Comparison of Supervised Machine Learning Models to Logistic Regression Model Using Tooth-Related Factors to Predict the Outcome of Nonsurgical Periodontal Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Tooth-Related Parameters and Intervention
2.4. Outcomes and Sample Size
2.5. Statistical Analysis
2.5.1. Conventional Statistical Analysis
2.5.2. Machine Learning Models Algorithms
3. Results
3.1. Basic Demographic and Tooth-Related Parameters
3.2. Logistic Regression Model
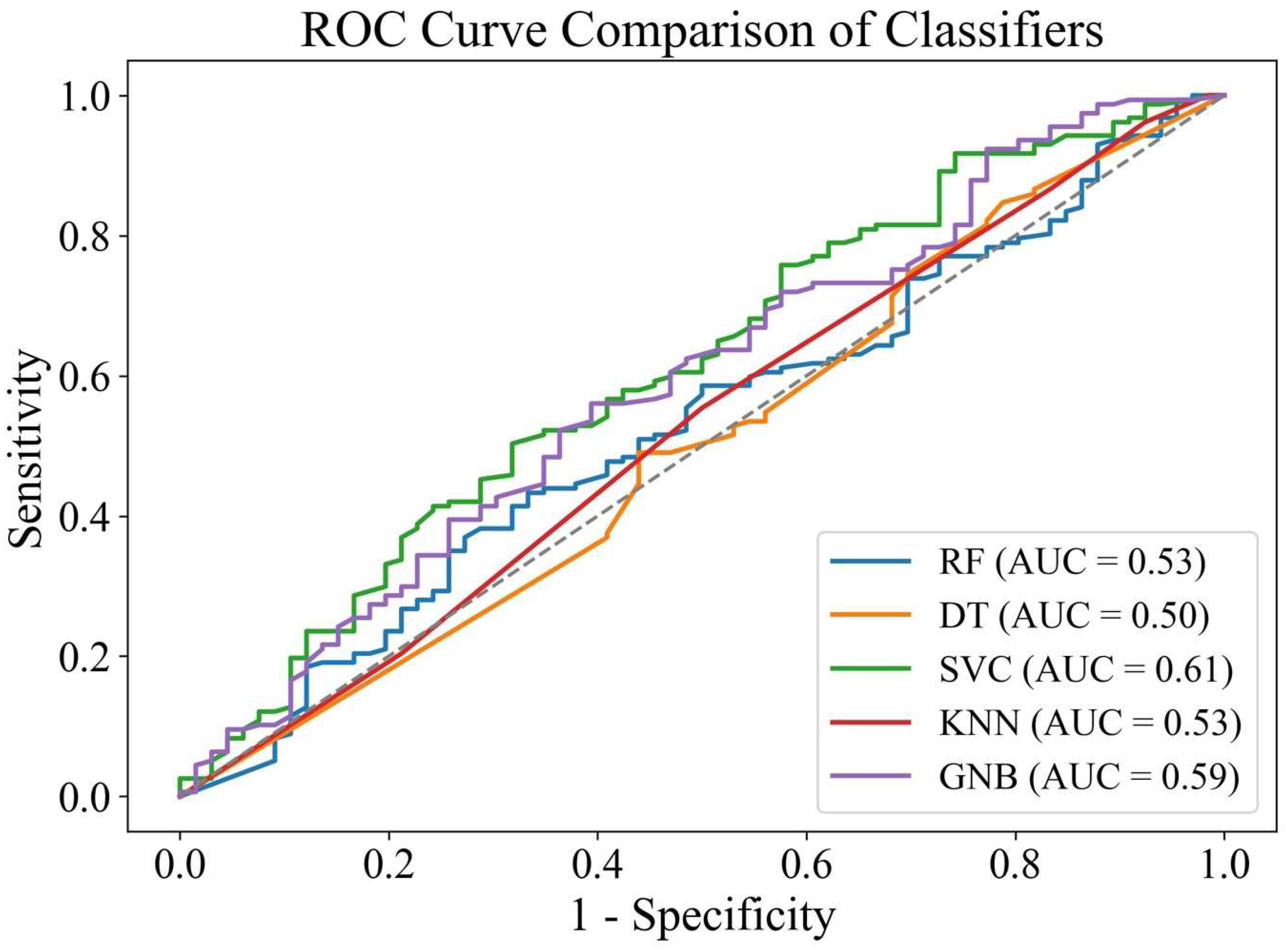
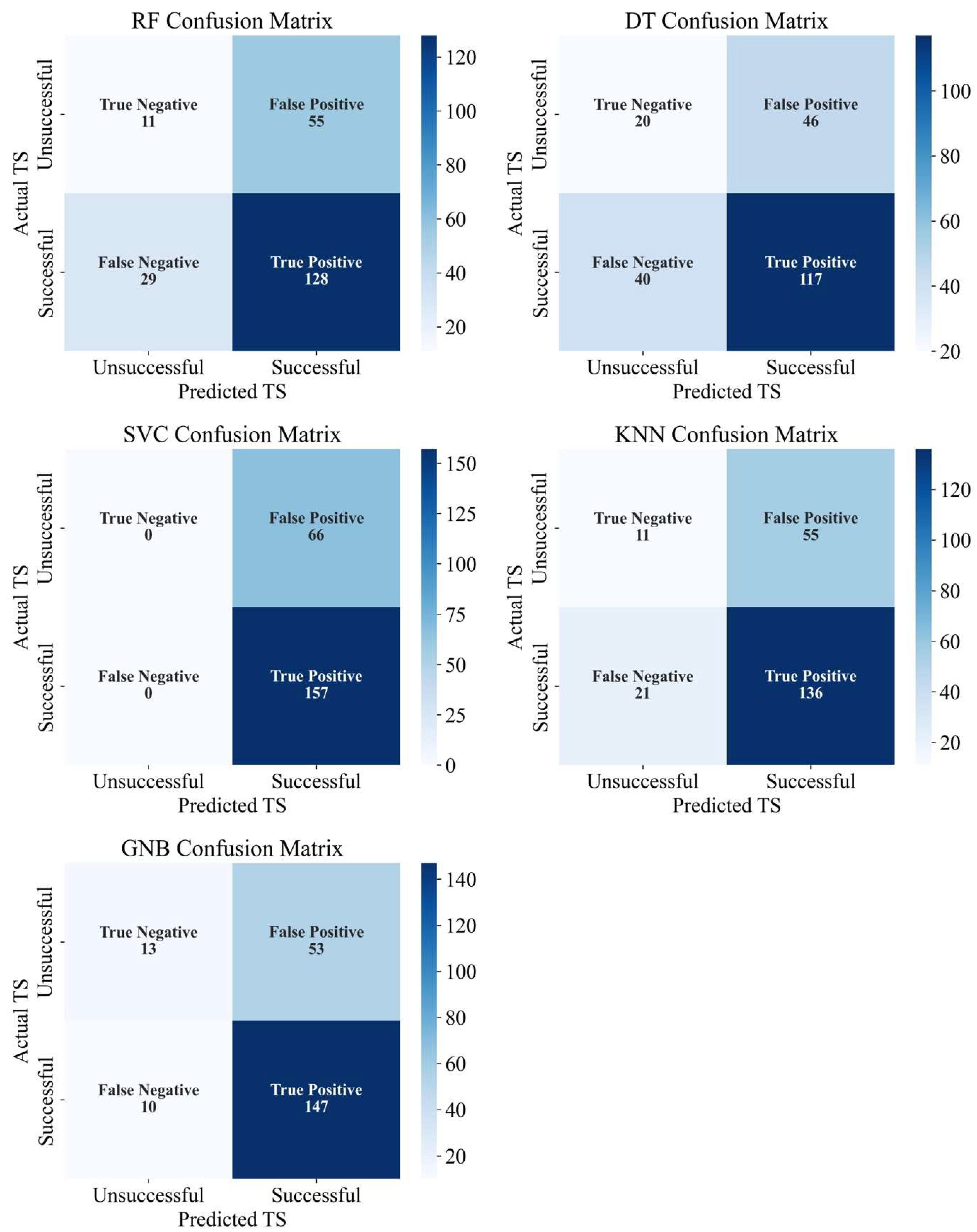
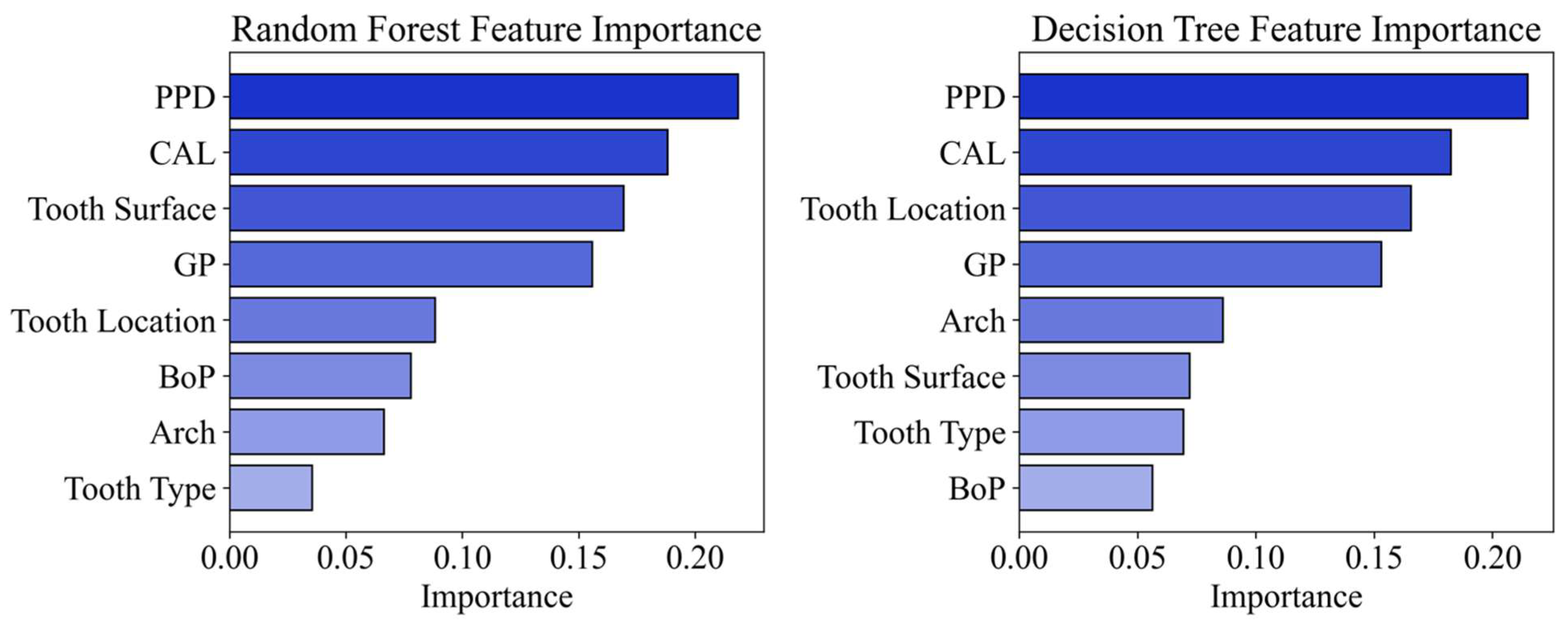
3.3. Machine Learning Models
3.4. Logistic Regression vs. Machine Learning Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cobb, C.M. Non-surgical pocket therapy: Mechanical. Ann. Periodontol. 1996, 1, 443–490. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef]
- Belstrøm, D.; Grande, M.A.; Sembler-Møller, M.L.; Kirkby, N.; Cotton, S.L.; Paster, B.J.; Holmstrup, P. Influence of periodontal treatment on subgingival and salivary microbiotas. J. Periodontol. 2018, 89, 531–539. [Google Scholar] [CrossRef]
- Byrne, S.J.; Chang, D.; Adams, G.G.; Butler, C.A.; Reynolds, E.C.; Darby, I.B.; Dashper, S.G. Microbiome profiles of non-responding and responding paired periodontitis sites within the same participants following non-surgical treatment. J. Oral Microbiol. 2022, 14, 2043595. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Montero, E.; Citterio, F.; Romano, F.; Molina, A.; Aimetti, M. Efficacy of access flap procedures compared to subgingival debridement in the treatment of periodontitis. A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 282–302. [Google Scholar] [CrossRef]
- Werner, N.; Heck, K.; Walter, E.; Ern, C.; Bumm, C.V.; Folwaczny, M. Probing pocket depth reduction after non-surgical periodontal therapy: Tooth-related factors. J. Periodontol. 2024, 95, 29–39. [Google Scholar] [CrossRef]
- Axtelius, B.; Söderfeldt, B.; Attström, R. A multilevel analysis of factors affecting pocket probing depth in patients responding differently to periodontal treatment. J. Clin. Periodontol. 1999, 26, 67–76. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Ready, D.; Parkar, M.; Tonetti, M.S. Relative Contribution of Patient-, Tooth-, and Site-Associated Variability on the Clinical Outcomes of Subgingival Debridement. I. Probing Depths. J. Periodontol. 2005, 76, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.J.; Syed, M.; Koshy, B.; Marinho, V.; Bostanci, N.; McKay, I.J.; Curtis, M.A.; Croucher, R.E.; Marcenes, W. Prognostic factors in the treatment of generalized aggressive periodontitis: I. Clinical features and initial outcome. J. Clin. Periodontol. 2006, 33, 663–670. [Google Scholar] [CrossRef]
- Pretzl, B.; Kaltschmitt, J.; Kim, T.-S.; Reitmeir, P.; Eickholz, P. Tooth loss after active periodontal therapy. 2: Tooth-related factors. J. Clin. Periodontol. 2008, 35, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bunæs, D.F.; Lie, S.A.; Åstrøm, A.N.; Mustafa, K.; Leknes, K.N. Site-specific treatment outcome in smokers following 12 months of supportive periodontal therapy. J. Clin. Periodontol. 2016, 43, 1086–1093. [Google Scholar] [CrossRef]
- Van der Weijden, G.A.F.; Dekkers, G.J.; Slot, D.E. Success of non-surgical periodontal therapy in adult periodontitis patients: A retrospective analysis. Int. J. Dent. Hyg. 2019, 17, 309–317. [Google Scholar] [CrossRef]
- Bumm, C.V.; Schwendicke, F.; Pitchika, V.; Heck, K.; Walter, E.; Ern, C.; Heym, R.; Werner, N.; Folwaczny, M. Effectiveness of nonsurgical re-instrumentation: Tooth-related factors. J. Periodontol. 2025, 96, 748–759. [Google Scholar] [CrossRef]
- Herz, M.M.; Hoffmann, N.; Braun, S.; Lachmann, S.; Bartha, V.; Petsos, H. Periodontal pockets: Predictors for site-related worsening after non-surgical therapy—A long-term retrospective cohort study. J. Clin. Periodontol. 2024, 51, 680–690. [Google Scholar] [CrossRef]
- Liu, H.-J.; Wang, B.; Wang, A.-C.; Zhang, D.-H.; Mao, C.; Li, Q.-H. Prognostic factors affecting the short-term efficacy of non-surgical treatment of chronic periodontitis: A multilevel modeling analysis. Eur. J. Med. Res. 2021, 26, 50. [Google Scholar] [CrossRef]
- Ferrarotti, F.; Baima, G.; Rendinelli, M.; Citterio, F.; Mariani, G.M.; Mussano, F.; Romano, F.; Romandini, M.; Aimetti, M. Pocket closure after repeated subgingival instrumentation: A stress test to the EFP guideline for stage III-IV periodontitis. Clin. Oral Investig. 2023, 27, 6701–6708. [Google Scholar] [CrossRef]
- Goldstein, H.; Browne, W.; Rasbash, J. Multilevel modelling of medical data. Stat. Med. 2002, 21, 3291–3315. [Google Scholar] [CrossRef]
- Leyland, A.H.; Groenewegen, P.P. Multilevel modelling and public health policy. Scand. J. Public Health 2003, 31, 267–274. [Google Scholar] [CrossRef]
- Tomasi, C.; Leyland, A.H.; Wennström, J.L. Factors influencing the outcome of non-surgical periodontal treatment: A multilevel approach. J. Clin. Periodontol. 2007, 34, 682–690. [Google Scholar] [CrossRef]
- Gul, S.S.; Griffiths, G.S.; Stafford, G.P.; Al-Zubidi, M.I.; Rawlinson, A.; Douglas, C.W. Investigation of a novel predictive biomarker profile for the outcome of periodontal treatment. J. Periodontol. 2017, 88, 1135–1144. [Google Scholar] [CrossRef]
- Pitchika, V.; Büttner, M.; Schwendicke, F. Artificial intelligence and personalized diagnostics in periodontology: A narrative review. Periodontol. 2000 2024, 95, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Bashir, N.Z.; Rahman, Z.; Chen, S.L.-S. Systematic comparison of machine learning algorithms to develop and validate predictive models for periodontitis. J. Clin. Periodontol. 2022, 49, 958–969. [Google Scholar] [CrossRef]
- Montero, E.; Sánchez, N.; Sanz-Sánchez, I.; López-Durán, M.; de Albornoz, A.C.; Dietrich, T. Emerging Technologies and Algorithms for Periodontal Screening and Risk of Disease Progression in Non-Dental Settings: A Scoping Review. J. Clin. Periodontol. 2025, 52, 246–291. [Google Scholar] [CrossRef]
- Farina, R.; Simonelli, A.; Trombelli, L.; Ettmayer, J.B.; Schmid, J.L.; Ramseier, C.A. Emerging Applications of Digital Technologies for Periodontal Screening, Diagnosis and Prognosis in the Dental Setting. J. Clin. Periodontol. 2025, 52, 211–245. [Google Scholar] [CrossRef]
- Baban, M.T.A.; Mohammad, D.N. A new approach for sex prediction by evaluating mandibular arch and canine dimensions with machine-learning classifiers and intraoral scanners (a retrospective study). Sci. Rep. 2024, 14, 27974. [Google Scholar] [CrossRef]
- Yan, Y.; Sharma, P.; Suvan, J.; D’Aiuto, F. The Association of Periodontal Inflammation and Systemic Health Indicators: A Machine Learning Approach. J. Clin. Periodontol. 2025, 52, 1466–1477. [Google Scholar] [CrossRef]
- Chow, D.Y.; Tay, J.R.H.; Nascimento, G.G. Systematic Review of Prognosis Models in Predicting Tooth Loss in Periodontitis. J. Dent. Res. 2024, 103, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Uysal, B.F.; Köse, T.; Gürkan, A. Gingival phenotype classification by visual and probe visibility assessments: Relationship with thickness and probe design. J. Periodontol. 2025, 96, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Needleman, I. Endpoints of active periodontal therapy. J. Clin. Periodontol. 2020, 47, 61–71. [Google Scholar] [CrossRef]
- Chiarito, M.; Luceri, L.; Oliva, A.; Stefanini, G.; Condorelli, G. Artificial Intelligence and Cardiovascular Risk Prediction: All That Glitters is not Gold. Eur. Cardiol. 2022, 17, e29. [Google Scholar] [CrossRef]
- Bostanci, N.; Manoil, D.; Van Holm, W.; Belibasakis, G.N.; Teughels, W. Microbial Markers for Diagnosis and Risk Assessment for Periodontal Diseases: A Systematic Literature Search and Narrative Synthesis. J. Clin. Periodontol. 2025, 52, 125–154. [Google Scholar] [CrossRef]
- Rakic, M.; Calciolari, E.; Grant, M.M.; Radovanovic, S.; Bostanci, N.; Preshaw, P.M. Host Markers of Periodontal Diseases: Meta-Analysis of Diagnostic Accuracy Studies. J. Clin. Periodontol. 2025, 52, 155–181. [Google Scholar] [CrossRef]
- Sha, A.M.; Abdulbaqi, H.R.; Qasim, S.S.B. Microbial and Inflammatory Salivary Biomarkers of Periodontal Diseases. Kurd. J. Appl. Res. 2024, 9, 113–125. [Google Scholar] [CrossRef]
- Badersten, A.; Nilveus, R.; Egelberg, J. Effect of nonsurgical periodontal therapy. J. Clin. Periodontol. 1984, 11, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Rosling, B.; Ramberg, P.; Socransky, S.S.; Lindhe, J. Initial outcome and long-term effect of surgical and non-surgical treatment of advanced periodontal disease. J. Clin. Periodontol. 2001, 28, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, G.A.; Timmerman, M.F. A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. J. Clin. Periodontol. 2002, 29, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Abdulkareem, A.A.; Zardawi, F.M.; Gul, S.S. Determination of the Accuracy of Salivary Biomarkers for Periodontal Diagnosis. Diagnostics 2022, 12, 2485. [Google Scholar] [CrossRef]
- Gul, S.S.; Abdulkareem, A.A.; Sha, A.M.; Rawlinson, A. Diagnostic Accuracy of Oral Fluids Biomarker Profile to Determine the Current and Future Status of Periodontal and Peri-Implant Diseases. Diagnostics 2020, 10, 838. [Google Scholar] [CrossRef]
- Baelum, V.; López, R. Defining and predicting outcomes of non-surgical periodontal treatment: A 1-yr follow-up study. Eur. J. Oral Sci. 2016, 124, 33–44. [Google Scholar] [CrossRef]
- Chen, M.H.; Yin, H.J.; Chang, H.H.; Kao, C.T.; Tu, C.C.; Chen, Y.W. Baseline probing depth and interproximal sites predict treatment outcomes of non-surgical periodontal therapy. J. Dent. Sci. 2020, 15, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol. 2000 2018, 76, 85–96. [Google Scholar] [CrossRef]
- Abdalla, M.; Peck, M.; Rayner, C.; Kimmie-Dhansay, F.; Jeftha, A. Factors that affected the efficacy of non-surgical periodontal treatment carried out by postgraduate periodontology students. S. Afr. Dent. J. 2023, 78, 126–129. [Google Scholar] [CrossRef]
- Killoy, W.J. The clinical significance of local chemotherapiesAbstract. J. Clin. Periodontol. 2002, 29, 6–13. [Google Scholar] [CrossRef]
- Olsson, M.; Lindhe, J. Periodontal characteristics in individuals with varying form of the upper central incisors. J. Clin. Periodontol. 1991, 18, 78–82. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Shameer, K.; Johnson, K.W.; Glicksberg, B.S.; Dudley, J.T.; Sengupta, P.P. Machine learning in cardiovascular medicine: Are we there yet? Heart 2018, 104, 1156–1164. [Google Scholar] [CrossRef]
- Ehnevid, H.; Jansson, L.E. Effects of Furcation Involvements on Periodontal Status and Healing in Adjacent Proximal Sites. J. Periodontol. 2001, 72, 871–876. [Google Scholar] [CrossRef]
- Faouzi, J.; Colliot, O. Classic Machine Learning Methods. In Machine Learning for Brain Disorders; Colliot, O., Ed.; Springer: New York, NY, USA, 2023; pp. 25–75. [Google Scholar]

| Variables | Baseline | Endpoint |
|---|---|---|
| Age † (years) | 56.72 ± 7.10 | |
| Sex ‡ | ||
| Male | 33, 49.30% | |
| Female | 34, 50.70% | |
| PPD † (mm) | 4.74 ± 0.66 | 3.83 ± 0.69 * |
| CAL † (mm) | 3.57 ± 0.66 | 3.19 ± 0.61 * |
| BoP ‡ | ||
| No bleeding | 231, 20.85% | 880, 79.42% ** |
| Bleeding | 877, 79.15% | 228, 20.58% ** |
| Gingival phenotype ‡ | ||
| Thin | 142, 12.82% | |
| Medium | 642, 57.94% | |
| Thick | 324, 29.24% | |
| Tooth type ‡ | ||
| Single-rooted | 696, 62.82% | |
| Multirooted | 412, 37.18% | |
| Tooth location ‡ | ||
| Incisors | 413, 37.27% | |
| Premolars | 371, 33.49% | |
| Molars | 324, 29.24% | |
| Arch ‡ | ||
| Maxillary | 637, 57.49% | |
| Mandibular | 471, 42.51% | |
| Tooth surface ‡ | ||
| Mesial | 497, 44.86% | |
| Distal | 487, 43.95% | |
| Facial | 116, 10.47% | |
| Oral | 8, 0.72% | |
| Site-specific outcomes (n = 1108) ‡ | ||
| Successful | 781, 70.49% | |
| Unsuccessful | 327, 29.51% |
| Tooth-Related Factors | Successful | Unsuccessful | p Value |
|---|---|---|---|
| PPD † (mm) | 4.65 ± 0.61 | 4.96 ± 0.71 | <0.001 * |
| CAL † (mm) | 3.57 ± 0.66 | 3.57 ± 0.64 | 0.9 |
| BoP ‡ | |||
| No bleeding | 621, 56.05% | 256, 23.10% | 0.57 |
| Bleeding | 159, 14.35% | 72, 6.50% | |
| Gingival phenotype ‡ | |||
| Thin | 100, 9.03% | 42, 3.79% | 0.93 |
| Medium | 450, 40.61% | 192, 17.33% | |
| Thick | 231, 20.85% | 93, 8.39% | |
| Tooth type ‡ | |||
| Single-rooted | 510, 46.03% | 186, 16.79% | 0.01 ** |
| Multirooted | 271, 24.46% | 141, 12.72% | |
| Tooth location ‡ | |||
| Incisors | 309, 27.80% | 105, 9.48% | 0.054 |
| Premolars | 255, 22.82% | 118, 10.65% | |
| Molars | 218, 19.68% | 106, 9.57% | |
| Arch ‡ | |||
| Maxillary | 436, 39.35% | 201, 18.14% | 0.11 |
| Mandibular | 344, 31.05% | 127, 11.46% | |
| Tooth surface ‡ | |||
| Mesial | 332, 29.96% | 165, 14.89% | 0.10 |
| Distal | 356, 32.13% | 131, 11.83% | |
| Facial | 87, 7.85% | 29, 2.62% | |
| Oral | 5, 0.45% | 3, 0.27% |
| Predictors | B | SE | p Value | Exp (B) | 95% CI for EXP (B) |
|---|---|---|---|---|---|
| PPD | −0.550 | 0.103 | 0.001 | 0.577 | 0.471 to 0.706 |
| CAL | 0.067 | 0.102 | 0.51 | 1.070 | 0.875 to 1.307 |
| BoP a | |||||
| Bleeding | −0.28 | 0.169 | 0.098 | 0.75 | 0.542 to 1.053 |
| Gingival phenotype b | |||||
| Medium | 0.009 | 0.234 | 0.971 | 1.009 | 0.637 to 1.579 |
| Thick | 0.049 | 0.167 | 0.772 | 1.05 | 0.756 to 1.457 |
| Tooth type c | |||||
| Multirooted | 0.4 | 0.281 | 0.155 | 1.491 | 0.86 to 2.585 |
| Tooth location d | |||||
| Premolars | −0.348 | 0.346 | 0.314 | 0.706 | 0.358 to 1.391 |
| Molars | −0.399 | 0.277 | 0.149 | 0.671 | 0.39 to 1.154 |
| Arch e | |||||
| Mandibular | −0.118 | 0.153 | 0.44 | 0.889 | 0.659 to 1.199 |
| Tooth surface f | |||||
| Distal | −0.16 | 0.741 | 0.983 | 0.984 | 0.23 to 4.206 |
| Facial | 0.261 | 0.743 | 0.725 | 1.299 | 0.303 to 5.568 |
| Oral | 0.28 | 0.767 | 0.715 | 1.323 | 0.294 to 5.942 |
| Accuracy of the model | 70.4% |
| ML Models | Treatment Outcome | Precision | Recall | F1-Score | Training Accuracy | Testing Accuracy |
|---|---|---|---|---|---|---|
| Random forest | Unsuccessful | 0.28 | 0.17 | 0.21 | 0.81 | 0.62 |
| Successful | 0.7 | 0.82 | 0.75 | |||
| Decision tree | Unsuccessful | 0.33 | 0.3 | 0.32 | 0.81 | 0.61 |
| Successful | 0.72 | 0.75 | 0.73 | |||
| Support vector classifier | Unsuccessful | 0 | 0 | 0 | 0.703 | 0.704 |
| Successful | 0.7 | 1 | 0.83 | |||
| K-nearest neighbors | Unsuccessful | 0.34 | 0.17 | 0.22 | 0.76 | 0.65 |
| Successful | 0.71 | 0.87 | 0.78 | |||
| Gaussian naïve Bayes | Unsuccessful | 0.57 | 0.2 | 0.29 | 0.72 | 0.71 |
| Successful | 0.73 | 0.94 | 0.82 |
| Training Set | RF | DT | SCV | KNN | GNB |
|---|---|---|---|---|---|
| 1 | 0.642 | 0.625 | 0.705 | 0.651 | 0.687 |
| 2 | 0.693 | 0.630 | 0.702 | 0.693 | 0.684 |
| 3 | 0.657 | 0.621 | 0.702 | 0.594 | 0.720 |
| 4 | 0.639 | 0.603 | 0.666 | 0.657 | 0.648 |
| 5 | 0.621 | 0.522 | 0.702 | 0.657 | 0.720 |
| 6 | 0.621 | 0.576 | 0.702 | 0.666 | 0.720 |
| 7 | 0.621 | 0.540 | 0.702 | 0.639 | 0.720 |
| 8 | 0.702 | 0.603 | 0.702 | 0.648 | 0.729 |
| 9 | 0.747 | 0.675 | 0.702 | 0.693 | 0.792 |
| 10 | 0.747 | 0.729 | 0.711 | 0.720 | 0.747 |
| Mean ± SD | 0.667 ± 0.049 | 0.613 ± 0.057 | 0.7 ± 0.011 | 0.662 ± 0.032 | 0.713 ± 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sharqi, A.J.B.; Baban, M.T.A.; Imran, N.K.; Gul, S.S.; Abdulkareem, A.A. Comparison of Supervised Machine Learning Models to Logistic Regression Model Using Tooth-Related Factors to Predict the Outcome of Nonsurgical Periodontal Treatment. Diagnostics 2025, 15, 2333. https://doi.org/10.3390/diagnostics15182333
Al-Sharqi AJB, Baban MTA, Imran NK, Gul SS, Abdulkareem AA. Comparison of Supervised Machine Learning Models to Logistic Regression Model Using Tooth-Related Factors to Predict the Outcome of Nonsurgical Periodontal Treatment. Diagnostics. 2025; 15(18):2333. https://doi.org/10.3390/diagnostics15182333
Chicago/Turabian StyleAl-Sharqi, Ali J. B., Mohammed Taha Ahmed Baban, Nada K. Imran, Sarhang S. Gul, and Ali A. Abdulkareem. 2025. "Comparison of Supervised Machine Learning Models to Logistic Regression Model Using Tooth-Related Factors to Predict the Outcome of Nonsurgical Periodontal Treatment" Diagnostics 15, no. 18: 2333. https://doi.org/10.3390/diagnostics15182333
APA StyleAl-Sharqi, A. J. B., Baban, M. T. A., Imran, N. K., Gul, S. S., & Abdulkareem, A. A. (2025). Comparison of Supervised Machine Learning Models to Logistic Regression Model Using Tooth-Related Factors to Predict the Outcome of Nonsurgical Periodontal Treatment. Diagnostics, 15(18), 2333. https://doi.org/10.3390/diagnostics15182333







