Wearables in ADHD: Monitoring and Intervention—Where Are We Now?
Abstract
1. Introduction
2. Physiology and Behavioral Pathology of ADHD
2.1. Autonomic Function and Emotional Regulation
2.2. Motor and Physical Activity
2.3. Neurocognitive Function and Brain Activity
2.4. Sleep and Circadian Rhythms
3. Materials and Methods
3.1. Sources and Search Strategy
3.2. Eligibility Criteria and Scope
3.3. Evidence Charting and Synthesis
3.4. Appraisal of Methodological Features
3.5. Methodological Limitations
4. Recent Advances in Wearable Technologies for ADHD
4.1. Monitoring Features of Wearables Devices in ADHD
4.2. Interventional Applications of Wearable Technologies
4.2.1. Behavioral Interventions
4.2.2. Biofeedback Mechanisms
4.2.3. Neuromodulation
4.2.4. Peripheral Visual Stimulation
4.3. Real-Time Data Collection and Integration with Digital Platforms
5. Efficacy and Challenges
5.1. Clinical Outcomes
5.2. Barriers to Adoption
5.3. Clinical Translation, Regulatory Pathway and Market Access Strategy
5.3.1. United States
5.3.2. European Union
5.3.3. Cost-Cutting Strategies
5.4. Future Directions
6. Risks and Ethical Considerations
6.1. Algorithmic Bias
6.2. Pediatric Consent and Assent
6.3. Data Privacy and Governance
7. Limitations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polanczyk, G.V.; Willcutt, E.G.; Salum, G.A.; Kieling, C.; Rohde, L.A. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int. J. Epidemiol. 2014, 43, 434–442. [Google Scholar] [CrossRef]
- CDCMMWR. QuickStats: Percentage of Children and Adolescents Aged 5–17 Years Who Had Ever Received a Diagnosis of Attention-Deficit/Hyperactivity Disorder, by Urbanization Level and Age Group—National Health Interview Survey, United States, 2020–2022. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 116. [Google Scholar] [CrossRef]
- Song, P.; Zha, M.; Yang, Q.; Zhang, Y.; Li, X.; Rudan, I. The prevalence of adult attention-deficit hyperactivity disorder: A global systematic review and meta-analysis. J. Glob. Health 2021, 11, 04009. [Google Scholar] [CrossRef]
- Gimbach, S.; Vogel, D.; Fried, R.; Faraone, S.V.; Banaschewski, T.; Buitelaar, J.; Döpfner, M.; Ammer, R. ADHD medicine consumption in Europe after COVID-19: Catch-up or trend change? BMC Psychiatry 2024, 24, 112. [Google Scholar] [CrossRef]
- Schein, J.; Adler, L.A.; Childress, A.; Gagnon-Sanschagrin, P.; Davidson, M.; Kinkead, F.; Cloutier, M.; Guérin, A.; Lefebvre, P. Economic burden of attention-deficit/hyperactivity disorder among adults in the United States: A societal perspective. J. Manag. Care Spec. Pharm. 2022, 28, 168–179. [Google Scholar] [CrossRef]
- French, B.; Nalbant, G.; Wright, H.; Sayal, K.; Daley, D.; Groom, M.J.; Cassidy, S.; Hall, C.L. The impacts associated with having ADHD: An umbrella review. Front. Psychiatry 2024, 15, 1343314. [Google Scholar] [CrossRef] [PubMed]
- Cibrian, F.L.; Hayes, G.R.; Lakes, K.D. Research Advances in ADHD and Technology; Synthesis Lectures on Assistive, Rehabilitative, and Health-Preserving Technologies; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Kooij, J.J.S.; Bijlenga, D.; Salerno, L.; Jaeschke, R.; Bitter, I.; Balázs, J.; Thome, J.; Dom, G.; Kasper, S.; Filipe, C.N.; et al. Updated European Consensus Statement on diagnosis and treatment of adult ADHD. Eur. Psychiatry 2019, 56, 14–34. [Google Scholar] [CrossRef]
- Welch, V.; Wy, T.J.; Ligezka, A.; Hassett, L.C.; Croarkin, P.E.; Athreya, A.P.; Romanowicz, M. Use of Mobile and Wearable Artificial Intelligence in Child and Adolescent Psychiatry: Scoping Review. J. Med. Internet Res. 2022, 24, e33560. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M. Unlocking the potential of wearable technology: Fitbit-derived measures for predicting ADHD in adolescents. Front. Child Adolesc. Psychiatry 2025, 4, 1504323. [Google Scholar] [CrossRef]
- Park, C.; Rouzi, M.D.; Atique, M.M.U.; Finco, M.G.; Mishra, R.K.; Barba-Villalobos, G.; Crossman, E.; Amushie, C.; Nguyen, J.; Calarge, C.; et al. Machine Learning-Based Aggression Detection in Children with ADHD Using Sensor-Based Physical Activity Monitoring. Sensors 2023, 23, 4949. [Google Scholar] [CrossRef]
- Ouyang, C.S.; Yang, R.C.; Chiang, C.T.; Wu, R.C.; Lin, L.C. Objective Evaluation of Therapeutic Effects of ADHD Medication Using a Smart Watch: A Pilot Study. Appl. Sci. 2020, 10, 5946. [Google Scholar] [CrossRef]
- Denyer, H.; Carr, E.; Deng, Q.; Asherson, P.; Bilbow, A.; Folarin, A.; Groom, M.J.; Hollis, C.; Sankesara, H.; Dobson, R.J.; et al. A 10-week remote monitoring study of sleep features and their variability in individuals with and without ADHD. BMC Psychiatry 2025, 25, 294. [Google Scholar] [CrossRef]
- Tosti, B.; Corrado, S.; Mancone, S.; Di Libero, T.; Rodio, A.; Andrade, A.; Diotaiuti, P. Integrated use of biofeedback and neurofeedback techniques in treating pathological conditions and improving performance: A narrative review. Front. Neurosci. 2024, 18, 1358481. [Google Scholar] [CrossRef] [PubMed]
- Santamaría-Vázquez, E.; Estudillo-Guerra, A.; Ali, L.; Martinez, D.; Hornero, R.; Morales-Quezada, L. Effects of a novel non-pharmacological intervention based on respiratory biofeedback, neurofeedback and median nerve stimulation to treat children with ADHD. Front. Hum. Neurosci. 2025, 19, 1478501. [Google Scholar] [CrossRef] [PubMed]
- Leikauf, J.E.; Correa, C.; Bueno, A.N.; Sempere, V.P.; Williams, L.M. StopWatch: Pilot study for an Apple Watch application for youth with ADHD. Digit. Health 2021, 7, 20552076211001215. [Google Scholar] [CrossRef] [PubMed]
- Koirala, S.; Grimsrud, G.; Mooney, M.A.; Larsen, B.; Feczko, E.; Elison, J.T.; Nelson, S.M.; Nigg, J.T.; Tervo-Clemmens, B.; Fair, D.A. Neurobiology of attention-deficit hyperactivity disorder: Historical challenges and emerging frontiers. Nat. Rev. Neurosci. 2024, 25, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Sonuga-Barke, E.J.S.; Castellanos, F.X. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci. Biobehav. Rev. 2007, 31, 977–986. [Google Scholar] [CrossRef]
- Mooney, M.A.; Hermosillo, R.J.M.; Feczko, E.; Miranda-Dominguez, O.; Moore, L.A.; Perrone, A.; Byington, N.; Grimsrud, G.; Rueter, A.; Nousen, E.; et al. Cumulative Effects of Resting-State Connectivity Across All Brain Networks Significantly Correlate with Attention-Deficit Hyperactivity Disorder Symptoms. J. Neurosci. 2024, 44, e1202232023. [Google Scholar] [CrossRef]
- Castellanos, F.X.; Proal, E. Large-scale brain systems in ADHD: Beyond the prefrontal-striatal model. Trends Cogn. Sci. 2012, 16, 17–26. [Google Scholar] [CrossRef]
- Robbins, T.W.; Sahakian, B.J. “Paradoxical” effects of psychomotor stimulant drugs in hyperactive children from the standpoint of behavioural pharmacology. Neuropharmacology 1979, 18, 931–950. [Google Scholar] [CrossRef]
- Faraone, S.V. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [CrossRef]
- Demontis, D.; Walters, G.B.; Athanasiadis, G.; Walters, R.; Therrien, K.; Nielsen, T.T.; Farajzadeh, L.; Voloudakis, G.; Bendl, J.; Zeng, B.; et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat. Genet. 2023, 55, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Nigg, J.T.; Karalunas, S.L.; Feczko, E.; Fair, D.A. Toward a Revised Nosology for Attention-Deficit/Hyperactivity Disorder Heterogeneity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 726–737. [Google Scholar] [CrossRef]
- Feczko, E.; Miranda-Dominguez, O.; Marr, M.; Graham, A.M.; Nigg, J.T.; Fair, D.A. The Heterogeneity Problem: Approaches to Identify Psychiatric Subtypes. Trends Cogn. Sci. 2019, 23, 584–601. [Google Scholar] [CrossRef]
- American Psychiatric Association (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; p. 947. [Google Scholar]
- Traunmuller, P.; Jahanjoo, A.; Khooyooz, S.; Aminifar, A.; TaheriNejad, N. Wearable Healthcare Devices for Monitoring Stress and Attention Level in Workplace Environments. arXiv 2024, arXiv:2406.05813. [Google Scholar] [CrossRef]
- Andrikopoulos, D.; Vassiliou, G.; Fatouros, P.; Tsirmpas, C.; Pehlivanidis, A.; Papageorgiou, C. Machine learning-enabled detection of attention-deficit/hyperactivity disorder with multimodal physiological data: A case-control study. BMC Psychiatry 2024, 24, 547. [Google Scholar] [CrossRef] [PubMed]
- Tim, P.L. Wearable device for determining and preempting the emotional dysregulation of ADHD adolescents. In Proceedings of the TENCON 2022—2022 IEEE Region 10 Conference (TENCON), Hong Kong, China, 1–4 November 2022; pp. 1–7. Available online: https://ieeexplore.ieee.org/document/9977770 (accessed on 5 July 2025).
- O’Connell, R.G.; Bellgrove, M.A.; Dockree, P.M.; Robertson, I.H. Reduced electrodermal response to errors predicts poor sustained attention performance in attention deficit hyperactivity disorder. Neuroreport 2004, 15, 2535–2538. [Google Scholar] [CrossRef] [PubMed]
- Te Lindert, B.H.W.; Van Someren, E.J.W. Skin temperature, sleep, and vigilance. Handb. Clin. Neurol. 2018, 156, 353–365. [Google Scholar] [CrossRef]
- Vavrinsky, E.; Stopjakova, V.; Kopani, M.; Kosnacova, H. The Concept of Advanced Multi-Sensor Monitoring of Human Stress. Sensors 2021, 21, 3499. [Google Scholar] [CrossRef]
- Gawrilow, C.; Kühnhausen, J.; Schmid, J.; Stadler, G. Hyperactivity and Motoric Activity in ADHD: Characterization, Assessment, and Intervention. Front. Psychiatry 2014, 5, 171. [Google Scholar] [CrossRef]
- Mokobane, M.; Pillay, B.J.; Meyer, A. Fine motor deficits and attention deficit hyperactivity disorder in primary school children. S. Afr. J. Psychiatry 2019, 25, 1232. [Google Scholar] [CrossRef]
- Barth, B.; Mayer, K.; Strehl, U.; Fallgatter, A.J.; Ehlis, A.C. EMG biofeedback training in adult attention-deficit/hyperactivity disorder: An active (control) training? Behav. Brain Res. 2017, 329, 58–66. [Google Scholar] [CrossRef]
- Arns, M.; Conners, C.K.; Kraemer, H.C. A decade of EEG Theta/Beta Ratio Research in ADHD: A meta-analysis. J. Atten. Disord. 2013, 17, 374–383. [Google Scholar] [CrossRef]
- Casson, A.; Yates, D.; Smith, S.; Duncan, J.; Rodriguez-Villegas, E. Wearable electroencephalography. What is it, why is it needed, and what does it entail? IEEE Eng. Med. Biol. Mag. 2010, 29, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Gajendiran, S.; Sivagnanam, Y. Acquisition and Detection of Eye Movement Using Electrooculography. In Proceedings of the 2024 International Conference on Signal Processing and Advance Research in Computing (SPARC), Lucknow, India, 12–13 September 2024; pp. 1–5. Available online: https://ieeexplore.ieee.org/document/10829113 (accessed on 5 July 2025).
- Demirci, E.; Yasin Esas, M.; Altıntop, C.G.; Tastepe, N.; Latifoglu, F. Diagnostic Support System for the Detection of Attention Deficit Hyperactivity Disorder using EOG signals. J. Psychiatry Psychiatr. Disord. 2023, 7, 118–127. [Google Scholar] [CrossRef]
- Prabhu, S.G.; Das, J.; Moncy, L.; Lalith Kumar, P.S.; M, C. Real-Time Attention Monitoring Using Smart Wearable for ADHD Patients. In Proceedings of the 2023 International Conference on Data Science and Network Security (ICDSNS), Tiptur, India, 28–29 July 2023; pp. 1–5. Available online: https://ieeexplore.ieee.org/document/10245407 (accessed on 5 July 2025). [CrossRef]
- De Crescenzo, F.; Licchelli, S.; Ciabattini, M.; Menghini, D.; Armando, M.; Alfieri, P.; Mazzone, L.; Pontrelli, G.; Livadiotti, S.; Foti, F.; et al. The use of actigraphy in the monitoring of sleep and activity in ADHD: A meta-analysis. Sleep Med. Rev. 2016, 26, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Haist, F.; Jernigan, T.L. Adolescent Brain Cognitive Development Study (ABCD)—Annual Release 5.1. Available online: https://nda.nih.gov/study.html?id=2313 (accessed on 5 July 2025). [CrossRef]
- Kim, W.P.; Kim, H.J.; Pack, S.P.; Lim, J.H.; Cho, C.H.; Lee, H.J. Machine Learning-Based Prediction of Attention-Deficit/Hyperactivity Disorder and Sleep Problems With Wearable Data in Children. JAMA Netw. Open. 2023, 6, e233502. [Google Scholar] [CrossRef]
- Jiang, Z.; Chan, A.Y.L.; Lum, D.; Wong, K.H.T.W.; Leung, J.C.N.; Ip, P.; Coghill, D.; Wong, R.S.; Ngai, E.C.; Wong, I.C. Wearable Signals for Diagnosing Attention-Deficit/Hyperactivity Disorder in Adolescents: A Feasibility Study. JAACAP Open 2024. [Google Scholar] [CrossRef]
- Lindhiem, O.; Goel, M.; Shaaban, S.; Mak, K.J.; Chikersal, P.; Feldman, J.; Harris, J.L. Objective Measurement of Hyperactivity Using Mobile Sensing and Machine Learning: Pilot Study. JMIR Form. Res. 2022, 6, e35803. [Google Scholar] [CrossRef]
- Arakawa, R.; Ahuja, K.; Mak, K.; Thompson, G.; Shaaban, S.; Lindhiem, O.; Goel, M. LemurDx: Using Unconstrained Passive Sensing for an Objective Measurement of Hyperactivity in Children with no Parent Input. Proc. ACM Interact. Mobile Wearable Ubiquitous Technol. 2023, 7, 46. [Google Scholar] [CrossRef]
- Muñoz-Organero, M.; Powell, L.; Heller, B.; Harpin, V.; Parker, J. Using Recurrent Neural Networks to Compare Movement Patterns in ADHD and Normally Developing Children Based on Acceleration Signals from the Wrist and Ankle. Sensors 2019, 19, 2935. [Google Scholar] [CrossRef]
- Ayearst, L.E.; Brancaccio, R.M.; Weiss, M.D. Improving On-Task Behavior in Children and Youth with ADHD: Wearable Technology as a Possible Solution. J. Pediatr. Neuropsychol. 2023, 9, 175–182. [Google Scholar] [CrossRef]
- Chen, I.C.; Chang, C.L.; Chang, M.H.; Ko, L.W. The utility of wearable electroencephalography combined with behavioral measures to establish a practical multi-domain model for facilitating the diagnosis of young children with attention-deficit/hyperactivity disorder. J. Neurodev. Disord. 2024, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Sonne, T.; Obel, C.; Grønbæk, K. Designing Real Time Assistive Technologies: A Study of Children with ADHD. In Proceedings of the Annual Meeting of the Australian Special Interest Group for Computer Human Interaction; Association for Computing Machinery: New York, NY, USA, 2015; pp. 34–38. [Google Scholar] [CrossRef]
- Whitehead, J.C.; Neeman, R.; Doniger, G.M. Preliminary Real-World Evidence Supporting the Efficacy of a Remote Neurofeedback System in Improving Mental Health: Retrospective Single-Group Pretest-Posttest Study. JMIR Form. Res. 2022, 6, e35636. [Google Scholar] [CrossRef]
- Huang, I.W.; Jheng, Y.C.; Chen, I.C.; Ko, L.W. Complexity Analysis based on Parietal Fuzzy Entropy to Facilitate ADHD Diagnosis in Young Children. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, FL, USA, 15–19 July 2024; Volume 2024, pp. 1–6. [Google Scholar] [CrossRef]
- Lin, J.W.; Fan, Z.C.; Tzou, S.C.; Wang, L.J.; Ko, L.W. Temporal Alpha Dissimilarity of ADHD Brain Network in Comparison With CPT and CATA. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 1333–1343. [Google Scholar] [CrossRef]
- Chen, I.C.; Chang, C.H.; Chang, Y.; Lin, D.S.; Lin, C.H.; Ko, L.W. Neural Dynamics for Facilitating ADHD Diagnosis in Preschoolers: Central and Parietal Delta Synchronization in the Kiddie Continuous Performance Test. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Santarrosa-López, I.; Alor-Hernández, G.; Bustos-López, M.; Hernández-Capistrán, J.; Sánchez-Morales, L.N.; Sánchez-Cervantes, J.L.; Marín-Vega, H. DETEC-ADHD: A Data-Driven Web App for Early ADHD Detection Using Machine Learning and Electroencephalography. Big Data Cogn. Comput. 2024, 9, 3. [Google Scholar] [CrossRef]
- Dibia, V. FOQUS: A Smartwatch Application for Individuals with ADHD and Mental Health Challenges. In Proceedings of the 18th International ACM SIGACCESS Conference on Computers and Accessibility; Association for Computing Machinery: New York, NY, USA, 2016; pp. 311–312. [Google Scholar] [CrossRef]
- Ayearst, L.E.; Brancaccio, R.; Weiss, M.D. An Open-Label Study of a Wearable Device Targeting ADHD, Executive Function, and Academic Performance. Brain Sci. 2023, 13, 1728. [Google Scholar] [CrossRef]
- Revibe Technologies. A Randomized Controlled Intervention Study to Assess a Wearable Digital Therapy for Youth with ADHD [Internet]. clinicaltrials.gov; Report No.: NCT05710965. 2023. Available online: https://clinicaltrials.gov/study/NCT05710965 (accessed on 5 July 2025).
- Garcia, J.J.; de Bruyckere, H.; Keyson, D.V.; Romero, N. Designing Personal Informatics for Self-reflection and Self-awareness: The Case of Children with Attention Deficit Hyperactivity Disorder. In Ambient Intelligence; Augusto, J.C., Wichert, R., Collier, R., Keyson, D., Salah, A.A., Tan, A.H., Eds.; Springer International Publishing: Cham, Switzerland, 2013; pp. 109–123. [Google Scholar] [CrossRef]
- Bartlett, G.; Frings, D.; Chaplin, E. Evaluating Doppel’s impact on Anxiety and Focus amongst adults with ADHD. PLoS Digit. Health 2024, 3, e0000555. [Google Scholar] [CrossRef]
- McGough, J.J.; Sturm, A.; Cowen, J.; Tung, K.; Salgari, G.C.; Leuchter, A.F.; Cook, I.A.; Sugar, C.A.; Loo, S.K. Double-Blind, Sham-Controlled, Pilot Study of Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 403–411.e3. [Google Scholar] [CrossRef]
- Arpaia, P.; Duraccio, L.; Moccaldi, N.; Rossi, S. Wearable Brain–Computer Interface Instrumentation for Robot-Based Rehabilitation by Augmented Reality. IEEE Trans. Instrum. Meas. 2020, 69, 6362–6371. [Google Scholar] [CrossRef]
- Arpaia, P.; Criscuolo, S.; De Benedetto, E.; Donato, N.; Duraccio, L. A Wearable AR-based BCI for Robot Control in ADHD Treatment: Preliminary Evaluation of Adherence to Therapy. In Proceedings of the 2021 15th International Conference on Advanced Technologies, Systems and Services in Telecommunications (℡SIKS), Niš, Serbia, 20–22 October 2021; pp. 321–324. Available online: https://ieeexplore.ieee.org/document/9606352 (accessed on 5 July 2025).
- Arpaia, P.; Criscuolo, S.; Benedetto, E.D.; Donato, N.; Duraccio, L. Evaluation of the Effectiveness of a Wearable, AR-based BCI for Robot Control in ADHD Treatment. In Proceedings of the 2022 IEEE International Conference on Metrology for Extended Reality, Artificial Intelligence and Neural Engineering (MetroXRAINE), Rome, Italy, 26–28 October 2022; pp. 630–634. Available online: https://ieeexplore.ieee.org/document/9967655 (accessed on 5 July 2025).
- McDermott, A.F.; Rose, M.; Norris, T.; Gordon, E. A Novel Feed-Forward Modeling System Leads to Sustained Improvements in Attention and Academic Performance. J. Atten. Disord. 2020, 24, 1443–1456. [Google Scholar] [CrossRef]
- Innosphere. tRNS Treatment for ADHD Symptoms [Internet]. clinicaltrials.gov; Report No.: NCT06189703. 2025. Available online: https://clinicaltrials.gov/study/NCT06189703 (accessed on 5 July 2025).
- Richter, Y.; Gordon, C.; Vainstein, G.; Bublil-Mor, C.; Geisinger, D.; Meital-Kfir, N.; Elyoseph, Z. A novel intervention for treating adults with ADHD using peripheral visual stimulation. Front. Psychiatry. 2023, 14, 1280440. [Google Scholar] [CrossRef]
- Stinson, L.; Liu, Y.; Dallery, J. Ecological Momentary Assessment: A Systematic Review of Validity Research. Perspect. Behav. Sci. 2022, 45, 469–493. [Google Scholar] [CrossRef] [PubMed]
- Lederer, L.; Breton, A.; Jeong, H.; Master, H.; Roghanizad, A.R.; Dunn, J. The Importance of Data Quality Control in Using Fitbit Device Data From the All of Us Research Program. JMIR mHealth uHealth 2023, 11, e45103. [Google Scholar] [CrossRef] [PubMed]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef] [PubMed]
- Shafi, I.; Din, S.; Farooq, S.; Díez I de la, T.; Breñosa, J.; Espinosa, J.C.M.; Ashraf, I.; Islam, M. Design and development of patient health tracking, monitoring and big data storage using Internet of Things and real time cloud computing. PLoS ONE 2024, 19, e0298582. [Google Scholar] [CrossRef]
- Rahman, M.M. Enhancing ADHD Prediction in Adolescents through Fitbit-Derived Wearable Data. medRxiv 2024. [Google Scholar] [CrossRef]
- Hyun, A.; Takashima, M.; Hall, S.; Lee, L.; Dufficy, M.; Ruppel, H.; Ullman, A. Wearable biosensors for pediatric hospitals: A scoping review. Pediatr. Res. 2024, 98, 90–99. [Google Scholar] [CrossRef]
- Herrera, N.; Cibrian, F.L.; Silva, L.M.; Beltran, J.A.; Schuck, S.E.B.; Hayes, G.R.; Lakes, K.D. Digital health intervention for children with ADHD to improve mental health intervention, patient experiences, and outcomes: A study protocol. BMC Digit. Health 2024, 2, 78. [Google Scholar] [CrossRef]
- Tran, B. Wearable Health Tech Costs: What Consumers Pay. PatentPC Blog, 6 March 2025. Available online: https://patentpc.com/blog/wearable-health-tech-costs-what-consumers-pay (accessed on 5 July 2025).
- Kam, H.J.; Shin, Y.M.; Cho, S.M.; Kim, S.Y.; Kim, K.W.; Park, R.W. Development of a Decision Support Model for Screening Attention-deficit Hyperactivity Disorder with Actigraph-based Measurements of Classroom Activity. Appl. Clin. Inform. 2010, 1, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Center for Devices and Radiological Health; Center for Biologics Evaluation and Research. Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program. FDA. 2025. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/requests-feedback-and-meetings-medical-device-submissions-q-submission-program (accessed on 21 August 2025).
- ISO 13485:2016; Medical devices—Quality management systems—Requirements for regulatory purposes. International Organization for Standardization: Geneva, Switzerland, 2016.
- Regulation—2017/745—EN—Medical Device Regulation—EUR-Lex [Internet]. Available online: https://eur-lex.europa.eu/eli/reg/2017/745/oj/eng (accessed on 24 August 2025).
- ISO 14971:2019; Medical devices—Application of risk management to medical devices. International Organization for Standardization: Geneva, Switzerland, 2019.
- IEC 62304:2006+A1:2015; Medical Device Software—Software life cycle processes. International Electrotechnical Commission: Geneva, Switzerland, 2015.
- IEC 62366-1:2015+A1:2020; Medical Devices—Part 1: Application of Usability Engineering to Medical Devices. International Electrotechnical Commission: Geneva, Switzerland, 2020.
- Medical Device Coordination Group. MDCG 2019-11: Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745—MDR and Regulation (EU) 2017/746—IVDR. October 2019. Available online: https://ec.europa.eu/growth/sectors/medical-devices/new-regulations/guidance_en (accessed on 3 September 2025).
- Billing for Remote Patient Monitoring | Telehealth.HHS.gov [Internet]. Available online: https://telehealth.hhs.gov/providers/best-practice-guides/telehealth-and-remote-patient-monitoring/billing-remote-patient (accessed on 25 August 2025).
- Federal Register [Internet]. Medicare Program; Transitional Coverage for Emerging Technologies. 2024. Available online: https://www.federalregister.gov/documents/2024/08/12/2024-17603/medicare-program-transitional-coverage-for-emerging-technologies (accessed on 27 August 2025).
- Mäder, M.; Timpel, P.; Schönfelder, T.; Militzer-Horstmann, C.; Scheibe, S.; Heinrich, R.; Häckl, D. Evidence requirements of permanently listed digital health applications (DiGA) and their implementation in the German DiGA directory: An analysis. BMC Health Serv. Res. 2023, 23, 369. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, R.; Srivastava, D.; Kyriopoulos, I.; Monti, G.; Novillo-Ortiz, D.; Milman, R.; Zhang-Czabanowski, W.W.; Nasi, G.; Stern, A.D.; Wharton, G.; et al. Digital Health Reimbursement Strategies of 8 European Countries and Israel: Scoping Review and Policy Mapping. JMIR mHealth uHealth 2023, 11, e49003. [Google Scholar] [CrossRef]
- Rajkomar, A.; Hardt, M.; Howell, M.D.; Corrado, G.; Chin, M.H. Ensuring Fairness in Machine Learning to Advance Health Equity. Ann. Intern. Med. 2018, 169, 866–872. [Google Scholar] [CrossRef]
- Suresh, H.; Guttag, J. A Framework for Understanding Sources of Harm throughout the Machine Learning Life Cycle. In Proceedings of the 1st ACM Conference on Equity and Access in Algorithms, Mechanisms, and Optimization; Association for Computing Machinery: New York, NY, USA, 2021; pp. 1–9. [Google Scholar] [CrossRef]
- Sifaoui, A.; Eastin, M.S. “Whispers from the Wrist”: Wearable Health Monitoring Devices and Privacy Regulations in the U.S.: The Loopholes, the Challenges, and the Opportunities. Cryptography 2024, 8, 26. [Google Scholar] [CrossRef]
- Katz, A.L.; Webb, S.A.; Committee on Bioethics; Macauley, R.C.; Mercurio, M.R.; Moon, M.R.; Okun, A.L.; Opel, D.J.; Statter, M.B. Informed Consent in Decision-Making in Pediatric Practice. Pediatrics 2016, 138, e20161485. [Google Scholar] [CrossRef] [PubMed]
- Regulation—2016/679—EN—Gdpr—EUR-Lex [Internet]. Available online: https://eur-lex.europa.eu/eli/reg/2016/679/oj/eng (accessed on 28 August 2025).
- 16 CFR Part 312—Children’s Online Privacy Protection Rule (Coppa Rule) [Internet]. Available online: https://www.ecfr.gov/current/title-16/part-312 (accessed on 28 August 2025).
- ISO/IEC 27701:2019; Security Techniques—Extension to ISO/IEC 27001 and ISO/IEC 27002 for privacy information management—Requirements and guidelines. International Organization for Standardization: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/71670.html (accessed on 28 August 2025).
- National Institute of Standards and Technology. NIST Privacy Framework: A Tool for Improving Privacy Through Enterprise Risk Management, Version 1.0; Report No.: NIST CSWP 01162020; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020. Available online: https://nvlpubs.nist.gov/nistpubs/CSWP/NIST.CSWP.01162020.pdf (accessed on 28 August 2025).
- Age Appropriate Design: A Code of Practice for Online Services [Internet]. ICO. 2025. Available online: https://ico.org.uk/for-organisations/uk-gdpr-guidance-and-resources/childrens-information/childrens-code-guidance-and-resources/age-appropriate-design-a-code-of-practice-for-online-services/ (accessed on 28 August 2025).
- Privacy Data Ethics of Wearable Digital Health Technology | Center for Digital Health | Engineering | Brown University [Internet]. Available online: https://cdh.brown.edu/news/2023-05-04/ethics-wearables (accessed on 5 July 2025).
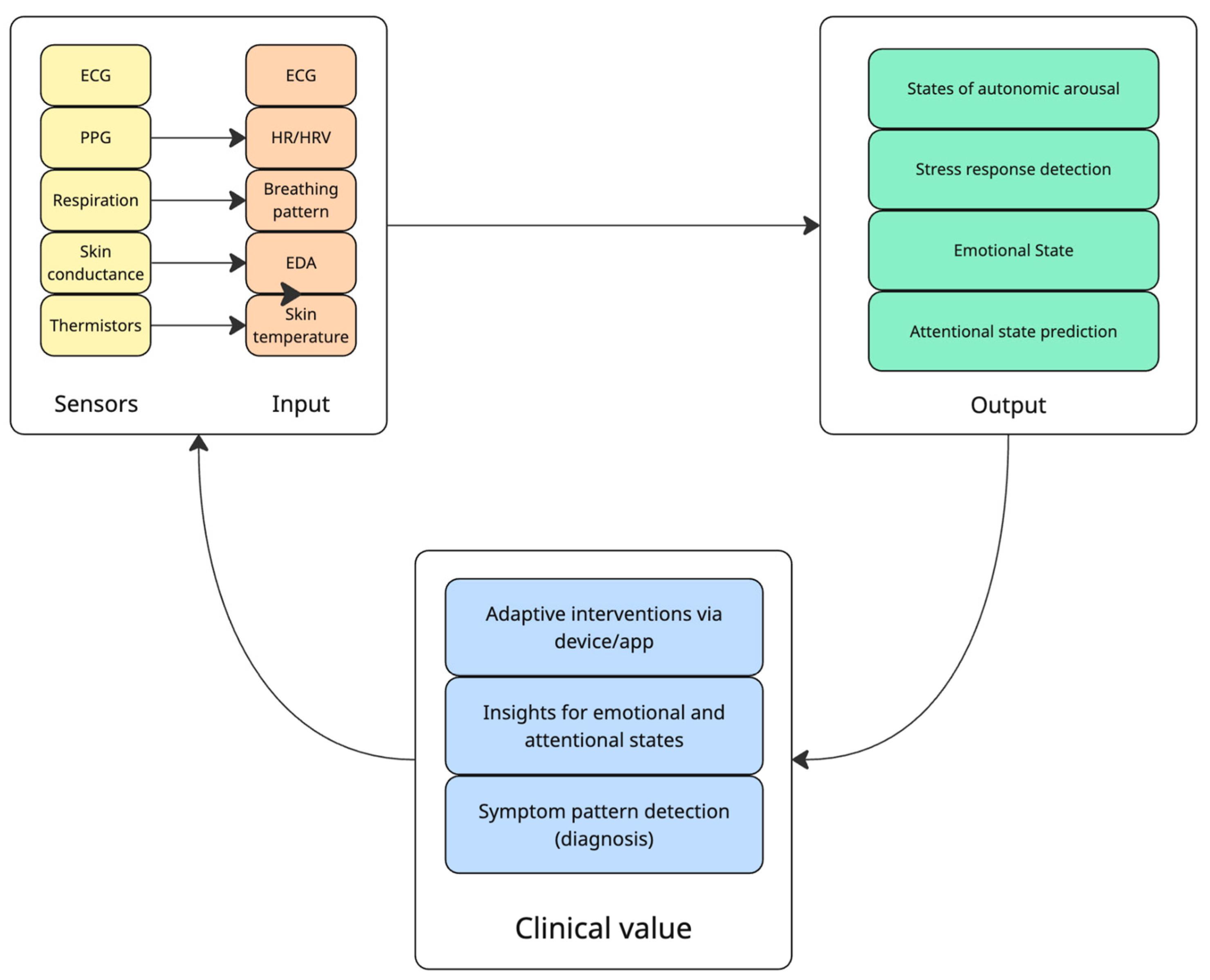
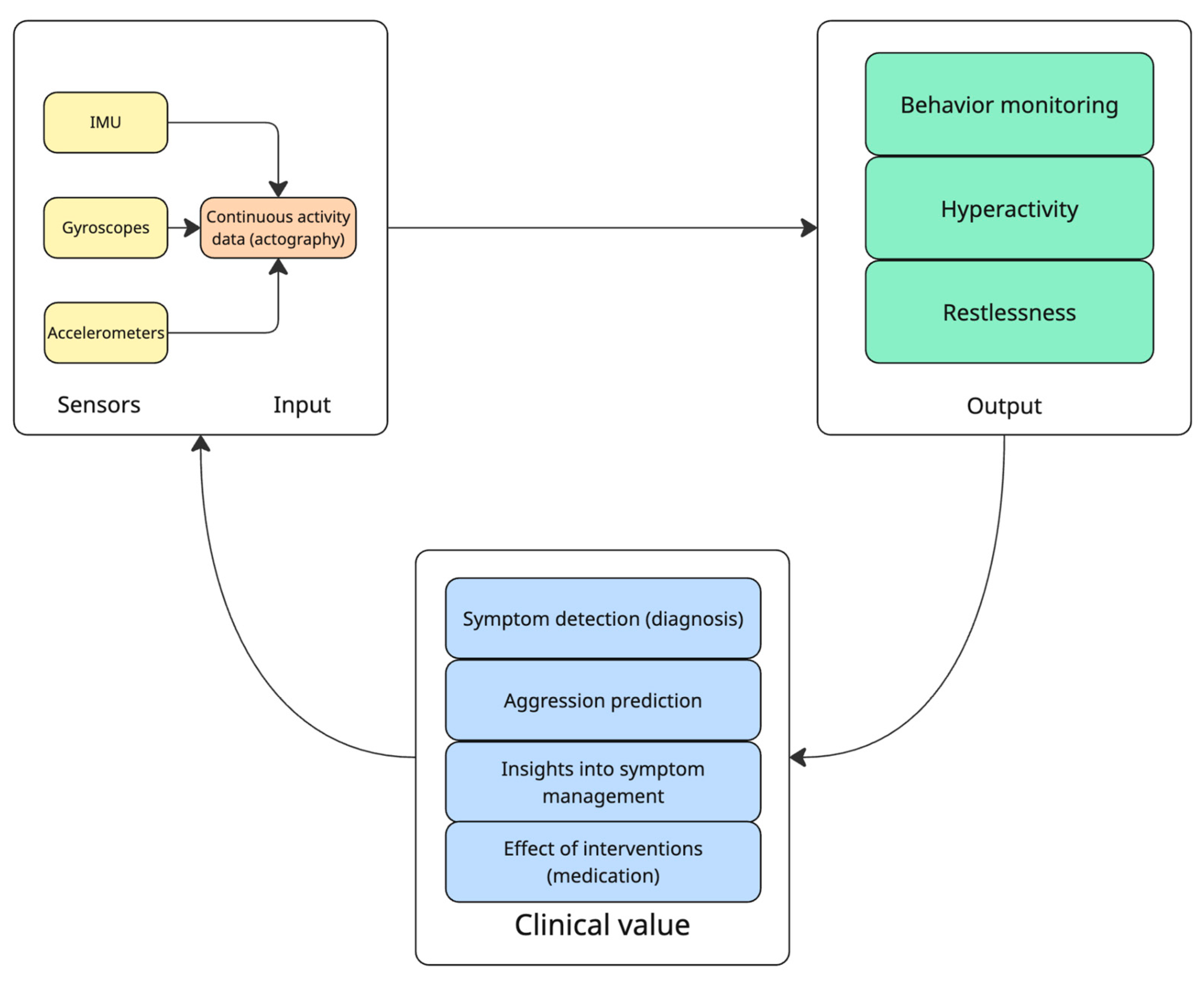
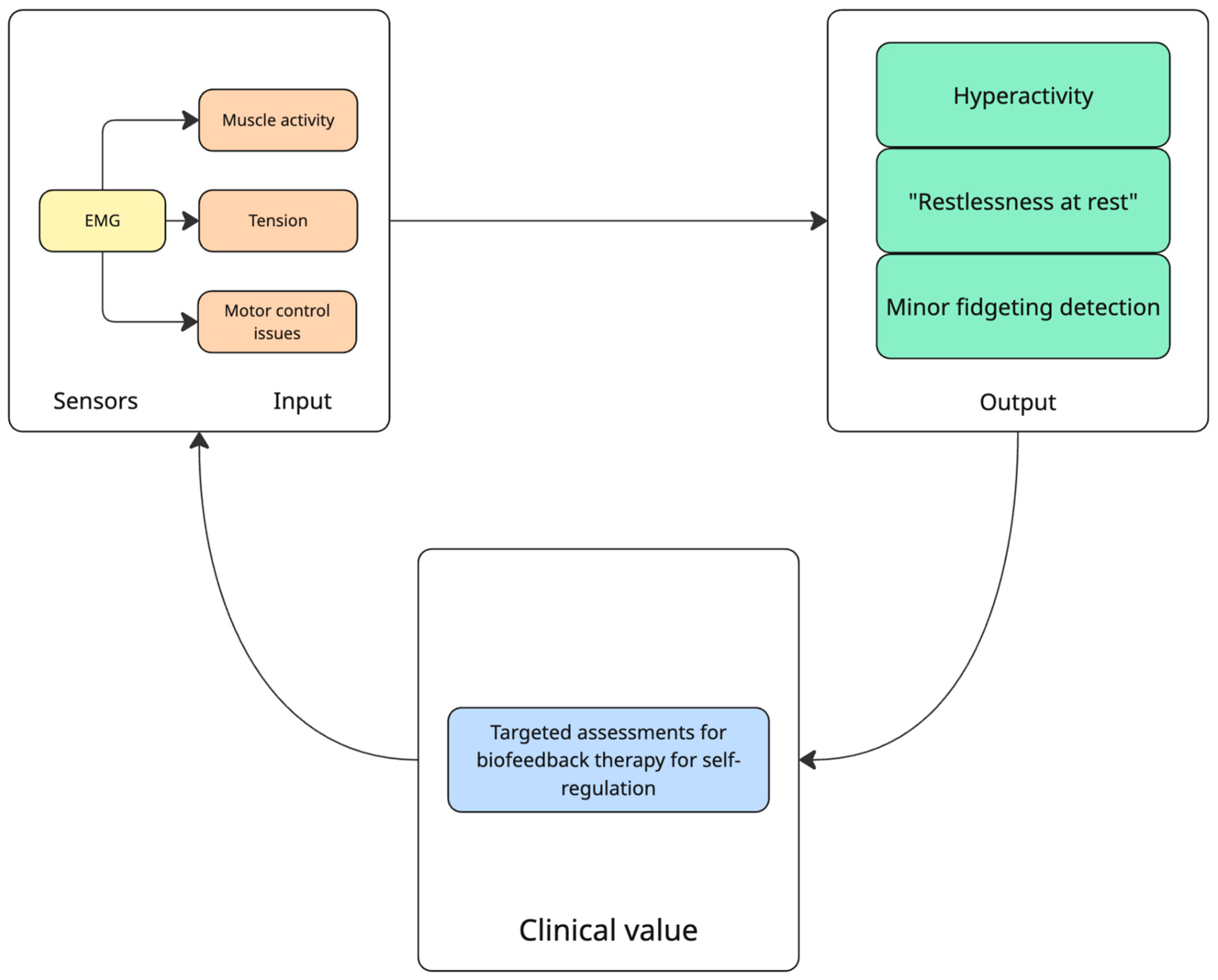
| Clinical Target | Study | Objective | Sample | Study Design | Device/Sensors Involved | Results |
|---|---|---|---|---|---|---|
| Hyperactivity | Kim WP et al., 2023 [43] | ML prediction of ADHD/sleep problems from wearable data. | ABCD cohort subset; ADHD/controls: 79/1011; Sleep/controls: 68/3346. | Case-control ML classification (RF/XGB/LGBM); internal validation. | Fitbit (Google LLC) PPG HR/HRV, 3-axis accelerometer (activity/sleep) | ADHD: AUC 0.80; sens 0.76; spec 0.72; NPV 0.98. Sleep: AUC 0.74; sens 0.74; spec 0.63; NPV 0.99; heart rate strongest predictor |
| Rahman MM et al. 2025 [10] | Fitbit-derived measures to predict adolescent ADHD via ML | ABCD cohort (release 5.0); N = 450 adolescents | Cross-sectional secondary analysis; logistic regression + ML classification with internal cross-validation (CV) and held-out test | Fitbit (Google LLC) PPG HR (resting HR), 3-axis accelerometer (activity/sedentary; energy expenditure derived) | RF (CV): AUC 0.95; acc 0.89; precision 0.88; recall 0.90; F1 0.89; held-out test acc 0.88; Fitbit metrics showed significant associations with ADHD in regression | |
| Jiang Z et al., 2024 [44] | Feasibility ML classification of ADHD and medication status from wearable actigraphy/HR in adolescents | ADHD/controls: 17/13; ages 16–17 (N = 30) | Longitudinal pilot; case–control ML (XGBoost) with internal validation | Fitbit (Google LLC) PPG HR; 3-axis accelerometer; actigraphy-derived sleep | ADHD (objective only): AUC 0.844; objective + subjective: AUC 0.933; medication-status classification: AUC 1.00. Key predictors: HR (resting/mean) & very active minutes (medication status); irritability/sex/QoL (ADHD) | |
| Lindhiem O et al., 2022 [45] | Objective measurement of hyperactivity in children using a smartwatch + ML (LemurDx) | N = 30 (ADHD-H/I or combined/controls: 15/15), ages 6–11; 2 days wear | Pilot observational case–control; supervised ML classification; usability assessed | Apple Watch (LemurDx app) 3-axis accelerometer (primary signal); contextual: heart rate, GPS, Bluetooth; App with parent input | Diagnostic accuracy 0.89; sensitivity 0.93; specificity 0.86 (with motion features + parent activity labels) | |
| Arakawa R et al., 2023 [46] | Objective hyperactivity measurement from smartwatch sensing (LemurDx) | Children 5–12 y; ADHD/controls: 25/36; N = 61; wear 2–7 days | Observational case–control ML classification; context filtering vs. none; leave-one-participant-out cross-validation (CV) for evaluation; 5-fold CV for hyperparameter tuning | Apple Watch (LemurDx app) 3-axis accelerometer (primary); HR (PPG), GPS, Bluetooth recorded for context (not used in final ML) | With parent-provided context filtering: AUC 0.85; acc 85.2%; F1 0.816. Without context: AUC 0.70; acc 67.2%; F1 0.630. Automated context (no parent input): acc 82.0%; F1 0.784. Threshold 0.505 → TPR 0.80, FPR 0.11. Slight correlation of risk score with VADPRS | |
| Muñoz-Organero M et al., 2019 [47] | Comparison (RNN-based) of movement patterns in ADHD vs. typically developing children from wrist/ankle accelerometry; medicated vs. non-medicated contrasts | N = 36; ADHD/controls: 18 (9 medicated, 9 non-medicated)/18; ages 6–16 | Observational case–control; 24-h wear; RNN trained on 9 controls, evaluated on remaining 9 controls | Runscribe inertial sensors (Scribe Labs, CA, USA) 3i-axial accelerometers (wrists, ankles) | Non-medicated ADHD > “non-similar” fragments vs. controls: d ≈ 0.80 (estimated). Medicated vs. controls: d ≈ 0.50 (estimated) | |
| Aggression/ agitation | Park C et al., 2023 [11] | ML detection of aggression episodes from waist-worn actigraphy in children with/without ADHD | N = 39; ages 7–16; repeated 1-week wear (3 times/12 months) | Observational monitoring; parent episode logs as labels; Random Forest model; internal validation | ActiGraph GT3X+ (ActiGraph Corp.)—triaxial accelerometer (waist) | AUC 0.893; accuracy 0.820; recall 0.850; precision 0.802; F1 0.824. Vector-magnitude acceleration higher during aggression vs. non-aggression (means 1580.7 ± 1831.1 vs. 873.3 ± 1137.2; approx. Cohen’s d ≈ 0.46, epoch-level; p = 0.027) |
| Attention/alertness/arousal | Chen IC et al., 2024 [49] | Multimodal ADHD detection in preschoolers using wearable EEG + behavioral measures | Preschoolers; ADHD/controls: 43/35 (N = 78) | Case–control ML/DL classification (Decision Tree/Random Forest/bi-LSTM); 5-fold internal validation; ensemble model | Wearable wireless EEG (Mindo BR8; 8-ch) | Ensemble accuracy 0.974 Sensitivity 92.3%, specificity 90.0% Effect sizes: K-CPT-2 HRT SD (ADHD 52.05 ± 8.45 vs. TD 47.94 ± 6.49) Cohen’s d ≈ 0.54; HRT ISI change (52.44 ± 8.89 vs. 47.69 ± 6.82) Cohen’s d ≈ 0.59 |
| Huang IW et al., 2024 [52] | Assess EEG complexity (parietal fuzzy entropy) to aid ADHD diagnosis | Children 4–7 y; ADHD/controls: 30/30 | 8-ch dry-EEG headband | 8-channel wireless wearable EEG | Best feature set (right occipital beta PSD + parietal FuEn) achieved accuracy = 0.90; | |
| Lin JW et al., 2024 [53] | Characterize EEG functional-connectivity patterns—focusing on temporal alpha dissimilarity/coherence for potential diagnosis marker | N-72; Ages 8–16 y; ADHD/controls: 53/19 | Case–control, task-evoked EEG study (visual CPT and auditory CATA tasks) | EEG sensors 16-ch EEG cap | Temporal-lobe FC in alpha during CATA was higher in TD vs. ADHD (p < 0.05). | |
| Santarrosa-López I et al., 2025 [55] | Develop and validate DETEC-ADHD, a web-based application that integrates machine learning with personal, clinical, psychological and EEG data to detect ADHD and its subtypes in real time. | N = 19 (Children n = 10; Adults n = 9; mixed ADHD and non-ADHD) | Proof-of-concept case study; Logistic Regression model | Webapp + Muse S headband (InteraXon)—EEG (dry electrodes) | Logistic Regression: accuracy = 90%; AUC = 0.92; case-study detection rates—children: 100%; adults: 90%. | |
| Autonomic arousal | Andrikopoulos D et al., 2024 [28] | ML detection of adult ADHD from multimodal wearable signals during Stroop tasks | Adults; ADHD/controls: 32/44 (N = 76) | Case–control ML classification (LR/KNN/RF/SVM); internal cross-validation; i-KNN filtering, data collected during Stroop tests | Feel Monitoring Device + app (Feel Therapeutics)—EDA, PPG HR/HRV, skin temperature (9-axis IMU present; not modeled). | SVM (multimodal): accuracy 0.816; sensitivity 0.814; specificity 0.819. Unimodal models lower/less balanced |
| Sleep/ circadian rhythm | Denyer H et al. 2025 [13] | Remote 10-week monitoring of sleep in ADHD vs. controls; test group differences in mean vs. night-to-night variability and links with anxiety/depression. | N = 40 (ADHD/controls: 20/20), ages 16–39; 2428 nights total (median nights: ADHD 62; controls 68). | Observational non-interventional cohort; linear mixed models for mean sleep features | Fitbit Charge 3 (Fitbit/Google LLC)—3-axis accelerometer (sleep duration, onset, offset, efficiency) | ADHD showed greater night-to-night variability: SD duration 1:33 vs. 1:10; SD onset 2:02 vs. 1:43; SD offset 1:50 vs. 1:37; SD efficiency 4.23 vs. 3.67 (all p < 0.001); within-person anxiety/depression associations were non-significant |
| Monitoring medication | Ouyang CS et al., 2020 [12] | Objective evaluation of methylphenidate effects via smartwatch accelerometry | N = 10 children with ADHD (9M/1F); mean age ≈ 7.4 ± 1.3 y | Pre–post within-subject (baseline vs. 1-month methylphenidate 10 mg/day, weekdays); paired t-tests (Bonferroni α = 0.0167); correlation with SNAP-IV (teacher) | Garmin Vivosmart 3-axis accelerometer, HRV | Variance decreased after treatment: Y-axis 4.42 ± 2.17 → 2.32 ± 0.65 (p = 0.0119); Z-axis 4.09 ± 1.57 → 2.41 ± 0.81 (p = 0.0140). SNAP hyperactivity reduction correlated with Y-axis variance reduction (r = 0.605); other subscales weak/non-significant |
| Clinical Target | Study | Objective | Population | Study Design | Device/Sensors Involved | Key Findings |
|---|---|---|---|---|---|---|
| Multidomain clinical targets: attention, hyperactivity, impulsivity, executive function, behavior | Ayearst LE et al., 2023 [48] | Wearable digital intervention to improve on-task behavior—specifically attention, hyperactivity/impulsivity, executive function, and academic performance | ADHD, 8–12 y; N = 38 (parent raters N = 38; teacher raters N = 26); 4-week school wear; unmedicated | Single-arm, open-label pre–post pilot (4-week wearable use) in unmedicated children with ADHD; baseline → post parent/teacher ratings; no randomization, blinding, or control | Revibe Connect (Revibe Technologies)—haptic prompts; tap-back self-reports; step logging, 3-axis accelerometer, gyroscope | Parent ADHD-RS-5 inattention d = 1.07; hyperactivity/impulsivity d = 0.70. Teacher ADHD-RS-5 inattention d = 0.54. WFIRS-P school learning r ≈ 0.58 (large). APRS academic productivity d = 0.59 (moderate) |
| Garcia JJ et al., 2013 [59] | Design-driven personal informatics (KITA/WRISTWIT) to support self-awareness and on-task behavior in ADHD | Children (KITA: 4–7 yrs N = 2, WRISTWIT: 8–12 yrs N = 5) Context informants N = 15 | Empirical Research Through Design; iterative prototyping; in-situ school testing; exploratory sensing—no control group | KITA: waist-worn toy + “nest” (3-axis accelerometer; vibration motor; 31 LEDs; IR link; microcontroller/speaker in nest). WRISTWIT: bracelet (3-axis accelerometer; 12-LED time display) | KITA pilot: ~16% reduction in in-class activity vs. baseline; high engagement reported. WRISTWIT concept: accelerometry distinguished on-/off-task states; supports time awareness. | |
| Sonne T et al., 2015 [50] | Design and preliminary evaluation of CASTT—a real-time assistive wearable to help children with ADHD regain attention in school | Children 2nd-5tth grade (n = 20, ADHD/controls: 11/9) | Non-randomized, uncontrolled observational feasibility pilot study | CASTT (Child Activity Sensing and Training Tool) custom wearable + smartphone system: Heart rate monitor, accelerometers (limbs), EEG | CASTT was wearable in class and captured physical activity continuously in real time; preliminary evidence indicated practical feasibility in authentic school contexts. | |
| Santamaría-Vázquez E. et al. 2025 [15] | Test whether combined respiratory biofeedback, neurofeedback and median nerve stimulation improve ADHD symptoms | N = 60; ADHD randomized active group(AG)/sham group(SG): 31/29; ages 8–18; | Exploratory randomized, double-blind, sham-controlled, two-arm parallel trial; 10 sessions over 2 weeks; pre/post/1-mo follow-up; resting-state EEG | Qey-DTx NMS (median nerve stimulation) stimulator (wrist electrodes); ProComp Infiniti with respiration belt (breathing sensor); EEG Neuroamp II | Within-group improvements in AG post-treatment and at 1-mo follow-up (Cohen’s d: post—hyperactivity index −0.45, anxiety −0.34, impulsivity-hyperactivity −0.40; follow-up—learning −0.62, hyperactivity index −0.50, impulsivity-hyperactivity −0.53) | |
| Attention, hyperactivity | Leikauf JE et al., 2021 [16] | Feasibility study of an Apple Watch app, tracking movement and delivering visual/haptic feedback to manage hyperactivity/attention in youth with ADHD | ADHD; N = 32; ages 8–17; 6-week follow-up | Open-label single-arm pilot; weekly ADHD-RS via parent report; linear mixed models for symptom trajectories; exit interviews (feasibility/acceptability) | Apple Watch Series 0 (Apple Inc., Cupertino, CA, USA) 3-axis accelerometer (actigraphy for movement); haptic motor (biofeedback) | ADHD-RS total β −1.2 units/week (95% CI −1.88 to −0.56; F = 13.4; p = 0.0004); Inattentive β −0.8/week (p = 7 × 10−5); Hyperactive/Impulsive β −0.4/week (p = 0.02); no adverse events; older age associated with greater improvement |
| Anxiety, arousal, emotional dysregulation | Dibia V, 2016 [56] | Smartwatch app (FOQUS) to support focus and reduce anxiety in adults with ADHD/attention difficulties | Survey n = 27 (ages 16–40) + 7-day usability study n = 10 (ages 21–30) | User-centred design; cognitive walkthrough + 7-day prototype usability test (no control) | Samsung Gear 2 (Samsung Electronics). PPG heart rate (pre/post meditation feedback); vibrotactile cues; positive-message priming | 80% reported reduced stress/anxiety after meditation; observed HR decreases pre→post |
| Whitehead JC et al., 2022 [51] | Remote EEG-neurofeedback efficacy for ADHD-related symptoms, cognition, and EEG markers | N = 593 (560 included), age > 13 Questionnaire pre–post n = 301; CPT pre–post n = 99 with known ADHD status (plus n = 104 unknown status); resting EEG baseline n = 271; pre–post EEG n = 41 | Retrospective single-group pretest–posttest; home/clinic use | Muse EEG headband (InteraXon) via Myndlift app—4 dry electrodes | (Cohen’s d): questionnaires—Large pre–post improvements: ADHD-RS-IV abnormal d = 2.41, GAD-7 abnormal d = 1.24, PHQ-9 abnormal d = 1.13, ASRS abnormal d = 1.05, GHQ-12 abnormal d = 0.99; CPT—response-time variability d = 1.02–1.24, average RT d = 0.56 (healthy), commission d = 0.55–0.62, omission d = 0.34–0.48; EEG—baseline DAR higher in abnormal ASRS (d = 0.37); pre-post DAR reduced in abnormal group (d = 0.70) |
| Clinical Target | Study | Objective | Sample | Study Design | Device/Sensors Involved | Key Findings |
|---|---|---|---|---|---|---|
| Overall ADHD symptom severity | McGough JJ et al., 2019 [61] | Non-invasive neuromodulation during sleep for symptom improvement in ADHD | Children 8–12 y; randomized: active/sham = 32/30 (N = 62) | Double-blind RCT; 4 weeks nightly eTNS + 1-week blinded discontinuation; weekly ADHD-RS & CGI; mechanistic qEEG | Monarch eTNS System (NeuroSigma): external stimulator with adhesive forehead patch electrodes | ADHD-RS: significant group × time (F(1, 228) = 8.12, p = 0.005); Cohen’s d = 0.50 at week 4. Clinical Global Impression-Improvement responders at week 4: 52% AG vs. 14% SG (NNT = 3). qEEG: increased frontal spectral power with active eTNS; partial r (EEG change ↔ ADHD-RS change) = −0.34 to −0.41 |
| McDermott AF et al., 2016 [65] | EEG feed-forward modeling (Atentiv/CogoLand) attention-training for pediatric ADHD; Neurofeedback training via EEG) | ADHD, 8–12 y; randomized: 46 (immediate FFM = 21; wait-list control = 19; total randomized = 46; 32M/14F) | Randomized parallel-group trial (8-week FFM vs. non-pharmacological community care), waitlist crossover; outcomes at post and 3-month follow-up | EEG headband with three frontal electrodes (Zeo Sleep Manager™) + PC game (CogoLand®®) | Clinician ADHD-RS: −36% vs. control; partial η2 (Group × Time) = 0.434. Parent ADHD-RS: −31%; partial η2 = 0.141. CGI: partial η2 (Group × Time) = 0.282. PERMP problems attempted: +26% (η2 > 0.150). Effects largely maintained at 3 months; Quotient® (Pearson Education, Inc., Westford, MA, USA) ADHD no improvement | |
| Attention, executive function | Richter Y et al., 2023 [67] | Evaluate efficacy of peripheral visual stimulation “Neuro-glasses” for adult ADHD | ADHD, 18–40 y; enrolled N = 108; per-protocol N = 97; wear ≥2 h/day | Open-label single-arm clinical trial; pre–post assessments (ASRS, BRIEF-A, CPT-3); CGI-I at endpoint | Neuro-glasses (Sparkles™, VIZO Specs Ltd., Tel Aviv, Israel)—standard lenses with semi-transparent peripheral stimuli; personalization with eye-tracking | ASRS-Inattention improved (p = 0.037), Cohen’s d = 0.22; BRIEF-A Metacognition improved (p = 0.029), d = 0.23; CPT-3 detectability d′ improved (p = 0.027), d = 0.23; CPT-3 commission errors reduced (p = 0.004), d = 0.30; 62% CGI-I responders |
| Clinical trial NCT06189703 [66] | Examine the safety and effectiveness of tRNS on unmedicated pediatric patients | Children (7–12 yrs) | Randomized, sham-controlled, double-blind clinical trial | Novostim 2—Transcranial random noise stimulation device | Subjects will undergo either tRNS or sham treatment for 10 days during a two-week period in a home-simulated environment. Each treatment session is 20 min, during which their attention will be maintained using a software game. | |
| Anxiety, focus | Bartlett G et al., 2024 [60] | Evaluate whether a wrist-worn haptic device (Doppel) reduces anxiety and improves focus in adults with ADHD over 8 weeks | Adults 18–25 y with self-reported ADHD; N = 49 at baseline; 4-week n = 37; 8-week n = 32 (active 14/comparator 18) | Double-blind randomized controlled trial; active HR-matched vibrations vs. fixed-pattern comparator; intention-to-treat | Doppel wristband + smartphone app; haptic actuator delivering heartbeat-like vibrations | No superiority of active vs. comparator at 4 or 8 wk (all p ≥ 0.31; partial η2 ≤ 0.03). Time effects across groups: anxiety ↓ (η2 = 0.10) and focus ↑ (η2 = 0.22) |
| Multidomain clinical targets: attention, hyperactivity, impulsivity, executive function, cognitive skills | Arpaia P et al., 2020 [62] | Wearable single-channel SSVEP BCI with AR glasses for robot-based rehabilitation in ADHD; evaluate accuracy/latency and feasibility | Algorithm tuning: N = 20 healthy adults; Robot-control test: N = 10 healthy adults; Clinical preliminary: N = 4 children with ADHD (6–8 y) | Instrumentation study + observational case study; training-less single-channel SSVEP with eye-blink detection; lab evaluation and rehab-center pilot | Epson Moverio BT-200 AR glasses (eye-blink detection); Olimex EEG-SMT (single-channel EEG); Sanbot Elf robot. | Accuracy–latency trade-off (e.g., 92.6% at ~3.71 s vs. 70.8% at ~0.64 s); clinical target setting selected ~1.5 s response time; case study average accuracy >83% with ITR up to 39 bits/min; preliminary ADHD tests reported positive acceptability/attentional engagement. |
| Arpaia P et al., 2021 [63] | Wearable AR-based single-channel EEG (SSVEP) BCI to control a social robot for ADHD therapy; preliminary adherence evaluation | Children 5–10 y; N = 18 (ADHD); plus adult benchmark N = 10 | Pilot case study (task-based robot control); descriptive outcomes on acceptance/adherence; no inferential testing. | Epson Moverio BT-200 AR glasses (eye-blink detection); Olimex EEG-SMT (single-channel EEG); Sanbot Elf robot. | Adherence: 18/18 accepted wearing; completion: all 8–10 y finished tasks; some 5–7 y had ergonomics/attention issues; prior adult test accuracy ≈83.5% for command detection | |
| Arpaia P et al., 2022 [64] | Evaluate a wearable EEG-based brain computer interface for rehabilitation/training ADHD therapy, assessing adherence and preliminary cognitive/attentional gains | Adherence/acceptability: N = 18 ADHD children; Therapy cohort: N = 7 ADHD children | Single-arm pilot (within-subject pre–post); task-based sessions (planning, path-following, inhibition) while controlling a social robot | Epson Moverio BT-200 AR glasses (eye-blink detection); Olimex EEG-SMT (single-channel EEG); Sanbot Elf robot. | High acceptability/adherence (18 screened). All 7 treated children showed improvement across BIA subtests after 1 month (e.g., higher semantic/phonological fluency, better visual-sequential and Span-4; fewer reading errors) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olinic, M.-S.; Stretea, R.; Cherecheș, C. Wearables in ADHD: Monitoring and Intervention—Where Are We Now? Diagnostics 2025, 15, 2359. https://doi.org/10.3390/diagnostics15182359
Olinic M-S, Stretea R, Cherecheș C. Wearables in ADHD: Monitoring and Intervention—Where Are We Now? Diagnostics. 2025; 15(18):2359. https://doi.org/10.3390/diagnostics15182359
Chicago/Turabian StyleOlinic, Mara-Simina, Roland Stretea, and Cristian Cherecheș. 2025. "Wearables in ADHD: Monitoring and Intervention—Where Are We Now?" Diagnostics 15, no. 18: 2359. https://doi.org/10.3390/diagnostics15182359
APA StyleOlinic, M.-S., Stretea, R., & Cherecheș, C. (2025). Wearables in ADHD: Monitoring and Intervention—Where Are We Now? Diagnostics, 15(18), 2359. https://doi.org/10.3390/diagnostics15182359





