Endometriosis and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration and Reporting Guidelines
2.2. Data Sources and Search Strategy
2.3. Study Selection and Eligibility Criteria
2.4. Study Outcomes and Data Extraction
2.5. Statistical Analysis
2.6. Risk of Bias (Quality) Assessment
3. Results
3.1. Study Characteristics
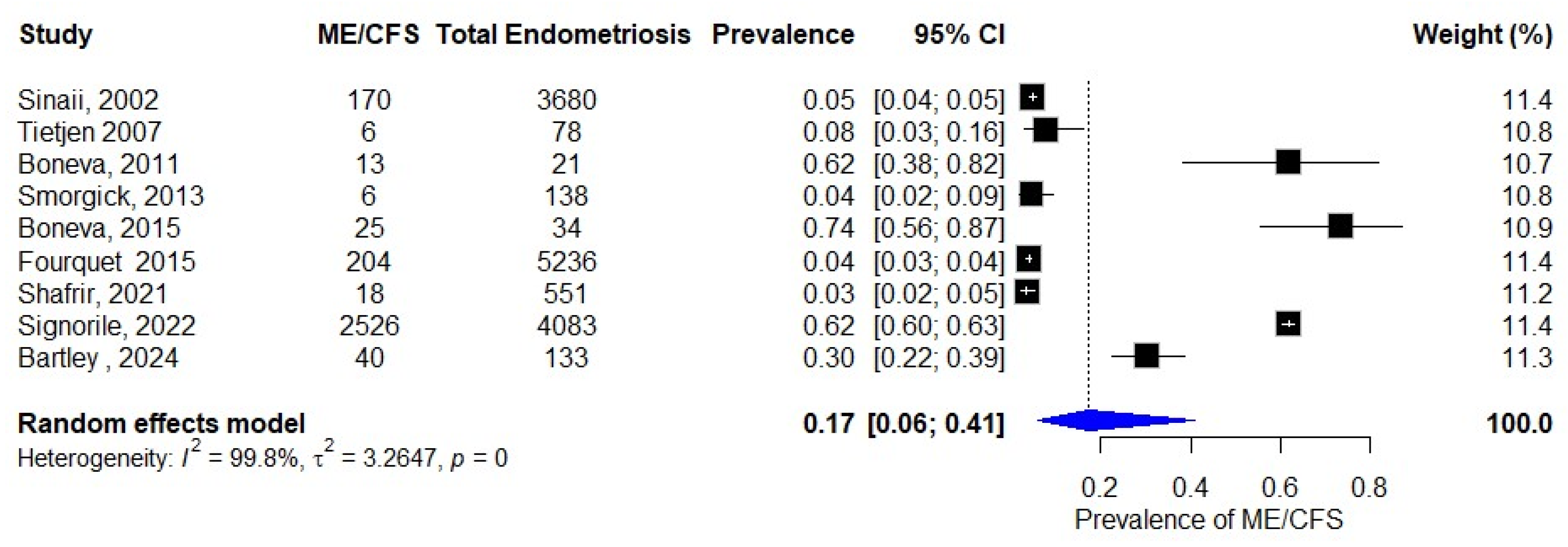
3.2. Prevalence of ME/CFS in Patients with Endometriosis
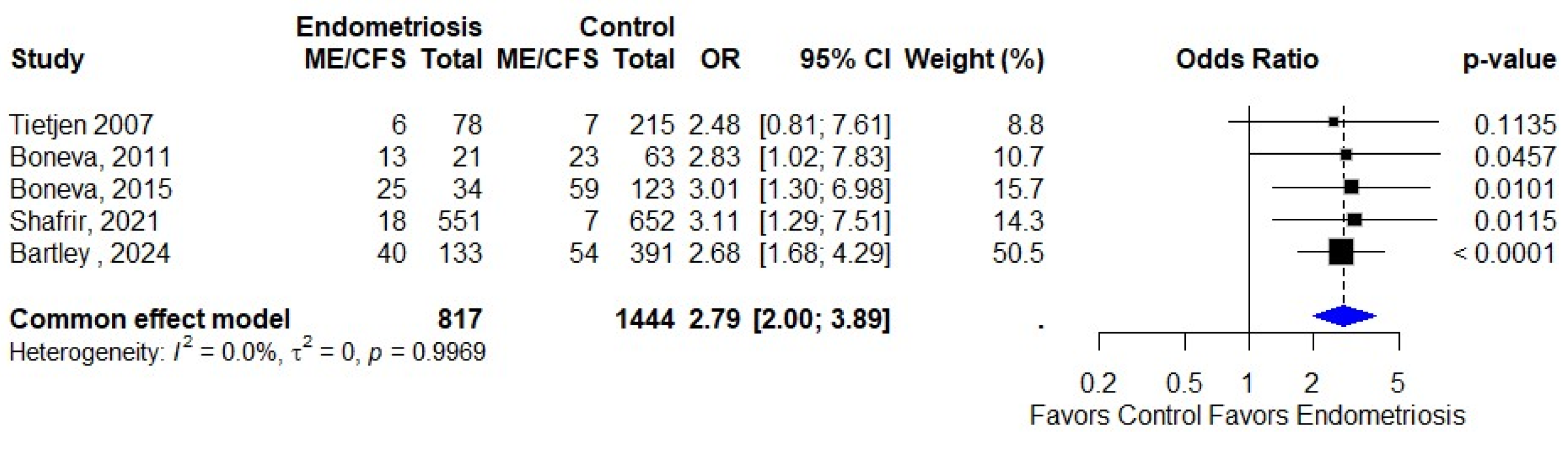
3.3. Association of Endometriosis and ME/CFS
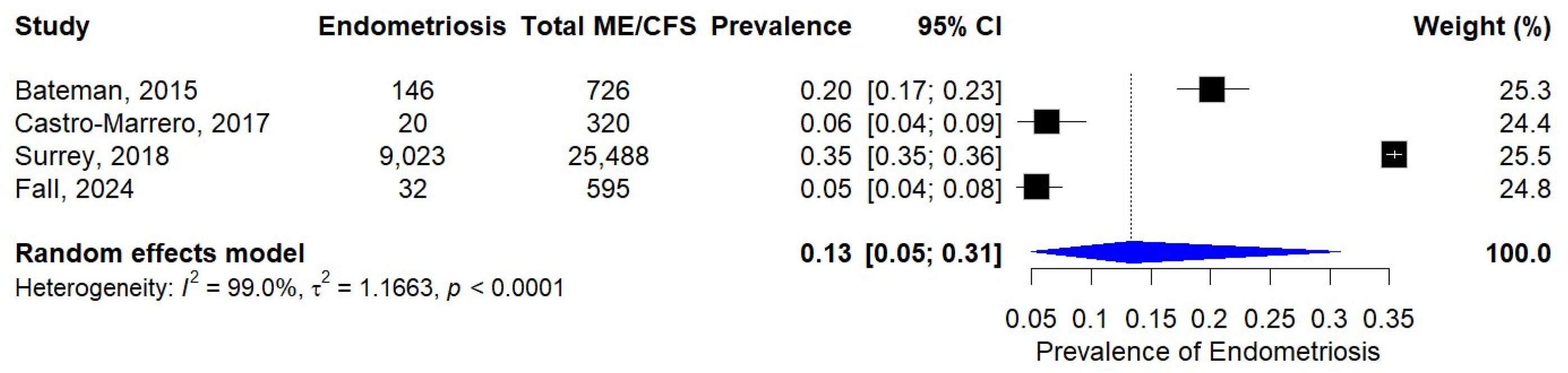
3.4. Prevalence of Endometriosis in Patients with ME/CFS
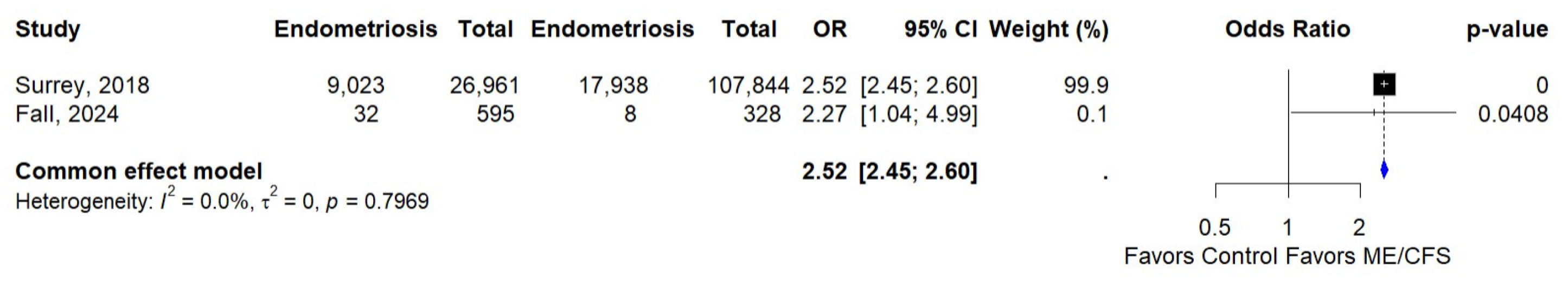
3.5. Association of ME/CFS and Endometriosis
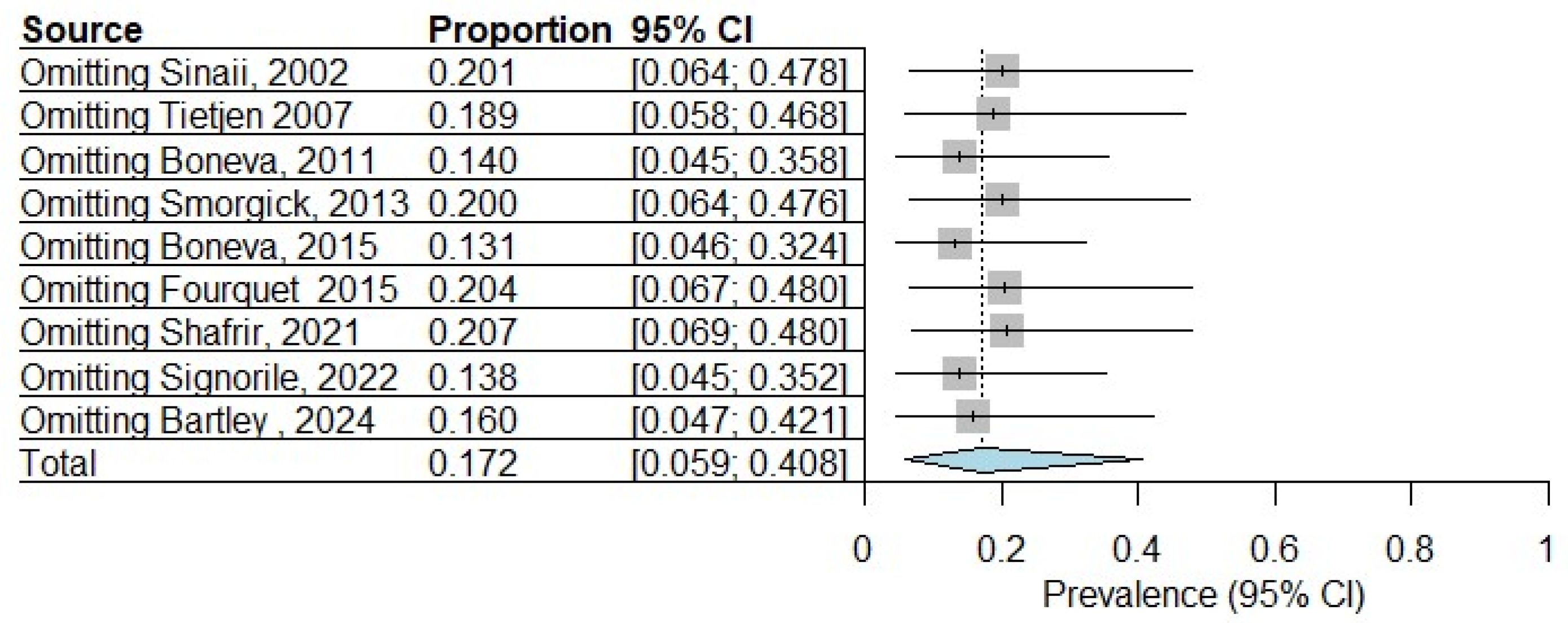
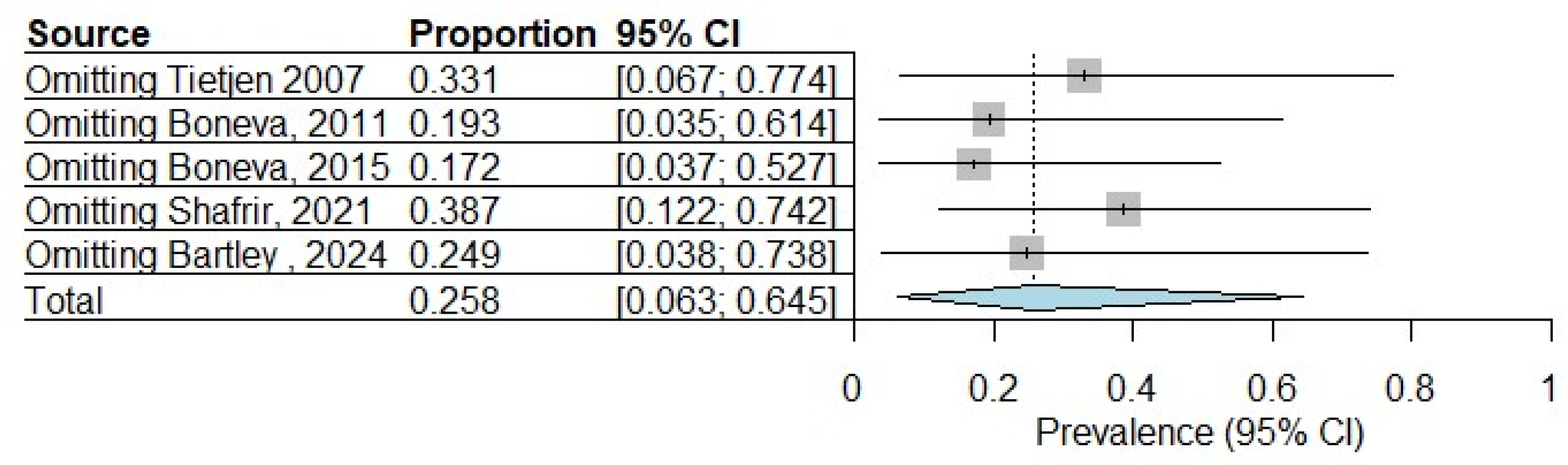
3.6. Sensitivity Analysis
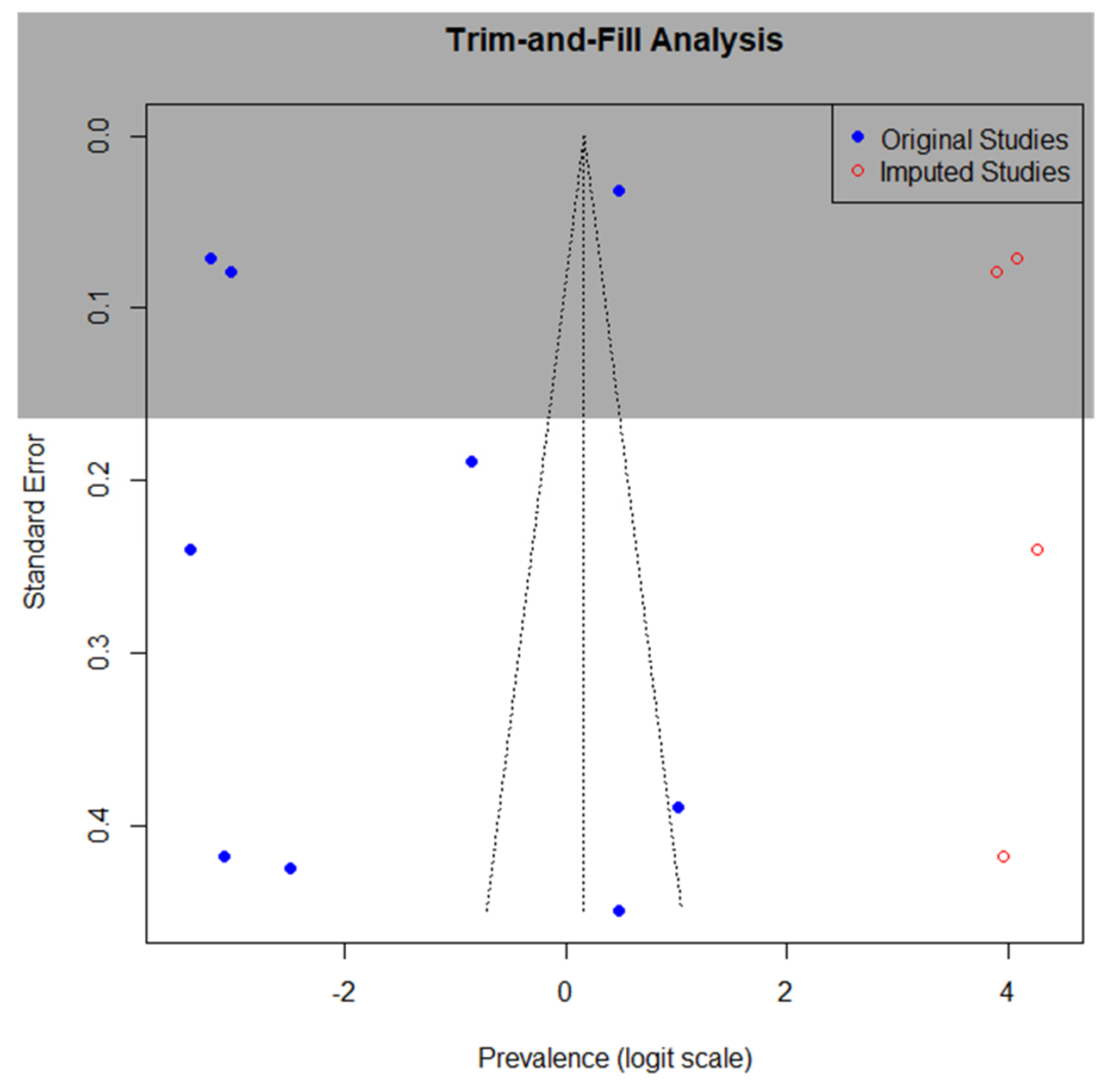
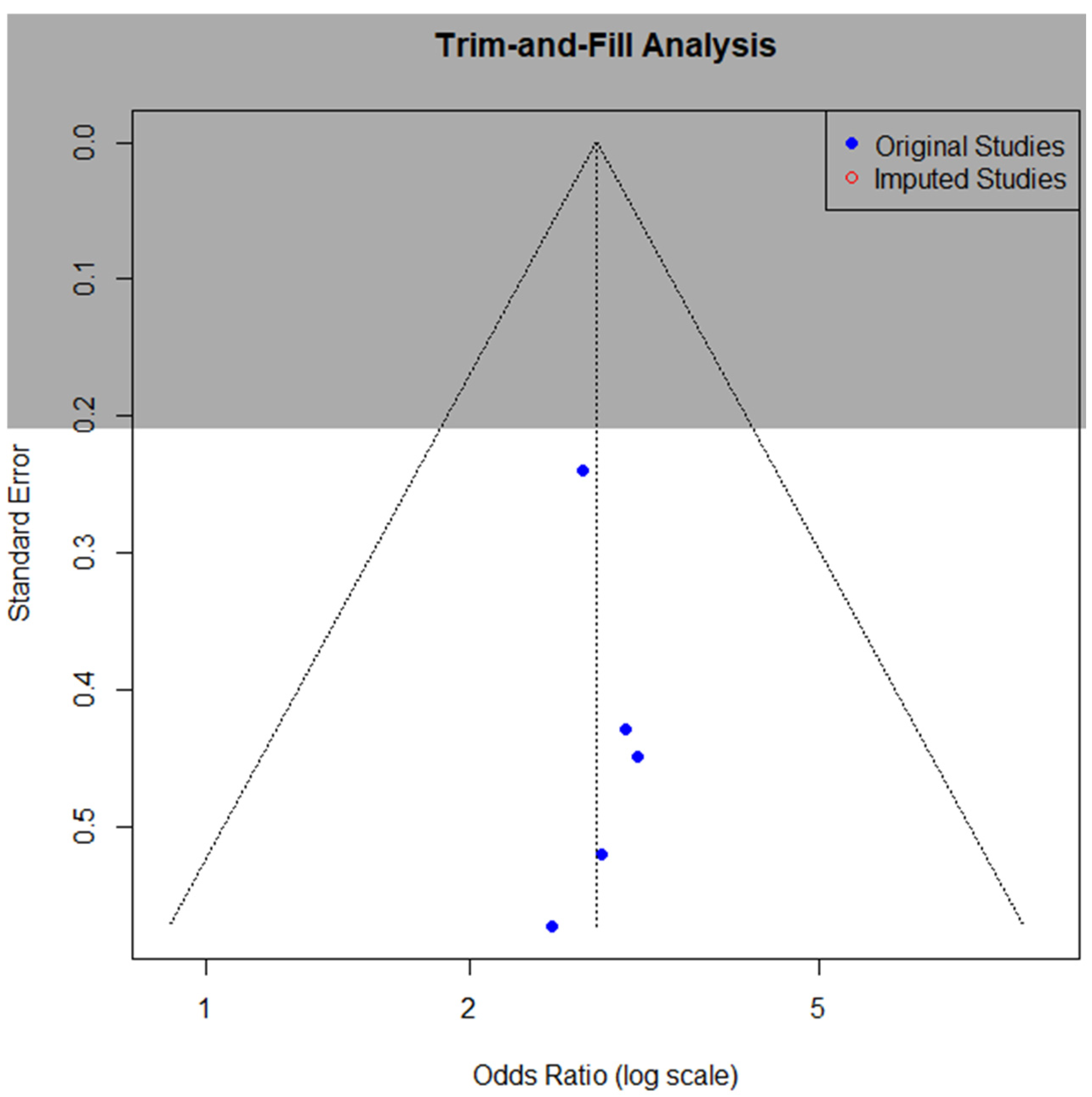
3.7. Publication Bias
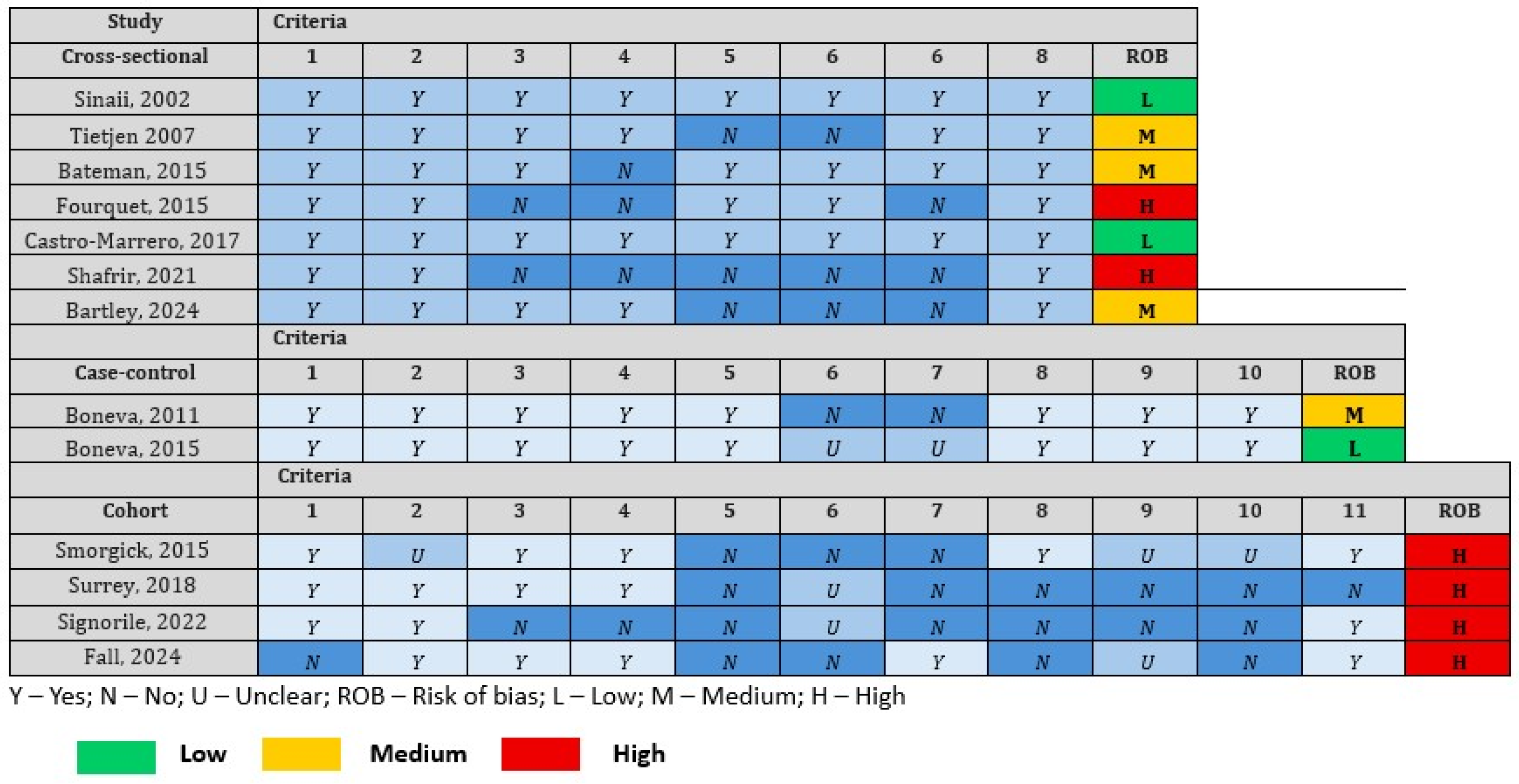
3.8. Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ME/CFS | Myalgic Encephalomyelitis/Chronic Fatigue Syndrome |
| JBI | Joanna Briggs Institute |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| HPA | Hypothalamic–Pituitary–Adrenal |
| IL | Interleukin |
| TNF | Tumor Necrosis Factor |
| ICD-9-CM | International Classification of Diseases, 9th Revision, Clinical Modification |
| CDC-SI | Centers for Disease Control and Prevention Symptom Inventory |
References
- Lim, E.-J.; Son, C.-G. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2020, 18, 289. [Google Scholar] [CrossRef]
- Vardaman, M.; Gilmour, S. Letter: Time to correct the record on the global burden of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2025, 23, 331. [Google Scholar] [CrossRef]
- Jason, L.A.; Mirin, A.A. Updating the National Academy of Medicine ME/CFS prevalence and economic impact figures to account for population growth and inflation. Fatigue Biomed. Health Behav. 2021, 9, 9–13. [Google Scholar] [CrossRef]
- Hanson, M.R. The viral origin of myalgic encephalomyelitis/chronic fatigue syndrome. PLoS Pathog. 2023, 19, e1011523. [Google Scholar] [CrossRef] [PubMed]
- Rohrhofer, J.; Hauser, L.; Lettenmaier, L.; Lutz, L.; Koidl, L.; Gentile, S.A.; Ret, D.; Stingl, M.; Untersmayr, E. Immunological Patient Stratification in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Clin. Med. 2024, 13, 275. [Google Scholar] [CrossRef]
- Kemp, J.; Sunnquist, M.; Jason, L.A.; Newton, J.L. Autonomic dysfunction in myalgic encephalomyelitis and chronic fatigue syndrome: Comparing self-report and objective measures. Clin. Auton. Res. 2019, 29, 475–477. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Taylor, H.S.; Adamson, G.D.; Diamond, M.P.; Goldstein, S.R.; Horne, A.W.; Missmer, S.A.; Snabes, M.C.; Surrey, E.; Taylor, R.N. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int. J. Gynecol. Obstet. 2018, 142, 131–142. [Google Scholar] [CrossRef]
- Tang, W.-Z.; Cai, Q.-Y.; Huang, K.-J.; Xu, W.-Z.; Li, J.-Z.; Pan, Y.-R.; Xu, H.-Y.; Zhao, Y.-F.; Sheng, T.-H.; Li, Z.-M.; et al. The global burden of polycystic ovary syndrome, endometriosis, uterine fibroids, cervical cancer, uterine cancer, and ovarian cancer from 1990 to 2021. BMC Public Health 2025, 25, 1774. [Google Scholar] [CrossRef]
- Kuan, K.K.W.; Heinzl, F.; Horne, A.W.; Whitaker, L.H.R.; Heine, J.; Bekos, C. Perceived effectiveness of endometriosis therapies on fatigue: An international survey. Reprod. Fertil. 2025, 6, e250010. [Google Scholar] [CrossRef]
- Mokhtari, T.; Irandoost, E.; Sheikhbahaei, F. Stress, pain, anxiety, and depression in endometriosis–Targeting glial activation and inflammation. Int. Immunopharmacol. 2024, 132, 111942. [Google Scholar] [CrossRef]
- Xu, X.; Mei, J.; Zhang, B.; Jiang, X.; Wang, L.; Zhang, A.; Li, J.; Chen, S.; He, Y.; Fang, Y.; et al. Association Between Circulating Cytokines and Endometriosis: A Mendelian Randomization Study. J. Cell Mol. Med. 2025, 29, e70532. [Google Scholar] [CrossRef]
- Corbitt, M.; Eaton-Fitch, N.; Staines, D.; Cabanas, H.; Marshall-Gradisnik, S. A systematic review of cytokines in chronic fatigue syndrome/myalgic encephalomyelitis/systemic exertion intolerance disease (CFS/ME/SEID). BMC Neurol. 2019, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Miller, A.H. Malaise, melancholia and madness: The evolutionary legacy of an inflammatory bias. Brain Behav. Immun. 2013, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zhang, W.; Leng, J.; Lang, J. Research on central sensitization of endometriosis-associated pain: A systematic review of the literature. J. Pain Res. 2019, 12, 1447–1456. [Google Scholar] [CrossRef]
- Wirth, K.J.; Löhn, M. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Comorbidities: Linked by Vascular Pathomechanisms and Vasoactive Mediators? Medicina 2023, 59, 978. [Google Scholar] [CrossRef]
- Álvarez-Salvago, F.; Lara-Ramos, A.; Cantarero-Villanueva, I.; Mazheika, M.; Mundo-López, A.; Galiano-Castillo, N.; Fernández-Lao, C.; Arroyo-Morales, M.; Ocón-Hernández, O.; Artacho-Cordón, F. Chronic fatigue, physical impairments and quality of life in women with endometriosis: A case-control study. Int. J. Environ. Res. Public Health 2020, 17, 3610. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Board on the Health of Select Populations and Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press: Washington, DC, USA, 2015; ISBN 0-309-31689-8. [Google Scholar]
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. JBI Manual for Evidence Synthesis; JBI: Kingston, Jamaica, 2024. [Google Scholar]
- Sinaii, N.; Cleary, S.D.; Ballweg, M.L.; Nieman, L.K.; Stratton, P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum. Reprod. 2002, 17, 2715–2724. [Google Scholar] [CrossRef]
- Tietjen, G.E.; Bushnell, C.D.; Herial, N.A.; Utley, C.; White, L.; Hafeez, F. Endometriosis is associated with prevalence of comorbid conditions in migraine: CME. Headache 2007, 47, 1069–1078. [Google Scholar] [CrossRef]
- Boneva, R.S.; Maloney, E.M.; Lin, J.-M.; Jones, J.F.; Wieser, F.; Nater, U.M.; Heim, C.M.; Reeves, W.C. Gynecological history in chronic fatigue syndrome: A population-based case-control study. J. Women’s Health 2011, 20, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Smorgick, N.; Marsh, C.A.; As-Sanie, S.; Smith, Y.R.; Quint, E.H. Prevalence of Pain Syndromes, Mood Conditions, and Asthma in Adolescents and Young Women with Endometriosis. J. Pediatr. Adolesc. Gynecol. 2013, 26, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.N.; Smith, M.A. Validation of ICD-9 Codes for Identification of Chronic Overlapping Pain Conditions. J. Pain Palliat. Care Pharmacother. 2022, 36, 166–177. [Google Scholar] [CrossRef]
- Bateman, L.; Darakjy, S.; Klimas, N.; Peterson, D.; Levine, S.M.; Allen, A.; Carlson, S.A.; Balbin, E.; Gottschalk, G.; March, D. Chronic fatigue syndrome and co-morbid and consequent conditions: Evidence from a multi-site clinical epidemiology study. Fatigue Biomed. Health Behav. 2015, 3, 1–15. [Google Scholar] [CrossRef]
- Boneva, R.S.; Lin, J.-M.S.; Unger, E.R. Early menopause and other gynecologic risk indicators for chronic fatigue syndrome in women. Menopause 2015, 22, 826–834. [Google Scholar] [CrossRef]
- Fourquet, J.; Sinaii, N.; Stratton, P.; Khayel, F.; Alvarez-Garriga, C.; Bayona, M.; Ballweg, M.L.; Flores, I. Characteristics of women with endometriosis from the USA and Puerto Rico. J. Endometr. 2015, 7, 129–135. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Palmor, M.C.; Fourquet, J.; DiVasta, A.D.; Farland, L.V.; Vitonis, A.F.; Harris, H.R.; Laufer, M.R.; Cramer, D.W.; Terry, K.L.; et al. Co-occurrence of immune-mediated conditions and endometriosis among adolescents and adult women. Am. J. Reprod. Immunol. 2021, 86, e13404. [Google Scholar] [CrossRef]
- Signorile, P.G.; Cassano, M.; Viceconte, R.; Marcattilj, V.; Baldi, A. Endometriosis: A Retrospective Analysis of Clinical Data from a Cohort of 4,083 Patients, with Focus on Symptoms. Vivo 2022, 36, 874–883. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Faro, M.; Aliste, L.; Sáez-Francàs, N.; Calvo, N.; Martínez-Martínez, A.; de Sevilla, T.F.; Alegre, J. Comorbidity in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: A Nationwide Population-Based Cohort Study. Psychosomatics 2017, 58, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Surrey, E.S.; Soliman, A.M.; Johnson, S.J.; Davis, M.; Castelli-Haley, J.; Snabes, M.C. Risk of Developing Comorbidities Among Women with Endometriosis: A Retrospective Matched Cohort Study. J. Women’s Health 2018, 27, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Alappattu, M.J.; Manko, K.; Lewis, H.; Vasilopoulos, T.; Lamvu, G. Presence of endometriosis and chronic overlapping pain conditions negatively impacts the pain experience in women with chronic pelvic–abdominal pain: A cross-sectional survey. Women’s Health 2024, 20, 17455057241248017. [Google Scholar] [CrossRef]
- Fall, E.A.; Chen, Y.; Lin, J.-M.S.; Issa, A.; Brimmer, D.J.; Bateman, L.; Lapp, C.W.; Podell, R.N.; Natelson, B.H.; Kogelnik, A.M.; et al. Chronic Overlapping Pain Conditions in people with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A sample from the Multi-site Clinical Assessment of ME/CFS (MCAM) study. BMC Neurol. 2024, 24, 399. [Google Scholar] [CrossRef]
- Strauss, B.; Löschau, M.; Seidel, T.; Stallmach, A.; Thomas, A. Are fatigue symptoms and chronic fatigue syndrome following Q fever infection related to psychosocial variables? J. Psychosom. Res. 2012, 72, 300–304. [Google Scholar] [CrossRef]
- Murovska, M.L.; Stevens, S. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2017, 282, 353. [Google Scholar] [CrossRef]
- Son, C.-G. Differential diagnosis between “chronic fatigue” and “chronic fatigue syndrome”. Integr. Med. Res. 2019, 8, 89–91. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- DiBenedetti, D.; Soliman, A.M.; Gupta, C.; Surrey, E.S. Patients’ perspectives of endometriosis-related fatigue: Qualitative interviews. J. Patient-Rep. Outcomes 2020, 4, 33. [Google Scholar] [CrossRef]
- Jason, L.A.; Gaglio, C.L.; Furst, J.; Islam, M.; Sorenson, M.; Conroy, K.E.; Katz, B.Z. Cytokine network analysis in a community-based pediatric sample of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chronic Illn. 2023, 19, 571–580. [Google Scholar] [CrossRef]
- Miller, J.E.; Ahn, S.H.; Marks, R.M.; Monsanto, S.P.; Fazleabas, A.T.; Koti, M.; Tayade, C. IL-17A Modulates Peritoneal Macrophage Recruitment and M2 Polarization in Endometriosis. Front. Immunol. 2020, 11, 108. [Google Scholar] [CrossRef]
- Shi, J.-L.; Zheng, Z.-M.; Chen, M.; Shen, H.-H.; Li, M.-Q.; Shao, J. IL-17: An important pathogenic factor in endometriosis. Int. J. Med. Sci. 2022, 19, 769–778. [Google Scholar] [CrossRef]
- Akoum, A.; Al-Akoum, M.; Lemay, A.; Maheux, R.; Leboeuf, M. Imbalance in the peritoneal levels of interleukin 1 and its decoy inhibitory receptor type II in endometriosis women with infertility and pelvic pain. Fertil. Steril. 2008, 89, 1618–1624. [Google Scholar] [CrossRef]
- Hornig, M.; Montoya, J.G.; Klimas, N.G.; Levine, S.; Felsenstein, D.; Bateman, L.; Peterson, D.L.; Gottschalk, C.G.; Schultz, A.F.; Che, X.; et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci. Adv. 2015, 1, e1400121. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-M.; Ma, Z.-Y.; Song, N. Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2513–2518. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.T.; Redden, C.R.; Raj, K.; Arcanjo, R.B.; Stasiak, S.; Li, Q.; Steelman, A.J.; Nowak, R.A. Endometriosis leads to central nervous system-wide glial activation in a mouse model of endometriosis. J. Neuroinflammat. 2023, 20, 59. [Google Scholar] [CrossRef]
- Bansal, A.S.; Seton, K.A.; Brooks, J.C.W.; Carding, S.R. Cognitive Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome-Aetiology and Potential Treatments. Int. J. Mol. Sci. 2025, 26, 1896. [Google Scholar] [CrossRef]
- Ortiz, R.; Gemmill, J.A.L.; Sinaii, N.; Stegmann, B.; Khachikyan, I.; Chrousos, G.; Segars, J.; Stratton, P. Hypothalamic-Pituitary-Adrenal Axis Responses in Women with Endometriosis-Related Chronic Pelvic Pain. Reprod. Sci. Thousand Oaks Calif. 2020, 27, 1839–1847. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kang, J.-Y.; Park, S.-Y.; Hwang, S.-J.; Bae, S.-J.; Son, C.-G. Central 5-HTergic hyperactivity induces myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-like pathophysiology. J. Transl. Med. 2024, 22, 34. [Google Scholar] [CrossRef]
- Sabbadin, C.; Saccardi, C.; Andrisani, A.; Vitagliano, A.; Marin, L.; Ragazzi, E.; Bordin, L.; Ambrosini, G.; Armanini, D. Role of Renin-Angiotensin-Aldosterone System and Cortisol in Endometriosis: A Preliminary Report. Int. J. Mol. Sci. 2022, 24, 310. [Google Scholar] [CrossRef]
- Taccori, A.; Maksoud, R.; Eaton-Fitch, N.; Patel, M.; Marshall-Gradisnik, S. A systematic review and meta-analysis of urinary biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2023, 21, 440. [Google Scholar] [CrossRef]
- Greygoose, E.; Metharom, P.; Kula, H.; Seckin, T.K.; Seckin, T.A.; Ayhan, A.; Yu, Y. The Estrogen–Immune Interface in Endometriosis. Cells 2025, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- McNamara, H.C.; Frawley, H.C.; Donoghue, J.F.; Readman, E.; Healey, M.; Ellett, L.; Reddington, C.; Hicks, L.J.; Harlow, K.; Rogers, P.A.W.; et al. Peripheral, Central, and Cross Sensitization in Endometriosis-Associated Pain and Comorbid Pain Syndromes. Front. Reprod. Health 2021, 3, 729642. [Google Scholar] [CrossRef]
- Nacul, L.; Authier, F.J.; Scheibenbogen, C.; Lorusso, L.; Helland, I.B.; Martin, J.A.; Sirbu, C.A.; Mengshoel, A.M.; Polo, O.; Behrends, U.; et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): Expert Consensus on the Diagnosis, Service Provision, and Care of People with ME/CFS in Europe. Medicina 2021, 57, 510. [Google Scholar] [CrossRef] [PubMed]

| Study | Country | Study Design | Criteria for Endometriosis | Criteria for ME/CFS | Sample Size |
|---|---|---|---|---|---|
| ME/CFS in Patients with Endometriosis | |||||
| Sinaii, 2002 [23] | USA | Cross-sectional | Surgical diagnosis | Physician diagnosis | 3680 |
| Tietjen 2007 [24] | USA | Cross-sectional | Self-reported | Self-reported | 275 |
| Boneva, 2011 [25] | USA | Case–control | Self-reported | Fukuda 1994 [19] | 84 |
| Smorgick, 2013 [26] | USA | Retrospective cohort | Surgical diagnosis | ICD-9-CM [27] | 138 |
| Bateman, 2015 [28] | USA | Cross-sectional | Self-reported | Fukuda 1994 [19] | 960 |
| Boneva, 2015 [29] | USA | Case–control | Self-reported | Fukuda 1994 [19] | 157 |
| Fourquet, 2015 [30] | USA/PR | Cross-sectional | Self-reported | Self-reported | 4358 |
| Shafrir, 2021 [31] | USA | Cross-sectional | Surgical diagnosis | Self-reported | 1203 |
| Signorile, 2022 [32] | Italy | Retrospective cohort | Self-reported | Physical exam, MRI | 4083 |
| Endometriosis in Patients with ME/CFS | |||||
| Castro-Marrero, 2017 [33] | Spain | Cross-sectional | Surgical | Fukuda 1994 [19] | 320 |
| Surrey, 2018 [34] | USA | Retrospective cohort | Clinically diagnosed | Clinical diagnosis | 134,805 |
| Bartley, 2024 [35] | USA | Cross-sectional | Self-reported | Self-reported | 525 |
| Fall, 2024 [36] | USA | Cross-sectional | Self-reported | CDC-SI [37] | 923 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Compton, S.; Alkabalan, R.; Cadet, J.; Mastali, A.; Ramdass, P.V.A.K. Endometriosis and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 2332. https://doi.org/10.3390/diagnostics15182332
Compton S, Alkabalan R, Cadet J, Mastali A, Ramdass PVAK. Endometriosis and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(18):2332. https://doi.org/10.3390/diagnostics15182332
Chicago/Turabian StyleCompton, Sabrina, Rodolf Alkabalan, Judd Cadet, Azin Mastali, and Prakash V. A. K. Ramdass. 2025. "Endometriosis and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review and Meta-Analysis" Diagnostics 15, no. 18: 2332. https://doi.org/10.3390/diagnostics15182332
APA StyleCompton, S., Alkabalan, R., Cadet, J., Mastali, A., & Ramdass, P. V. A. K. (2025). Endometriosis and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review and Meta-Analysis. Diagnostics, 15(18), 2332. https://doi.org/10.3390/diagnostics15182332