileusis |
| LASEK | Laser-assisted epithelial keratomileusis |
References
- Bonini, S.; Rama, P.; Olzi, D.; Lambiase, A. Neurotrophic keratitis. Eye 2003, 17, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, K.; Tajdaran, K.; Mirmoeini, K.; Daeschler, S.C.; Henriquez, M.A.; Stevens, K.E.; Mulenga, C.M.; Hussain, A.; Hamrah, P.; Ali, A.; et al. The Role of Sensory Innervation in Homeostatic and Injury-Induced Corneal Epithelial Renewal. Int. J. Mol. Sci. 2023, 24, 12615. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.R.; Tannir, S.; Tran, K.; Boente, C.S.; Ali, A.; Borschel, G.H. Corneal Nerve Assessment by Aesthesiometry: History, Advancements, and Future Directions. Vision 2024, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, M.; Lambiase, A. Diagnosis and management of neurotrophic keratitis. Clin. Ophthalmol. 2014, 8, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.Y.; Chow, J.; Liu, J. Corneal Innervation and Sensation: The Eye and Beyond. Yale J. Biol. Med. 2018, 91, 13–21. [Google Scholar] [PubMed]
- Liu, Y.C.; Jung, A.S.J.; Chin, J.Y.; Yang, L.W.Y.; Mehta, J.S. Cross-sectional Study on Corneal Denervation in Contralateral Eyes Following SMILE Versus LASIK. J. Refract. Surg. 2020, 36, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, M.T.; Lee, I.X.Y.; Wong, J.H.F.; Lu, D.; Lam, T.C.; Zhou, L.; Mehta, J.S.; Ong, H.S.; Ang, M.; et al. Neuropathic Corneal Pain: Tear Proteomic and Neuromediator Profiles, Imaging Features, and Clinical Manifestations. Am. J. Ophthalmol. 2024, 265, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díaz de León, L.; Morales-León, J.E.; Ledesma-Gil, J.; Navas, A. Conjunctival and corneal sensitivity in patients under topical antiglaucoma treatment. Int. Ophthalmol. 2016, 36, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Polat, O.A.; Şener, H.; Erkiliç, K. Corneal Nerve Fiber and Sensitivity Loss After Repeated Intravitreal Anti-VEGF Injections: An In Vivo Confocal Microscopy Study. Cornea 2022, 41, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.D.; Kennedy, J.; Wittpenn, J.R. Topical NSAIDs effect on corneal sensitivity. Cornea 2015, 34, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.C.B.; Goldstein, D.; Trinh, T.; Au, K.; Mizrahi, D.; Muhlmann, M.; Crowe, P.; O’Neill, S.; Edwards, K.; Park, S.B.; et al. A Cross-Sectional Study of Sub-Basal Corneal Nerve Reduction Following Neurotoxic Chemotherapy. Transl. Vis. Sci. Technol. 2021, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H.; Tan, H.C.; Lin, M.T.; Mehta, J.S.; Liu, Y.C. Diabetic Corneal Neuropathy. J. Clin. Med. 2020, 9, 3956. [Google Scholar] [CrossRef] [PubMed]
- So, W.Z.; Qi Wong, N.S.; Tan, H.C.; Yu Lin, M.T.; Yu Lee, I.X.; Mehta, J.S.; Liu, Y.C. Diabetic corneal neuropathy as a surrogate marker for diabetic peripheral neuropathy. Neural Regen. Res. 2022, 17, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Lee, J.H.; Kim, S.S. Reduced corneal sensitivity in patients with rheumatoid arthritis. Cornea 2012, 31, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; Gutierrez, D.R. Hypovitaminosis A: A hidden cause of neurotrophic keratitis. Arq. Bras. Oftalmol. 2024, 87, e2023. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, N.; Assarzadegan, F.; Shahriari, M.; Norouzi, H.; Kavousnezhad, S.; Nassiri, N.; Sheibani, K. Vitamin B12 Deficiency as a Cause of Neurotrophic Keratopathy. Open Ophthalmol. J. 2018, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, A.M.; Colosi, P.; Ferreri, F.M.; Galasso, S. Age-related modifications of corneal sensitivity. Ophthalmologica 2004, 218, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Nosch, D.S.; Käser, E.; Bracher, T.; Joos, R.E. Age-Related Changes in Corneal Sensitivity. Cornea 2023, 42, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.C.; Alfaro, M.L.; Borrás, F.; Belmonte, C.; Gallar, J. Influence of age, gender and iris color on mechanical and chemical sensitivity of the cornea and conjunctiva. Exp. Eye Res. 2006, 83, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Said, D.G.; Messmer, E.M.; Rolando, M.; Benitez-Del-Castillo, J.M.; Hossain, P.N.; Shortt, A.J.; Geerling, G.; Nubile, M.; Figueiredo, F.C.; et al. Neurotrophic keratopathy. Prog. Retin. Eye Res. 2018, 66, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Lin, M.T.; Mehta, J.S. Analysis of corneal nerve plexus in corneal confocal microscopy images. Neural Regen. Res. 2021, 16, 690–691. [Google Scholar] [CrossRef] [PubMed]
- Draeger, J. Development of the Various Methods of Esthesiometry. In Corneal Sensitivity: Measurement and Clinical Importance; Draeger, J., Ed.; Springer: Vienna, Austria, 1984; pp. 8–34. [Google Scholar] [CrossRef]
- Murphy, P.J.; Patel, S.; Marshall, J. A new non-contact corneal aesthesiometer (NCCA). Ophthalmic Physiol. Opt. 1996, 16, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Seyed-Razavi, Y.; Kenyon, B.M.; Qiu, F.; Harris, D.L.; Hamrah, P. A novel animal model of neuropathic corneal pain-the ciliary nerve constriction model. Front. Neurosci. 2023, 17, 1265708. [Google Scholar] [CrossRef] [PubMed]
- De Silva, M.E.H.; Hill, L.J.; Downie, L.E.; Chinnery, H.R. The Effects of Aging on Corneal and Ocular Surface Homeostasis in Mice. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H.; Lee, I.X.Y.; Lin, M.T.-Y.; Ang, H.P.; Xue, Y.C.; Krishaa, L.; Patil, M.; Koh, S.-K.; Tan, H.C.; Zhou, L.; et al. Topical and oral peroxisome proliferator-activated receptor-α agonist ameliorates diabetic corneal neuropathy. Sci. Rep. 2024, 14, 13435. [Google Scholar] [CrossRef] [PubMed]
- Nosch, D.S.; Käser, E.; Bracher, T.; Joos, R.E. Clinical application of the Swiss Liquid Jet Aesthesiometer for corneal sensitivity measurement. Clin. Exp. Optom. 2024, 107, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Acosta, M.C.; Schmelz, M.; Gallar, J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Investig. Ophthalmol. Vis. Sci. 1999, 40, 513–519. [Google Scholar]
- Stapleton, F.; Tan, M.E.; Papas, E.B.; Ehrmann, K.; Golebiowski, B.; Vega, J.; Holden, B.A. Corneal and conjunctival sensitivity to air stimuli. Br. J. Ophthalmol. 2004, 88, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, K.; Saha, M.; Falk, D. A novel method to stimulate mechanoreceptors and quantify their threshold values. Biomed. Phys. Eng. Express 2018, 4, 025004. [Google Scholar] [CrossRef]
- Nosch, D.S.; Oscity, M.; Steigmeier, P.; Käser, E.; Loepfe, M.; Joos, R.E. Working principle and relevant physical properties of the Swiss Liquid Jet Aesthesiometer for Corneal Sensitivity (SLACS) evaluation. Ophthalmic Physiol. Opt. 2022, 42, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Merayo-Lloves, J.; Gómez Martín, C.; Lozano-Sanroma, J.; Renedo Laguna, C. Assessment and safety of the new esthesiometer BRILL: Comparison with the Cochet-Bonnet Esthesiometer. Eur. J. Ophthalmol. 2024, 34, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Lum, E.; Murphy, P.J. Effects of ambient humidity on the Cochet-Bonnet aesthesiometer. Eye 2018, 32, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M.; Bartoli, E. Corneal sensitivity after small-incision lenticule extraction and laser in situ keratomileusis. J. Cataract. Refract. Surg. 2015, 41, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Bucher, F.; Adler, W.; Lehmann, H.C.; Hos, D.; Steven, P.; Cursiefen, C.; Heindl, L.M. Corneal nerve alterations in different stages of Fuchs’ endothelial corneal dystrophy: An in vivo confocal microscopy study. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, A.; Hamrah, P.; Cavalcanti, B.M.; Zheng, L.; Colby, K.; Pavan-Langston, D. Corneal Reinnervation and Sensation Recovery in Patients With Herpes Zoster Ophthalmicus: An In Vivo and Ex Vivo Study of Corneal Nerves. Cornea 2016, 35, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.J.; Lawrenson, J.G.; Patel, S.; Marshall, J. Reliability of the non-contact corneal aesthesiometer and its comparison with the Cochet-Bonnet aesthesiometer. Ophthalmic Physiol. Opt. 1998, 18, 532–539. [Google Scholar] [PubMed]
- Golebiowski, B.; Papas, E.; Stapleton, F. Assessing the sensory function of the ocular surface: Implications of use of a non-contact air jet aesthesiometer versus the Cochet-Bonnet aesthesiometer. Exp. Eye Res. 2011, 92, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Tesón, M.; Calonge, M.; Fernández, I.; Stern, M.E.; González-García, M.J. Characterization by Belmonte’s gas esthesiometer of mechanical, chemical, and thermal corneal sensitivity thresholds in a normal population. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3154–3160. [Google Scholar] [CrossRef] [PubMed]
- Golebiowski, B.; Lim, M.; Papas, E.; Stapleton, F. Understanding the stimulus of an air-jet aesthesiometer: Computerised modelling and subjective interpretation. Ophthalmic Physiol. Opt. 2013, 33, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.; Sabates, V.; Orgul, S.; Perez, V.L.; Swaminathan, S.S.; Sabater, A.L. Detection of Subclinical Neurotrophic Keratopathy by Noncontact Esthesiometry. Ophthalmol. Ther. 2024, 13, 2393–2404. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, R.E.; Quiroga-Garza, M.E.; Ramos-DÁVila, E.M.; PantaleÓN-GarcÍA, J.; Khodor, A.L.I.; Komai, S.; Rodriguez-Gutierrez, L.A.; Ma, S.; Mousa, H.M.; Mattes, R.; et al. Comparative Evaluation of the Corneal Sensitivity Thresholds between the Novel Non-Contact and Cochet-Bonnet Esthesiometers. Am. J. Ophthalmol. 2025, 271, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Surico, P.L.; Saricay, L.Y.; Singh, R.B.; Kahale, F.; Romano, F.; Dana, R. Corneal Sensitivity and Neuropathy in Patients With Ocular Graft-Versus-Host Disease. Cornea 2022, 44, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Del-Castillo, J.M.; Acosta, M.C.; Wassfi, M.A.; Díaz-Valle, D.; Gegúndez, J.A.; Fernandez, C.; García-Sánchez, J. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Martone, G.; Frezzotti, P.; Tosi, G.M.; Traversi, C.; Mittica, V.; Malandrini, A.; Pichierri, P.; Balestrazzi, A.; Motolese, P.A.; Motolese, I.; et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am. J. Ophthalmol. 2009, 147, 725–735.el. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.V.; Ku, J.Y.; Johnson, R.; McGhee, C.N. Laser scanning in vivo confocal microscopy and quantitative aesthesiometry reveal decreased corneal innervation and sensation in keratoconus. Eye 2009, 23, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Cruzat, A.; Dastjerdi, M.H.; Zheng, L.; Shahatit, B.M.; Bayhan, H.A.; Dana, R.; Pavan-Langston, D. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: An in vivo confocal microscopy study. Ophthalmology 2010, 117, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Stachs, O.; Zhivov, A.; Kraak, R.; Hovakimyan, M.; Wree, A.; Guthoff, R. Structural-functional correlations of corneal innervation after LASIK and penetrating keratoplasty. J. Refract. Surg. 2010, 26, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.E.; Tervo, T.M.; Immonen, I.J.; Müller, L.J.; Grönhagen-Riska, C.; Vesaluoma, M.H. Corneal structure and sensitivity in type 1 diabetes mellitus. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2915–2921. [Google Scholar]
- Aggarwal, S.; Cavalcanti, B.M.; Regali, L.; Cruzat, A.; Trinidad, M.; Williams, C.; Jurkunas, U.V.; Hamrah, P. In Vivo Confocal Microscopy Shows Alterations in Nerve Density and Dendritiform Cell Density in Fuchs’ Endothelial Corneal Dystrophy. Am. J. Ophthalmol. 2018, 196, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Kallinikos, P.A.; Efron, N.; Boulton, A.J.; Malik, R.A. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care 2007, 30, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Dikmetas, O.; Kocabeyoglu, S.; Mocan, M.C.; Karahan, S.; İrkec, M. The relationship between corneal subbasal nerve density and corneal sensitivity in patients with Fuchs endothelial corneal dystrophy. Indian J. Ophthalmol. 2021, 69, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Labbé, A.; Alalwani, H.; Van Went, C.; Brasnu, E.; Georgescu, D.; Baudouin, C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4926–4931. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, J.K.; Seo, K.Y.; Kim, E.K.; Lee, H.K. Comparison of corneal nerve regeneration and sensitivity between LASIK and laser epithelial keratomileusis (LASEK). Am. J. Ophthalmol. 2006, 141, 1009–1015.el. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Liu, Y.C.; Liu, C.; Mehta, J.S. Applications of Imaging Technologies in Fuchs Endothelial Corneal Dystrophy: A Narrative Literature Review. Bioengineering 2024, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.Y.; Yang, L.W.Y.; Ji, A.J.S.; Nubile, M.; Mastropasqua, L.; Allen, J.C.; Mehta, J.S.; Liu, Y.C. Validation of the Use of Automated and Manual Quantitative Analysis of Corneal Nerve Plexus Following Refractive Surgery. Diagnostics 2020, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Liu, Y.C.; Lin, M.T.; Mehta, J.S. Clinical Applications of In Vivo Confocal Microscopy in Keratorefractive Surgery. J. Refract. Surg. 2021, 37, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.V.; Tavakoli, M.; Craig, J.P.; Efron, N.; McGhee, C.N. Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea 2009, 28, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Erie, J.C.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M. The effect of age on the corneal subbasal nerve plexus. Cornea 2005, 24, 705–709. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
