AI-Augmented Quantitative MRI Predicts Spontaneous Intracranial Hypotension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Imaging Techniques
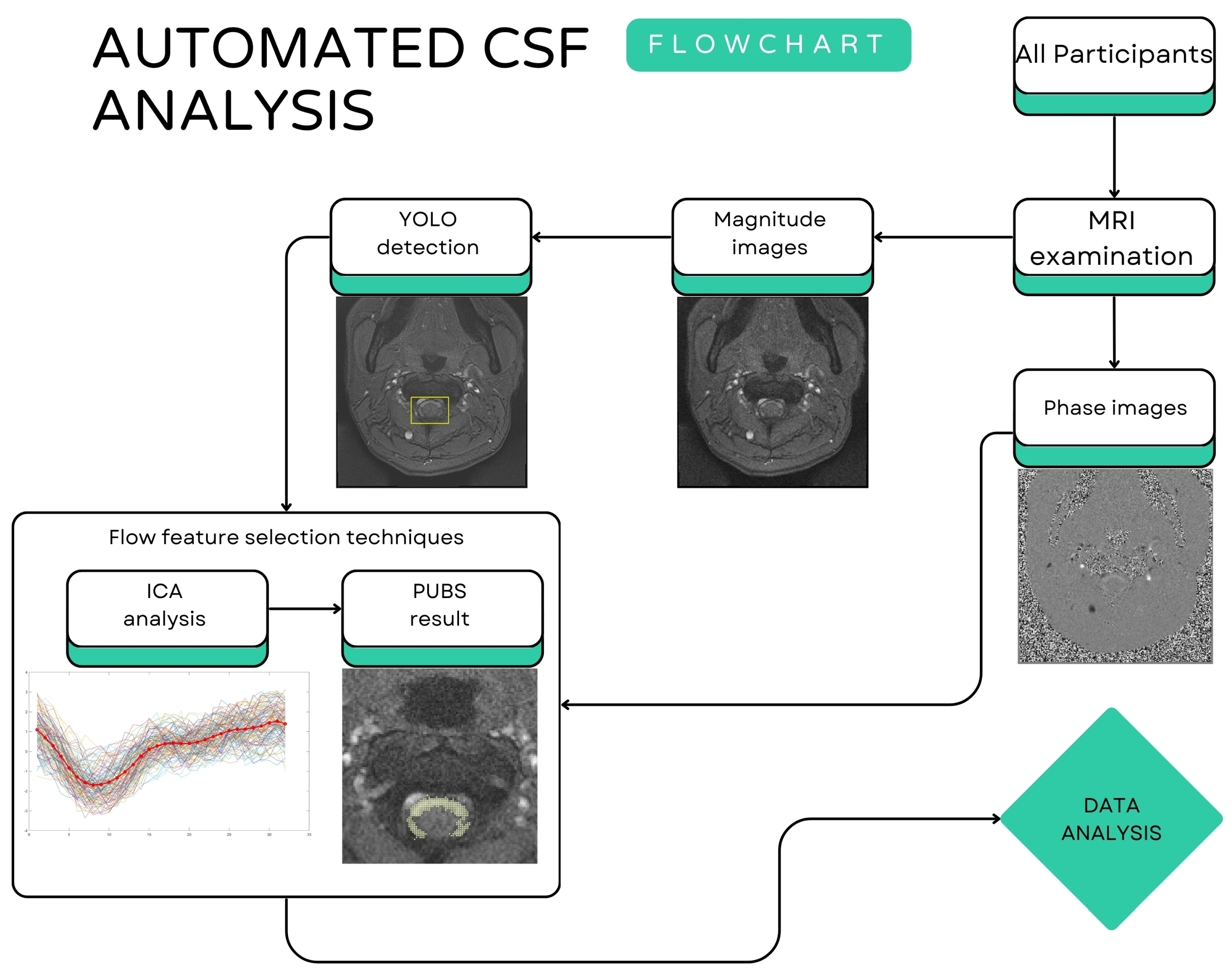
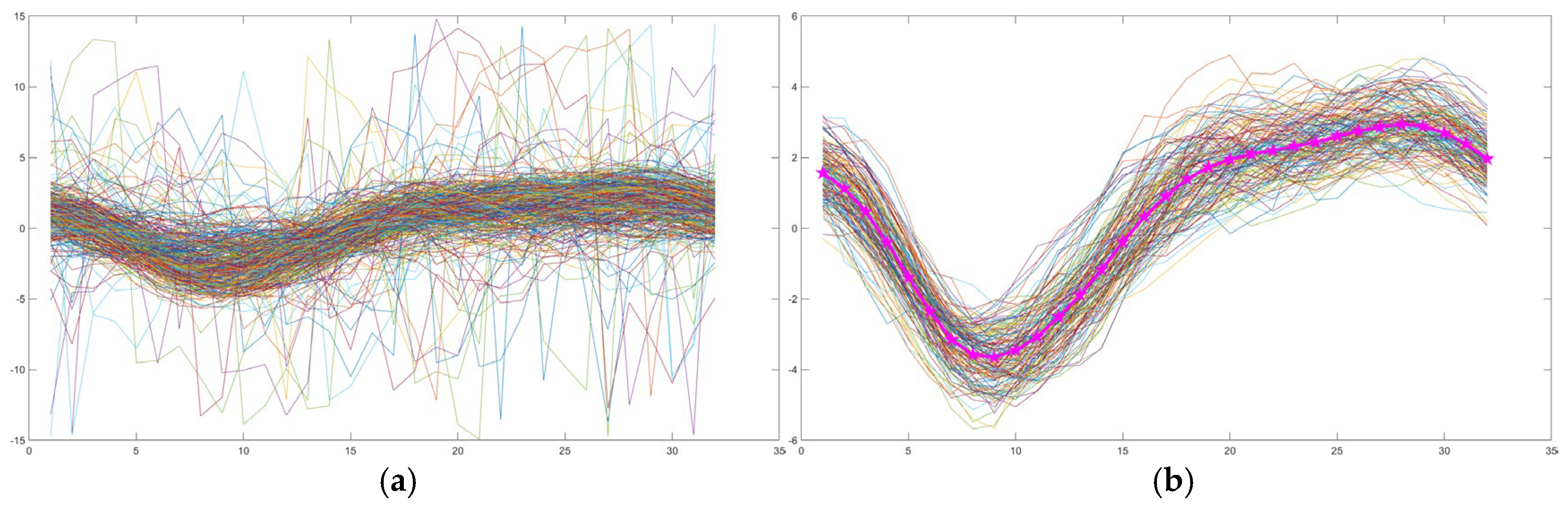
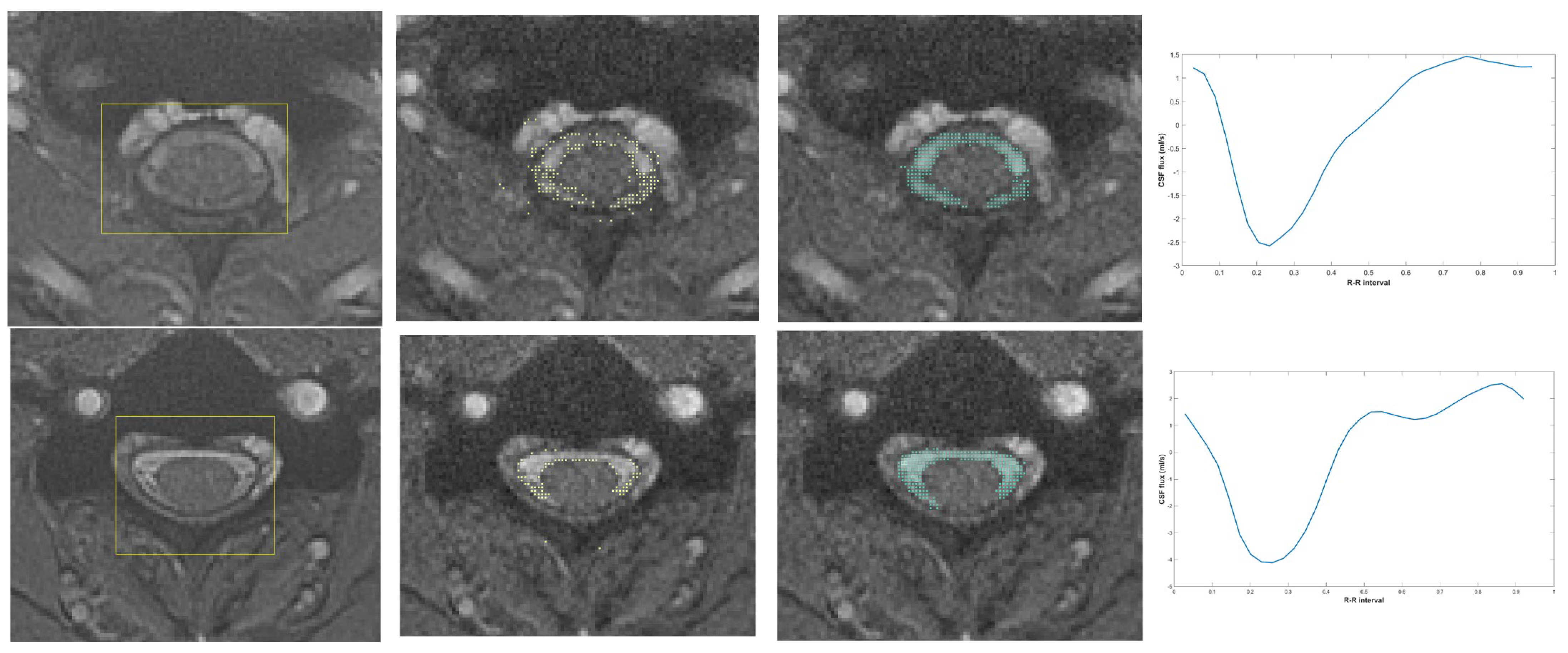
2.3. Artificial Intelligence-Based Flow Analysis
CSF Region Detection
2.4. Treatment and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics (Table 1)
| SIH | HVs | p | |
|---|---|---|---|
| Number of cases | 31 | 26 | |
| Sex (female/male) | 22/9 | 15/11 | 0.575 |
| Age (years old) (mean ± SD) | 39.58 ± 9.99 | 38.12 ± 6.82 | 0.724 |
| Days of Headache onset to the first MRI (range; median) | 2–60; 8 | Not applicable | |
| Epidural blood patch (EBP) | 25 | Not applicable | |
| Hydration only | 6 | Not applicable | |
| Times of EBP > 1 | 12 | Not applicable | |
| Days of the first MRI to the first EBP (range; median) | 1–7; 2 | Not applicable | |
| Days of the first EBP to the follow-up post-1st-EBP MRI (range; median) | 1–14; 2 | Not applicable |
3.2. Comparison of CSF Flow Parameters at Baseline MR Between SIH Patients and Healthy Volunteers (Table 2)
| Characteristic | Group | p Value | |
|---|---|---|---|
| SIH Patients (n = 31) | HVs (n = 26) | ||
| Mean ± SD | Mean ± SD | ||
| Upward mean flow (mL/s) | 0.76 ± 0.31 | 1.18 ± 0.34 | <0.001 ** |
| Downward mean flow (mL/s) | 1.01 ± 0.43 | 1.60 ± 0.54 | <0.001 ** |
| Summation of mean flow (mL/s) | 1.77 ± 0.72 | 2.78 ± 0.84 | <0.001 ** |
| Upward peak flow (mL/s) | 1.28 ± 0.50 | 1.87 ± 0.52 | <0.001 ** |
| Downward peak flow (mL/s) | 1.76 ± 0.77 | 2.96 ±0.92 | <0.001** |
| Summation of peak flow (mL/s) | 3.04 ± 1.26 | 4.83 ± 1.39 | <0.001 ** |
| Upward CSF total flow (mL/cycle) | 14.32 ± 5.59 | 22.24 ± 6.55 | <0.001 ** |
| Downward CSF total flow (mL/cycle) | 13.22 ± 5.65 | 20.74 ± 6.66 | <0.001 ** |
| Absolute stroke volume (mL/cycle) | 27.54 ± 11.03 | 42.98 ± 12.86 | <0.001 ** |
3.3. CSF Flow Comparison After Recovery (Table 3)
| Characteristic | Group | p Value | |
|---|---|---|---|
| Recovered SIH Patients (n = 24) | HVs (n = 26) | ||
| Mean ± SD | Mean ± SD | ||
| Upward mean flow (mL/s) | 1.29 ± 0.21 | 1.18 ± 0.34 | 0.193 |
| Downward mean flow (mL/s) | 1.84 ± 0.36 | 1.60 ± 0.54 | 0.068 |
| Summation of mean flow (mL/s) | 3.13 ± 0.52 | 2.78 ± 0.85 | 0.074 |
| Upward peak flow (mL/s) | 2.00 ± 0.32 | 1.87 ± 0.52 | 0.174 |
| Downward peak flow (mL/s) | 3.12 ± 0.52 | 2.96 ±0.92 | 0.294 |
| Summation of peak flow (mL/s) | 5.12 ± 0.78 | 4.83 ± 1.39 | 0.193 |
| Upward CSF total flow (mL/cycle) | 24.86 ± 4.86 | 22.24 ± 6.55 | 0.099 |
| Downward CSF total flow (mL/cycle) | 23.32 ± 3.56 | 20.74 ± 6.66 | 0.060 |
| Absolute stroke volume (mL/cycle) | 48.18 ± 7.22 | 42.98 ± 12.86 | 0.077 |
3.4. SIH Diagnostic Performance of CSF Flow Metrics at Baseline MR (Table 4)
| Parameter | AUC | Cutoff Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Upward mean flow (mL/s) | 0.825 | 0.8426 | 67.7 | 84.6 |
| Downward mean flow (mL/s) | 0.813 | 1.2314 | 77.4 | 73.1 |
| Summation of mean flow (mL/s) | 0.828 | 1.9812 | 71.0 | 88.5 |
| Upward peak flow (mL/s) | 0.793 | 1.4647 | 71.0 | 88.5 |
| Downward peak flow (mL/s) | 0.844 | 2.0964 | 71.0 | 92.3 |
| Summation of peak flow (mL/s) | 0.841 | 3.5265 | 74.2 | 92.3 |
| Upward CSF total flow (mL/cycle) | 0.819 | 16.7697 | 74.2 | 80.8 |
| Downward CSF total flow (mL/cycle) | 0.819 | 15.7528 | 71.0 | 84.6 |
| Absolute stroke volume (mL/cycle) | 0.829 | 30.8367 | 71.0 | 88.5 |
3.5. Association Between Post-1st-EBP PC-MRI CSF Flow Parameters and the Number of EBPs
3.6. Diagnostic Performance of CSF Flow Metrics at Post-1st-EBP MR for Predicting First EBP Effectiveness
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schievink, W.I. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. Jama 2006, 295, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Mokri, B. Spontaneous low pressure, low CSF volume headaches: Spontaneous CSF leaks. Headache 2013, 53, 1034–1053. [Google Scholar] [CrossRef]
- Benson, J.C.; Madhavan, A.A.; Mark, I.T.; Cutsforth-Gregory, J.K.; Brinjikji, W.; Verdoorn, J.T. Likelihood of Discovering a CSF Leak Based on Intracranial MRI Findings in Patients without a Spinal Longitudinal Extradural Collection: A New Probabilistic Scoring System. AJNR Am. J. Neuroradiol. 2023, 44, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Tung, H.; Liao, Y.C.; Wu, C.C.; Chang, M.H.; Chen, C.C.; Chen, P.L.; Chen, H.C. Usefulness of phase-contrast magnetic resonance imaging for diagnosis and treatment evaluation in patients with SIH. Cephalalgia 2014, 34, 584–593. [Google Scholar] [CrossRef]
- Yang, I.-H.; Chen, H.-L.; Chen, M.-H.; Lui, C.-C.; Su, C.-S.; Lan, M.-Y.; Cheng, Y.-F.; Lu, C.-H.; Lin, W.-C. Application of Cine Phase Contrast MRI in Spontaneous Intracranial Hypotension Before and After Treatment. J. Radiol. Sci. 2014, 39, 67–76. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Chen, H.C.; Tung, H.; Wu, Y.Y.; Chen, H.M.; Pan, K.J.; Cheng, D.C.; Chen, J.H.; Chen, C.C.; Chai, J.W.; et al. Noninvasive assessment of intracranial elastance and pressure in spontaneous intracranial hypotension by MRI. J. Magn. Reson. Imaging 2018, 48, 1255–1263. [Google Scholar] [CrossRef]
- Wolf, K.; Luetzen, N.; Mast, H.; Kremers, N.; Reisert, M.; Beltrán, S.; Fung, C.; Beck, J.; Urbach, H. CSF Flow and Spinal Cord Motion in Patients With Spontaneous Intracranial Hypotension: A Phase Contrast MRI Study. Neurology 2023, 100, e651–e660. [Google Scholar] [CrossRef]
- Wolf, K.; Volz, F.; Lützen, N.; Mast, H.; Reisert, M.; Rahal, A.E.; Fung, C.; Shah, M.J.; Beck, J.; Urbach, H. Non-invasive biomarkers for spontaneous intracranial hypotension (SIH) through phase-contrast MRI. J. Neurol. 2024, 271, 4336–4347. [Google Scholar] [CrossRef]
- Beltrán, S.; Reisert, M.; Krafft, A.J.; Frase, S.; Mast, H.; Urbach, H.; Luetzen, N.; Hohenhaus, M.; Wolf, K. Spinal cord motion and CSF flow in the cervical spine of 70 healthy participants. NMR Biomed. 2024, 37, e5013. [Google Scholar] [CrossRef]
- Grimm, F.; Edl, F.; Kerscher, S.R.; Nieselt, K.; Gugel, I.; Schuhmann, M.U. Semantic segmentation of cerebrospinal fluid and brain volume with a convolutional neural network in pediatric hydrocephalus-transfer learning from existing algorithms. Acta Neurochir. 2020, 162, 2463–2474. [Google Scholar] [CrossRef]
- Cui, J.; Yang, J.; Wang, Y.; Ma, M.; Zhang, N.; Wang, R.; Zhou, B.; Meng, C.; Yang, P.; Yang, J.; et al. Automatic segmentation of hemispheric CSF on MRI using deep learning: Quantifying cerebral edema following large hemispheric infarction. Heliyon 2024, 10, e26673. [Google Scholar] [CrossRef] [PubMed]
- Puzio, T.; Matera, K.; Wiśniewski, K.; Grobelna, M.; Wanibuchi, S.; Jaskólski, D.J.; Bobeff, E.J. Automated volumetric evaluation of intracranial compartments and cerebrospinal fluid distribution on emergency trauma head CT scans to quantify mass effect. Front. Neurosci. 2024, 18, 1341734. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Movahedi, M.M.; Kazemi, K.; Parsaei, H. 3D cerebral MR image segmentation using multiple-classifier system. Med. Biol. Eng. Comput. 2017, 55, 353–364. [Google Scholar] [CrossRef]
- Lundervold, A.; Storvik, G. Segmentation of brain parenchyma and cerebrospinal fluid in multispectral magnetic resonance images. IEEE Trans. Med. Imaging 1995, 14, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Ambrosius, W.; Qian, G.; Blazejewska, A.; Kazmierski, R.; Urbanik, A.; Nowinski, W.L. Automatic segmentation of cerebrospinal fluid, white and gray matter in unenhanced computed tomography images. Acad. Radiol. 2010, 17, 1350–1358. [Google Scholar] [CrossRef]
- Sáenz-Gamboa, J.J.; Domenech, J.; Alonso-Manjarrés, A.; Gómez, J.A.; de la Iglesia-Vayá, M. Automatic semantic segmentation of the lumbar spine: Clinical applicability in a multi-parametric and multi-center study on magnetic resonance images. Artif. Intell. Med. 2023, 140, 102559. [Google Scholar] [CrossRef]
- Liang, S.; Liu, H.; Chen, C.; Qin, C.; Yang, F.; Feng, Y.; Lin, Z. Research on multi-path dense networks for MRI spinal segmentation. PLoS ONE 2021, 16, e0248303. [Google Scholar] [CrossRef]
- Chen, M.; Carass, A.; Oh, J.; Nair, G.; Pham, D.L.; Reich, D.S.; Prince, J.L. Automatic magnetic resonance spinal cord segmentation with topology constraints for variable fields of view. Neuroimage 2013, 83, 1051–1062. [Google Scholar] [CrossRef]
- Jafrasteh, B.; Lubián-Gutiérrez, M.; Lubián-López, S.P.; Benavente-Fernández, I. Enhanced Spatial Fuzzy C-Means Algorithm for Brain Tissue Segmentation in T1 Images. Neuroinformatics 2024, 22, 407–420. [Google Scholar] [CrossRef]
- Fu, J.; Chai, J.W.; Chen, P.L.; Ding, Y.W.; Chen, H.C. Quantitative Measurement of Spinal Cerebrospinal Fluid by Cascade Artificial Intelligence Models in Patients with Spontaneous Intracranial Hypotension. Biomedicines 2022, 10, 2049. [Google Scholar] [CrossRef]
- Tsou, C.-H.; Cheng, Y.-C.; Huang, C.-Y.; Chen, J.-H.; Chen, W.-H.; Chai, J.-W.; Chen, C.C.-C. Using deep learning convolutional neural networks to automatically perform cerebral aqueduct CSF flow analysis. J. Clin. Neurosci. 2021, 90, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Flórez, Y.N.; Moratal, D.; Forner, J.; Martí-Bonmatí, L.; Arana, E.; Guajardo-Hernández, U.; Millet-Roig, J. Semiautomatic analysis of phase contrast magnetic resonance imaging of cerebrospinal fluid flow through the aqueduct of Sylvius. Magma 2006, 19, 78–87. [Google Scholar] [CrossRef]
- Le, R.F.D.; Karmonik, C.; Regnier-Golanov, A.S.; Golanov, E.V.; Britz, G.W. Quantitative evaluation of normal cerebrospinal fluid flow in Sylvian aqueduct and perivascular spaces of middle cerebral artery and circle of Willis using 2D phase-contrast MRI imaging. Front. Neurosci. 2025, 19, 1622351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, H.; Duan, W.; Li, X.; Wang, Y.; Cogswell, P.M.; Elder, B.D. Influence of the area of the aqueduct and region of interest on quantification of stroke volume in healthy volunteers using phase-contrast cine magnetic resonance imaging. Acta. Radiol. 2023, 64, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- The MathWorks Inc. MATLAB, Version: 24.1.0 (R2024a); The MathWorks Inc.: Natick, MA, USA, 2024.
- Bochkovskiy, A.; Wang, C.-Y.; Liao, H.-Y. YOLOv4: Optimal Speed and Accuracy of Object Detection. arXiv 2020, arXiv:2004.10934. [Google Scholar]
- Hyvärinen, A.; Oja, E. Independent component analysis: Algorithms and applications. Neural Netw. 2000, 13, 411–430. [Google Scholar] [CrossRef]
- Alperin, N.; Lee, S.H. PUBS: Pulsatility-based segmentation of lumens conducting non-steady flow. Magn. Reson. Med. 2003, 49, 934–944. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 21.0; IBM Corp.: Armonk, NY, UK, 2012.
- Cheema, S.; Anderson, J.; Angus-Leppan, H.; Armstrong, P.; Butteriss, D.; Carlton Jones, L.; Choi, D.; Chotai, A.; D’Antona, L.; Davagnanam, I.; et al. Multidisciplinary consensus guideline for the diagnosis and management of spontaneous intracranial hypotension. J. Neurol. Neurosurg. Psychiatry 2023, 94, 835–843. [Google Scholar] [CrossRef]
- Hazama, A.; Awawdeh, F.; Braley, A.; Loree, J.; Swarnkar, A.; Chin, L.S.; Krishnamurthy, S. Recurrent Spontaneous Intracranial Hypotension (SIH) and the Durability of Repeat Epidural Blood Patch (EBP). Cureus 2023, 15, e41457. [Google Scholar] [CrossRef]
- Wang, Y.W.; Teng, C.J.; Chai, J.W.; Wu, C.C.; Chen, P.L.; Chen, H.C. Prediction of Target Epidural Blood Patch Treatment Efficacy in Spontaneous Intracranial Hypotension Using Follow-Up MRI. Diagnostics 2022, 12, 1158. [Google Scholar] [CrossRef]
- Chen, H.C.; Chai, J.W.; Wu, C.C.; Chen, P.L.; Teng, C.L. Magnetic resonance imaging predicted the therapeutic response of patients with spinal cerebrospinal fluid leakage undergoing targeted epidural blood patch. Br. J. Radiol. 2022, 95, 20210841. [Google Scholar] [CrossRef]
- Lin, P.T.; Wang, Y.F.; Hseu, S.S.; Fuh, J.L.; Lirng, J.F.; Wu, J.W.; Chen, S.T.; Chen, S.P.; Chen, W.T.; Wang, S.J. The SIH-EBP Score: A grading scale to predict the response to the first epidural blood patch in spontaneous intracranial hypotension. Cephalalgia 2023, 43, 3331024221147488. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Hou, T.W.; Chen, P.L.; Wu, C.C.; Wang, S.J.; Wang, Y.F. SIH-EBP score for prediction of efficacy of epidural blood patching in patients with spontaneous intracranial hypotension. J. Chin. Med. Assoc. 2025, 88, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.M.; Elsorogy, L.; Abdelghaffar, R.; Naby, A.A.; Elmenshawi, I. Phase-Contrast MRI CSF Flow Measurements for the Diagnosis of Normal-Pressure Hydrocephalus: Observer Agreement of Velocity Versus Volume Parameters. AJR Am. J. Roentgenol. 2017, 208, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-Y.; Chung, H.-W.; Chen, M.-Y.; Giiang, L.-H.; Chin, S.-C.; Lee, C.-S.; Chen, C.-Y.; Liu, Y.-J. Supratentorial Cerebrospinal Fluid Production Rate in Healthy Adults: Quantification with Two-dimensional Cine Phase-Contrast MR Imaging with High Temporal and Spatial Resolution. Radiology 2004, 233, 603–608. [Google Scholar] [CrossRef]
- Owashi, K.P.; Liu, P.; Metanbou, S.; Capel, C.; Balédent, O. Phase-contrast MRI analysis of cerebral blood and CSF flow dynamic interactions. Fluids Barriers CNS 2024, 21, 88. [Google Scholar] [CrossRef]
- Sakhare, A.R.; Barisano, G.; Pa, J. Assessing test-retest reliability of phase contrast MRI for measuring cerebrospinal fluid and cerebral blood flow dynamics. Magn. Reson. Med. 2019, 82, 658–670. [Google Scholar] [CrossRef]
- van Pelt, R.; Nguyen, H.; ter Haar Romeny, B.; Vilanova, A. Automated segmentation of blood-flow regions in large thoracic arteries using 3D-cine PC-MRI measurements. Int. J. Comput. Assist. Radiol. Surg. 2012, 7, 217–224. [Google Scholar] [CrossRef]
- Keles, A.; Ozisik, P.A.; Algin, O.; Celebi, F.V.; Bendechache, M. Decoding pulsatile patterns of cerebrospinal fluid dynamics through enhancing interpretability in machine learning. Sci. Rep. 2024, 14, 17854. [Google Scholar] [CrossRef]
| Characteristic | Group | p Value | |
|---|---|---|---|
| EBP Failure Patients (n = 12) | EBP Successful Patients (n = 13) | ||
| Mean ± SD | Mean ± SD | ||
| Upward mean flow (mL/s) | 0.691 ± 0.14 | 1.26 ± 0.23 | <0.001 ** |
| Downward mean flow (mL/s) | 0.84 ± 0.24 | 1.81 ± 0.46 | <0.001 ** |
| Summation of mean flow (mL/s) | 1.53 ± 0.36 | 3.07 ± 0.65 | <0.001 ** |
| Upward peak flow (mL/s) | 1.13 ± 0.24 | 2.00 ± 0.34 | <0.001 ** |
| Downward peak flow (mL/s) | 1.45 ± 0.42 | 2.98 ± 0.64 | <0.001 ** |
| Summation of peak flow (mL/s) | 2.58 ± 0.61 | 4.98 ± 0.95 | <0.001** |
| Upward CSF total flow (mL/cycle) | 12.47 ± 2.64 | 24.26 ± 5.13 | <0.001 ** |
| Downward CSF total flow (mL/cycle) | 14.58 ± 2.95 | 22.92 ± 4.35 | <0.001 ** |
| Absolute stroke volume (mL/cycle) | 24.04 ± 5.45 | 47.17 ± 9.16 | <0.001 ** |
| Parameter | AUC | Cutoff Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Upward mean flow (mL/s) | 0.994 | 0.902 | 92.3 | 100 |
| Downward mean flow (mL/s) | 1 | 1.2511 | 100 | 100 |
| Summation of mean flow (mL/s) | 0.994 | 2.1521 | 92.3 | 100 |
| Upward peak flow (mL/s) | 0.994 | 1.4923 | 92.3 | 100 |
| Downward peak flow (mL/s) | 1 | 2.1968 | 100 | 100 |
| Summation of peak flow (mL/s) | 1 | 3.4623 | 100 | 100 |
| Upward CSF total flow (mL/cycle) | 0.974 | 17.1383 | 92.3 | 100 |
| Downward CSF total flow (mL/cycle) | 1 | 16.4993 | 100 | 100 |
| Absolute stroke volume (mL/cycle) | 1 | 33.3892 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-J.; Chai, J.-W.; Chen, W.-H.; Chen, H.-C.; Cheng, D.-C. AI-Augmented Quantitative MRI Predicts Spontaneous Intracranial Hypotension. Diagnostics 2025, 15, 2339. https://doi.org/10.3390/diagnostics15182339
Huang Y-J, Chai J-W, Chen W-H, Chen H-C, Cheng D-C. AI-Augmented Quantitative MRI Predicts Spontaneous Intracranial Hypotension. Diagnostics. 2025; 15(18):2339. https://doi.org/10.3390/diagnostics15182339
Chicago/Turabian StyleHuang, Yi-Jhe, Jyh-Wen Chai, Wen-Hsien Chen, Hung-Chieh Chen, and Da-Chuan Cheng. 2025. "AI-Augmented Quantitative MRI Predicts Spontaneous Intracranial Hypotension" Diagnostics 15, no. 18: 2339. https://doi.org/10.3390/diagnostics15182339
APA StyleHuang, Y.-J., Chai, J.-W., Chen, W.-H., Chen, H.-C., & Cheng, D.-C. (2025). AI-Augmented Quantitative MRI Predicts Spontaneous Intracranial Hypotension. Diagnostics, 15(18), 2339. https://doi.org/10.3390/diagnostics15182339








