CT-Based Morphometric Analysis of Zygomaticomaxillary Suture Symmetry: Implications for Diagnostic Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.2. Image Acquisition Protocol
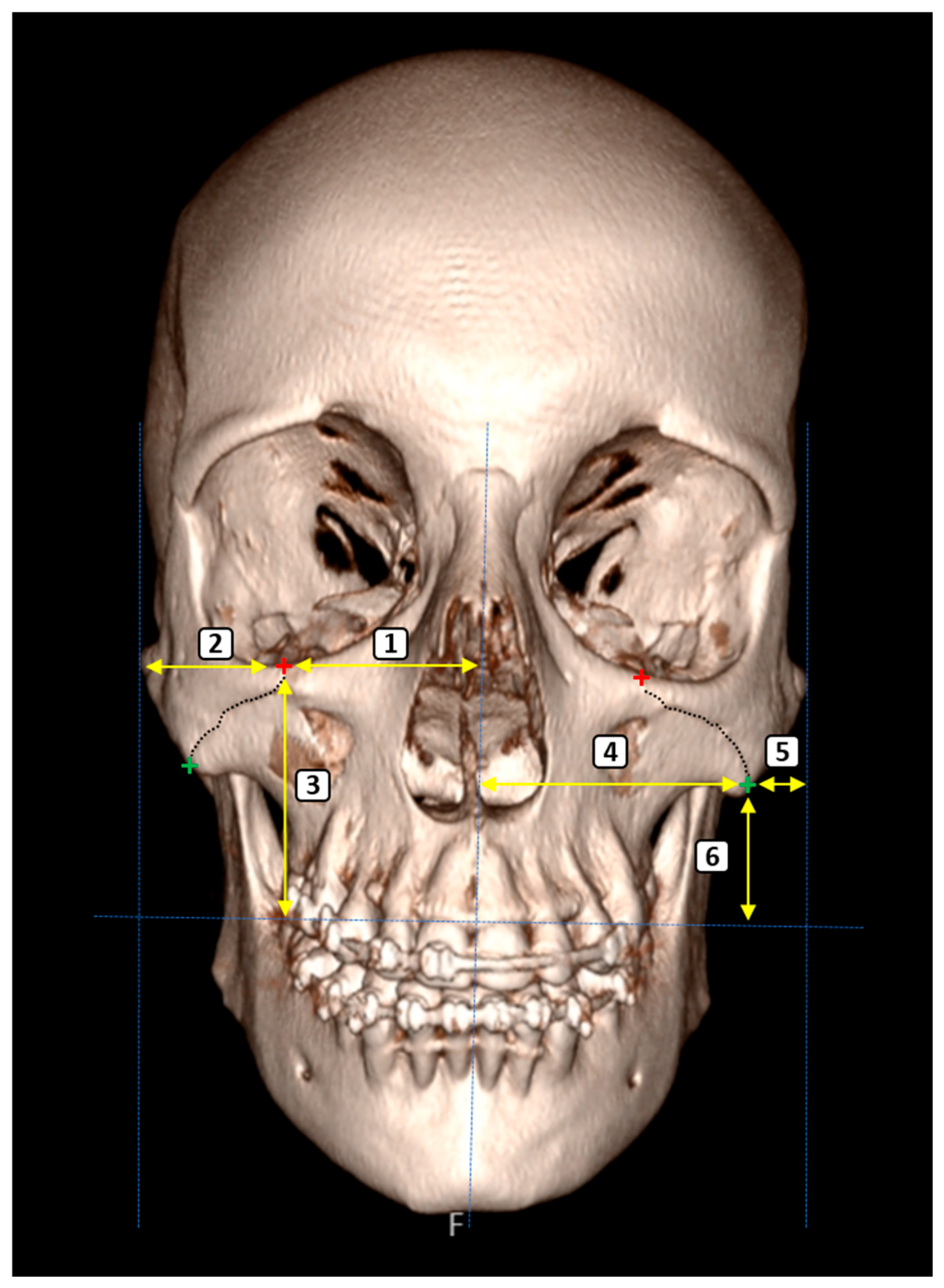
2.3. Morphometric Parameters
- ZMS-Related Measurements
- ZMS–Midline Distance (Superior): The horizontal distance from the superimargin of the ZMS at the infraorbital rim to the midsagittal plane.
- ZMS–Lateral Zygomatic Border (Superior): The distance between the superior ZMS and the lateral edge of the zygomatic bone.
- ZMS–Supradentale Plane (Superior): The vertical distance from the superior ZMS to the horizontal plane passing through the supradentale.
- ZMS–Midline Distance (Inferior): The horizontal distance from the inferior margin of the ZMS, located along the inferior zygomatic border, to the midsagittal plane.
- ZMS–Lateral Zygomatic Border (Inferior): The distance from the inferior ZMS to the lateral zygomatic border.
- ZMS–Supradentale Plane (Inferior): The vertical distance between the inferior ZMS and the horizontal plane aligned with the supradentale.
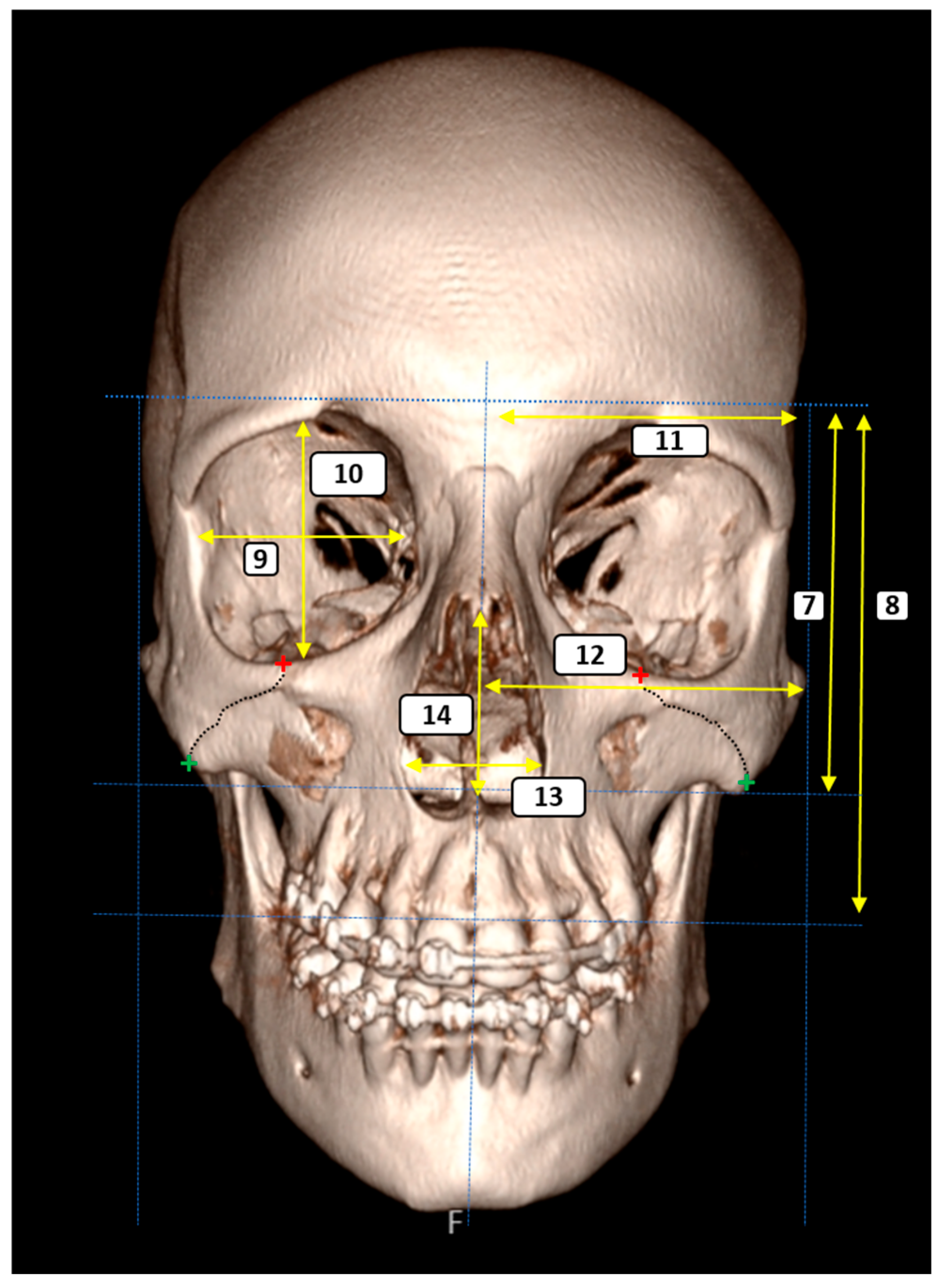
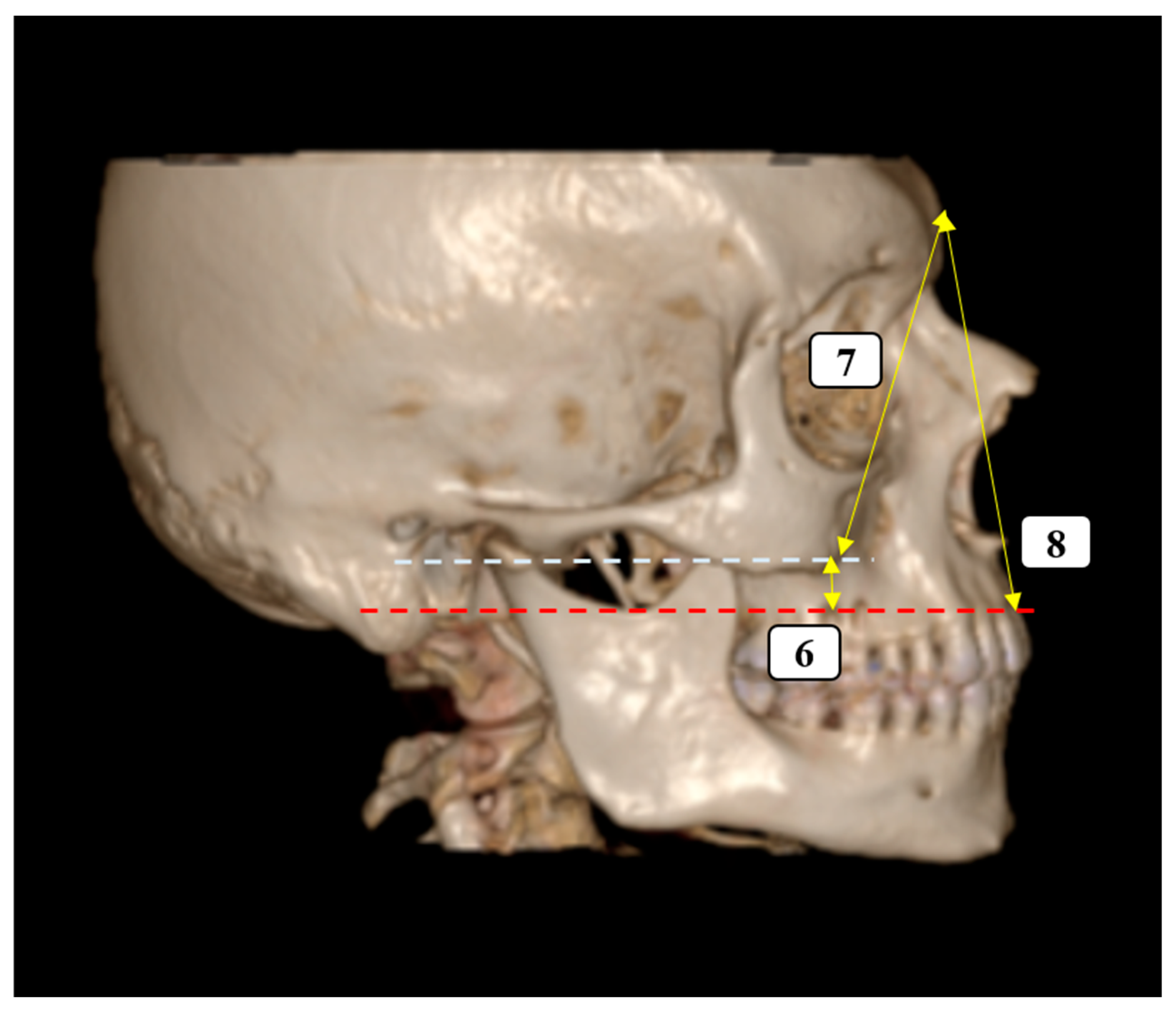
- Vertical Facial Proportions
- 7.
- Glabella–Inferior Zygomatic Plane Distance: The vertical distance between the horizontal planes passing through the glabella and the inferior zygomatic border.
- 8.
- Glabella–Supradentale Plane Distance: The vertical distance between the glabella and supradentale reference planes.
- Orbital Morphometry
- 9.
- Orbital Width: The maximum horizontal diameter of the orbit.
- 10.
- Orbital Height: The maximum vertical diameter of the orbit.
- Transverse Craniofacial Dimensions
- 11.
- Eurion–Midline Distance (bilateral): The distance from the eurion point (maximum cranial breadth) to the midsagittal plane.
- 12.
- Zygion–Midline Distance (bilateral): The distance from the zygion point (maximum facial breadth) to the midsagittal plane.
- Piriform Aperture Morphometry
- 13.
- Piriform Aperture Width: The distance between the alare points.
- 14.
- Piriform Aperture Height: The vertical distance between the nasospinale and rhinion.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ZMS | Zygomaticomaxillary suture |
| CT | Computed Tomography |
References
- Babacan, S.; Deniz, M. The harmony and balance of the facial organs for a natural face beauty: A novel perspective for cosmetic/aesthetic interventions. Medicina 2025, 61, 958. [Google Scholar] [CrossRef]
- Franke, A.; Hofmann, E.C.; Steinberg, A.; Lauer, G.; Kitzler, H.; Leonhardt, H. Probing real-world Central European population midfacial skeleton symmetry for maxillofacial surgery. Clin. Oral Investig. 2023, 27, 5637–5647. [Google Scholar] [CrossRef] [PubMed]
- Sholts, S.B.; Wärmländer, S.K. Zygomaticomaxillary suture shape analyzed with digital morphometrics: Reassessing patterns of variation in American Indian and European populations. Forensic Sci. Int. 2012, 217, 234.e1–234.e6. [Google Scholar] [CrossRef]
- Damstra, J.; Fourie, Z.; De Wit, M.; Ren, Y. A three-dimensional comparison of a morphometric and conventional cephalometric midsagittal planes for craniofacial asymmetry. Clin. Oral Investig. 2012, 16, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Captier, G.; Lethuilier, J.; Oussaid, M.; Canovas, F.; Bonnel, F. Neural symmetry and functional asymmetry of the mandible. Surg. Radiol. Anat. 2006, 28, 379–386. [Google Scholar] [CrossRef]
- Dubron, K.; Yang, L.H.; Jacobs, R.; Politis, C.; Willaert, R.; Shaheen, E. Symmetry recovery in zygomaticomaxillary complex fractures compared to normal unfractured population: A new reliable 3D evaluation. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101857. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; Matschke, J.B.; Bučkova, M.; Rahrisch, L.; Lauer, G.; Leonhardt, H. Symmetry-based analysis after surgical treatment of zygomaticomaxillary complex fractures using intraoperative cone-beam computed tomography: A retrospective case-control study. Sci. Rep. 2025, 15, 5898. [Google Scholar] [CrossRef]
- Bao, T.; Yu, D.; Luo, Q.; Wang, H.; Liu, J.; Zhu, H. Quantitative assessment of symmetry recovery in navigation-assisted surgical reduction of zygomaticomaxillary complex fractures. J. Craniomaxillofac. Surg. 2019, 47, 311–319. [Google Scholar] [CrossRef]
- Emara, A.; ElFetouh, A.A.; Hakam, M.; Mostafa, B. Midfacial reconstruction—A systematic review. Open Access Maced. J. Med. Sci. 2016, 4, 468–475. [Google Scholar] [CrossRef]
- Wilkat, M.; Kübler, N.; Rana, M. Advances in the resection and reconstruction of midfacial tumors through computer assisted surgery. Front. Oncol. 2021, 11, 719528. [Google Scholar] [CrossRef]
- Kessler, P.A.W.H.; Timmer, V.C.M.L.; Lie, S.A.N. Symmetry of the face: Review of a complex matter. J. Oral. Maxillofac. Surg. 2025, 37, 397–405. [Google Scholar] [CrossRef]
- Armengou, X.; Frank, K.; Kaye, K.; Brébant, V.; Möllhoff, N.; Cotofana, S.; Alfertshofer, M. Facial anthropometric measurements and principles—Overview and implications for aesthetic treatments. Facial Plast. Surg. 2024, 40, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Min, J.; Choi, J.W.; Ra, Y.S. Facial and cranial symmetry after one-piece fronto-orbital advancement with distraction for isolated unilateral coronal synostosis. Plast. Reconstr. Surg. 2023, 151, 1275–1284. [Google Scholar] [CrossRef]
- Silinevica, S.; Lokmane, K.; Vuollo, V.; Jakobsone, G.; Pirttiniemi, P. The association between dental and facial symmetry in adolescents. Am. J. Orthod. Dentofac. Orthop. 2023, 164, 340–350. [Google Scholar] [CrossRef]
- Kaur, P.; Krishan, K.; Sharma, S.K.; Kanchan, T. Integrating a profile of frontal face with its mirror image for facial reconstruction. J. Craniofac. Surg. 2018, 29, 1026–1030. [Google Scholar] [CrossRef]
- de Kort, W.W.B.; van Hout, W.M.M.T.; Ten Harkel, T.C.; van Cann, E.M.; Rosenberg, A.J.W.P. A novel method for quantitative three-dimensional analysis of zygomatico-maxillary complex symmetry. J. Craniofac. Surg. 2022, 33, 1474–1478. [Google Scholar] [CrossRef]
- Gibelli, D.; Cellina, M.; Gibelli, S.; Oliva, A.G.; Termine, G.; Pucciarelli, V.; Dolci, C.; Sforza, C. Assessing symmetry of zygomatic bone through three-dimensional segmentation on computed tomography scan and “mirroring” procedure: A contribution for reconstructive maxillofacial surgery. J. Craniomaxillofac. Surg. 2018, 46, 600–604. [Google Scholar] [CrossRef]
- Benedetti, S.; Frosolini, A.; Cascino, F.; Pignataro, L.V.; Franz, L.; Marioni, G.; Gabriele, G.; Gennaro, P. Computer-assisted evaluation of zygomatic fracture outcomes: Case series and proposal of a reproducible workflow. Tomography 2025, 11, 19. [Google Scholar] [CrossRef]
- Morgan, N.; Shujaat, S.; Jazil, O.; Jacobs, R. Three-dimensional quantification of skeletal midfacial complex symmetry. Int. J. Comput. Assist. Radiol. Surg. 2023, 18, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Moreddu, E.; Puymerail, L.; Michel, J.; Achache, M.; Dessi, P.; Adalian, P. Morphometric measurements and sexual dimorphism of the piriform aperture in adults. Surg. Radiol. Anat. 2013, 35, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Sarač Hadžihalilović, A.; Ajanović, Z.; Hasanbegović, I.; Šljuka, S.; Rakanović Todić, M.; Aganović, I.; Prazina, I.; Maleškić Kapo, S.; Hadžiselimović, R. Analysis of gender differences on pyriform aperture of human skulls using geometric morphometric method. Folia Morphol. 2022, 81, 707–714. [Google Scholar] [CrossRef]
- Toneva, D.; Nikolova, S.; Tasheva-Terzieva, E.; Zlatareva, D.; Lazarov, N. A geometric morphometric study on sexual dimorphism in viscerocranium. Biology 2022, 11, 1333. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, X.; Long, H.; Lai, W. Quantitative analysis of facial symmetry by using three-dimensional technology. BMC Oral Health 2022, 22, 277. [Google Scholar] [CrossRef]
- Cao, H.L.; Kang, M.H.; Lee, J.Y.; Park, W.J.; Choung, H.W.; Choung, P.H. Quantification of three-dimensional facial asymmetry for diagnosis and postoperative evaluation of orthognathic surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 17. [Google Scholar] [CrossRef]
- Djordjevic, J.; Toma, A.M.; Zhurov, A.I.; Richmond, S. Three-dimensional quantification of facial symmetry in adolescents using laser surface scanning. Eur. J. Orthod. 2014, 36, 125–132. [Google Scholar] [CrossRef]
- Lum, V.; Goonewardene, M.S.; Mian, A.; Eastwood, P. Three-dimensional assessment of facial asymmetry using dense correspondence, symmetry, and midline analysis. Am. J. Orthod. Dentofac. Orthop. 2020, 158, 134–146. [Google Scholar] [CrossRef]
- Pierrefeu, A.; Terzic, A.; Volz, A.; Courvoisier, D.; Scolozzi, P. How accurate is the treatment of midfacial fractures by a specific navigation system integrating “mirroring” computational planning? Beyond mere average difference analysis. J. Oral Maxillofac. Surg. 2015, 73, 315.e1–315.e10. [Google Scholar] [CrossRef] [PubMed]
- Scolozzi, P.; Terzic, A. “Mirroring” computational planning, navigation guidance system, and intraoperative mobile C-arm cone-beam computed tomography with flat-panel detector: A new rationale in primary and secondary treatment of midfacial fractures? J. Oral Maxillofac. Surg. 2011, 69, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Terzic, A.; Scolozzi, P. Image guided surgical navigation integrating “mirroring” computational planning based on intra-operative cone-beam CT imaging: A promising new approach for management of primary bilateral midfacial fractures. Comput. Aided Surg. 2011, 16, 170–180. [Google Scholar] [CrossRef]
- White, H.E.; Goswami, A.; Tucker, A.S. The intertwined evolution and development of sutures and cranial morphology. Front. Cell Dev. Biol. 2021, 9, 653579. [Google Scholar] [CrossRef] [PubMed]
| Female (n = 101) | Male (n = 99) | p Value Within Sex | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | p Value | Right | Left | p Value | Right | Left | |||||
| 1 | 33.00 (5.00) | 25.00–47.00 | 34.00 (4.50) | 25.00–46.00 | 0.496 | 38.00 (5.00) | 25.00–40.00 | 33.00 (5.00) | 25.00–43.00 | 0.657 | 0.954 | 0.956 |
| 2 | 32.0 (6.00) | 23.00–52.00 | 32.00 (5.00) | 24.00–56.00 | 0.892 | 33.00 (4.00) | 21.00–53.00 | 33.00 (5.00) | 20.00–53.00 | 0.749 | 0.024 | 0.046 |
| 3 | 45.43 ± 6.41 | 31.00–58.00 | 45.37 ± 6.10 | 32.00–59.00 | 0.946 | 45.97 ± 5.98 | 33.00–59.00 | 45.97 ± 5.99 | 29.00–59.00 | 0.895 | 0.536 | 0.401 |
| 4 | 48.33 ± 7.48 | 31.00–67.00 | 45.82 ± 7.50 | 32.00–65.00 | 0.851 | 47.84 ± 7.53 | 31.00–67.00 | 47.89 ± 7.55 | 31.00–69.00 | 0.962 | 0.646 | 0.551 |
| 5 | 9.00 (3.00) | 5.00–14.00 | 9.00 (4.00) | 4.00–16.00 | 0.805 | 9.00 (3.00) | 5.00–19.00 | 9.00 (3.00) | 4.00–19.00 | 0.361 | 0.287 | 0.202 |
| 6 | 28.00 (7.00) | 19.00–45.00 | 28.00 (8.00) | 20.00–47.00 | 0.998 | 29.00 (8.00) | 20.00–50.00 | 30.00 (7.00) | 20.00–50.00 | 0.971 | 0.428 | 0.491 |
| 7 | 59.00 (16.50) | 41.00–90.00 | 59.00 (16.50) | 42.00–91.00 | 0.854 | 59.00 (11.00) | 33.00–87.00 | 59.00 (9.00) | 29.00–89.00 | 0.592 | 0.719 | 0.824 |
| 8 | 79.00 (13.00) | 45.00–92.00 | 80.00 (13.50) | 45.00–91.00 | 0.410 | 80.00 (15.00) | 49.00–95.00 | 81.00 (14.00) | 48.00–95.00 | 0.533 | 0.194 | 0.122 |
| 9 | 42.00 (10.00) | 31.00–70.00 | 42.00 (9.50) | 33.00–72.00 | 0.500 | 42.00 (6.00) | 36.00–65.00 | 42.00 (6.00) | 6.00–65.00 | 0.883 | 0.428 | 0.683 |
| 10 | 43.00 (18.00) | 26.00–77.00 | 44.00 (16.00) | 29.00–79.00 | 0.757 | 43.00 (12.00) | 27.00–71.00 | 45.00 (13.00) | 27.00–72.00 | 0.717 | 0.577 | 0.475 |
| 11 | 55.00 (8.00) | 39.00–70.00 | 55.00 (7.50) | 39.00–72.00 | 0.839 | 55.00 (8.00) | 31.00–72.00 | 56.00 (7.00) | 31.00–74.00 | 0.558 | 0.994 | 0.709 |
| 12 | 52.54 ± 5.62 | 40.00–65.00 | 52.57 ± 5.93 | 39.00–69.00 | 0.971 | 52.57 ± 6.49 | 38.00–67.00 | 52.56 ± 6.30 | 36.00–67.00 | 0.981 | 0.768 | 0.759 |
| 13 | 25.00 (3.50) | 20.00–29.00 | - | 26.00 (3.00) | 21.00–29.00 | - | 0.017 | |||||
| 14 | 36.00 (3.00) | 29.00–40.00 | - | 36.00 (3.00) | 30.00–39.00 | - | 0.998 | |||||
| Female | |||||
|---|---|---|---|---|---|
| Formulas | Adjusted R2 | SE | Formulas | Adjusted R2 | SE |
| Right 1 = 4.21 + (0.862 × Left 1) | 0.821 | 1.591 | Left 1 = 1.93 + (0.954 × Right 1) | 0.821 | 1.674 |
| Right 2 = 2.11 + (0.933 × Left 2) | 0.930 | 1.694 | Left 2 = 0.24 + (0.998 × Right 2) | 0.930 | 1.752 |
| Right 3 = −0.499 + (1.01 × Left 3) | 0.927 | 1.732 | Left 3 = 3.37 + (0.916 × Right 3) | 0.927 | 1.648 |
| Right 4 = 1.23 + (0.97 × Left 4) | 0.946 | 1.738 | Left 4 = 1.38 + (0.975 × Right 4) | 0.946 | 1.742 |
| Right 5 = 3.07 + (0.638 × Left 5) | 0.605 | 1.442 | Left 5 = 0.62 + (0.954 × Right 5) | 0.605 | 1.764 |
| Right 6 = 1.77 + (0.935 × Left 6) | 0.931 | 1.533 | Left 6 = 0.24 + (0.996 × Right 6) | 0.931 | 1.582 |
| Right 7 = 1.54 + (0.97 × Left 7) | 0.981 | 1.604 | Left 7 = −0.351 + (1.011 × Right 7) | 0.981 | 1.638 |
| Right 8 = 0.34 + (0.988 × Left 8) | 0.976 | 1.666 | Left 8 = 1.49 + (0.988 × Right 8) | 0.976 | 1.666 |
| Right 9 = −2.02 + (1.038 × Left 9) | 0.961 | 1.544 | Left 9 = 3.59 + (0.926 × Right 9) | 0.961 | 1.459 |
| Right 10 = −0.199 + (0.996 × Left 10) | 0.964 | 1.809 | Left 10 = 1.85 + (0.969 × Right 10) | 0.964 | 1.785 |
| Right 11 = 5.36 + (0.896 × Left 11) | 0.803 | 2.642 | Left 11 = 5.75 + (0.898 × Right 11) | 0.803 | 2.644 |
| Right 12 = 5.75 + (0.890 × Left 12) | 0.881 | 1.939 | Left 12 = 0.50 + (0.991 × Right 12) | 0.881 | 2.046 |
| Male | |||||
|---|---|---|---|---|---|
| Formulas | Adjusted R2 | SE | Formulas | Adjusted R2 | SE |
| Right 1 = 35.57 + (−0.098 × Left 1) | 0.039 | 3.304 | Left 1 = 28.44 + (0.109 × Right 1) | 0.037 | 3.443 |
| Right 2 = 65.65 + (−0.876 × Left 2) | 0.084 | 5.418 | Left 2 = 25.10 + (0.318 × Right 2) | 0.091 | 5.371 |
| Right 3 = 1.11 + (0.973 × Left 3) | 0.921 | 1.678 | Left 3 = 2.53 + (0.947 × Right 3) | 0.921 | 1.656 |
| Right 4 = 1.42 + (0.969 × Left 4) | 0.942 | 1.814 | Left 4 = 1.36 + (0.972 × Right 4) | 0.942 | 1.817 |
| Right 5 = 2.72 + (0.680 × Left 5) | 0.648 | 1.404 | Left 5 = 0.74 + (0.958 × Right 5) | 0.648 | 1.667 |
| Right 6 = 1.79 + (0.937 × Left 6) | 0.930 | 1.489 | Left 6 = 0.30 + (0.993 × Right 6) | 0.930 | 1.533 |
| Right 7 = 3.07 + (0.944 × Left 7) | 0.976 | 1.640 | Left 7 = −1.67 + (1.034 × Right 7) | 0.976 | 1.716 |
| Right 8 = 0.533 + (0.984 × Left 8) | 0.977 | 1.666 | Left 8 = 1.23 + (0.993 × Right 8) | 0.977 | 1.674 |
| Right 9 = 10.58 + (0.765 × Left 9) | 0.734 | 3.592 | Left 9 = 1.46 + (0.963 × Right 9) | 0.734 | 4.032 |
| Right 10 = 1.18 + (0.946 × Left 10) | 0.958 | 1.766 | Left 10 = 0.72 + (0.995 × Right 10) | 0.958 | 1.794 |
| Right 11 = 2.62 + (0.943 × Left 11) | 0.933 | 1.561 | Left 11 = 1.03 + (0.99 × Right 11) | 0.935 | 1.574 |
| Right 12 = 0.50 + (0.991 × Left 12) | 0.923 | 1.795 | Left 12 = 3.51 + (0.933 × Right 12) | 0.923 | 1.742 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kırteke, A.; Gören, H.; Tuncel Çini, N. CT-Based Morphometric Analysis of Zygomaticomaxillary Suture Symmetry: Implications for Diagnostic Imaging. Diagnostics 2025, 15, 2330. https://doi.org/10.3390/diagnostics15182330
Kırteke A, Gören H, Tuncel Çini N. CT-Based Morphometric Analysis of Zygomaticomaxillary Suture Symmetry: Implications for Diagnostic Imaging. Diagnostics. 2025; 15(18):2330. https://doi.org/10.3390/diagnostics15182330
Chicago/Turabian StyleKırteke, Atakan, Hilal Gören, and Nilgün Tuncel Çini. 2025. "CT-Based Morphometric Analysis of Zygomaticomaxillary Suture Symmetry: Implications for Diagnostic Imaging" Diagnostics 15, no. 18: 2330. https://doi.org/10.3390/diagnostics15182330
APA StyleKırteke, A., Gören, H., & Tuncel Çini, N. (2025). CT-Based Morphometric Analysis of Zygomaticomaxillary Suture Symmetry: Implications for Diagnostic Imaging. Diagnostics, 15(18), 2330. https://doi.org/10.3390/diagnostics15182330







