Isolated Non-Progressive Hemidystonia in a Patient Homozygous for H63D Variant of Hereditary Hemochromatosis: A Case Report and Systematic Literature Review of Movement Disorders in Hereditary Hemochromatosis
Abstract
1. Introduction
2. Case Presentation
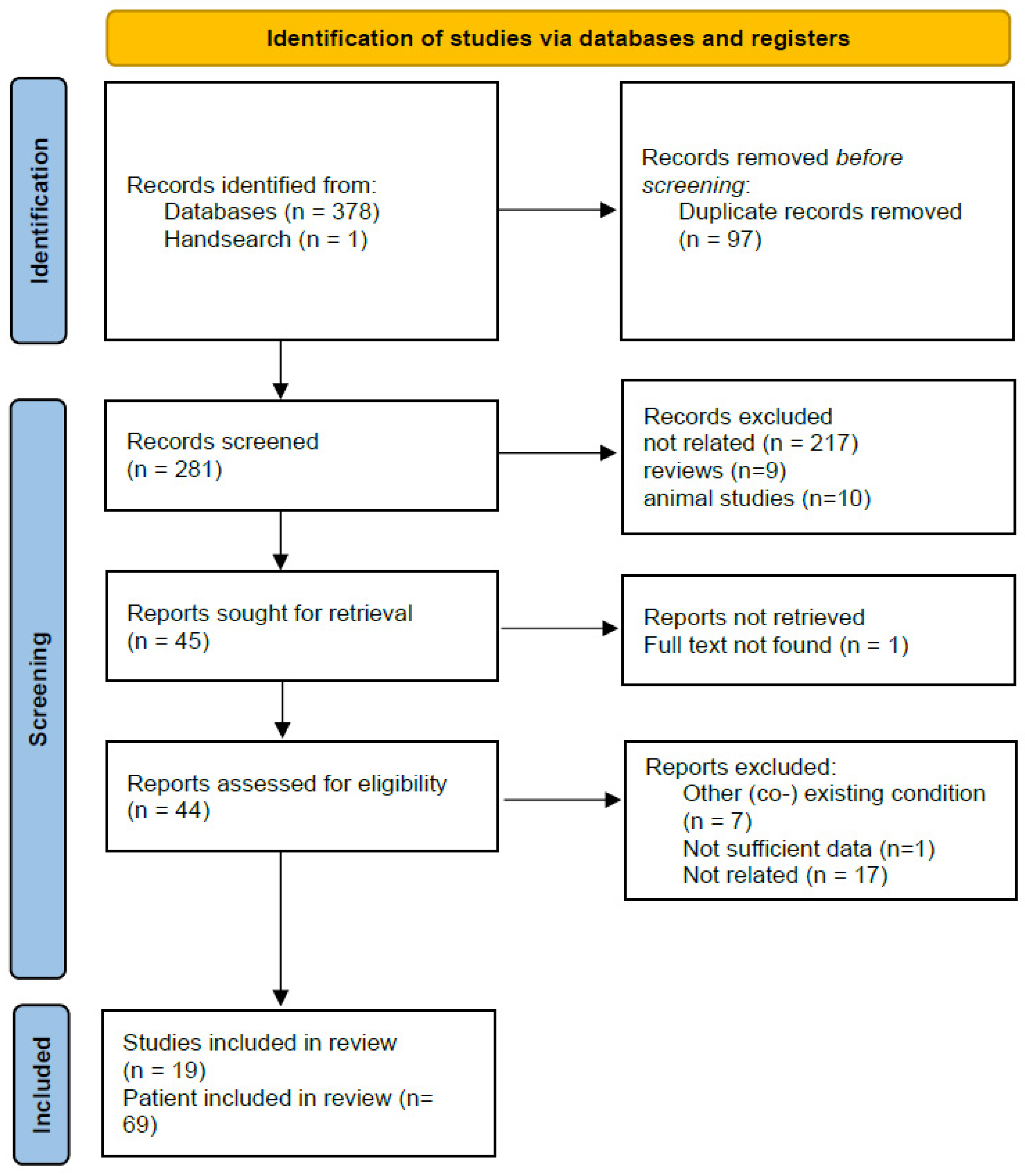
3. Materials and Methods
4. Discussion
| Authors, Year | Gender | Age at Presentation | Mutation | Movement Disorders | Other Neurological Symptoms | Presenting Symptom as Movement Disorder | Other Systemic Features | MRI Brain/Iron Deposition | Treatment | Outcome on Movement Disorder |
|---|---|---|---|---|---|---|---|---|---|---|
| Jones and Hedley-Whyte, 1983 [8] Case 1 | Male | 71 | n/a | Ataxia, rigidity, myoclonic jerks | dementia | no | Diabetes, hemochromatosis in liver biopsy | Basal ganglia calcification on CT | 40 gr protein/d, neomycin | died due to hepatic encephalopathy a few weeks later |
| Jones and Hedley-Whyte, 1983 [8] Case 2 | Male | 52 | n/a | Ataxia | Lethargy, dysarthria | no | Weight loss, skin pigmentation, hepatomegaly | n/a | Phlebotomies | Initial improvement, died after few months |
| Nielsen, 1995 [9] | Male | 50 | HLA-A2,3, B7, 44 heterozygosity | Left-sided parkinsonism, ataxia, postural and rest tremor | cognitive problems, headache, pyramidal weakness | yes | Fatigue, bronze skin pigmentation, heavy iron overload in liver, high ferritin and transferrin saturation | Reduced T2 signal/iron accumulation in the caudate, globus pallidus, red nucleus, substantia nigra, dentate nucleus | Levodopa Phlebotomies | Improvement of parkinsonism with levodopa |
| Demarquay et al., 2000 [7] | Male | 35 | Homozygous C282Y | Postural head and arm tremor, ataxia, cervical dystonia | Dysarthria | yes | Bronze skin pigmentation, heavy iron accumulation in liver biopsy | Moderate cerebellar atrophy | Phlebotomies, clonazepam, buspirone, trihexyphenidyl | No improvement with phlebotomy, little benefit of medication |
| Costello, 2004 [6] Case 1 | Male | 55 | Homozygous C282Y | resting tremor, parkinsonim | no | no | DM, elevated ferritin | n/a | Levodopa Phlebotomies | Good response to levodopa, no effect of phlebotomy |
| Costello, 2004 [6] Case 2 | Male | 61 | HLA A2A3B7B12 haplotype | Bilateral parkinsonism | no | no | DM II, abnormal skin pigmentation, elevated ferritin and transferrin saturation, cirrhosis on liver biopsy | Atrophy (not specified) on CT | Levodopa | Good response on levodopa |
| Costello, 2004 [6] Case 3 | Male | 63 | Homozygous C282Y | Lower limb and body tremor, bilateral parkinsonism | no | yes | Abnormal liver function tests, siderosis on liver biopsy, elevated ferritin | Normal brain MRI | Levodopa | Good response |
| Costello, 2004 [6] Case 4 | Female | 49 | Homozygous C282Y | Asymmetrical rest tremor and parkinsonism | no | no | DM II, elevated ferritin, heavy iron deposition on liver biopsy, angina | Cerebral infarcts on CT | Phlebotomies, levodopa | Iron overload corrected with phlebotomy/ Good response to levodopa |
| Dethy, 2004 [27] | Female | 62 | C282Y mutation | Restless legs, arm tremor, parkinsonism | no | yes | Increased ferritin, transferrin saturation, iron deposition on liver MRI | Normal brain MRI | Phlebotomies | After five phlebotomies neurological signs subsided |
| Haba-Rubio et al. 2004 [28] Case 1 | Female | 40 | Homozygous C282Y | Restless legs | no | yes | no | Reduced R2’ values (reduced iron level) in substantia nigra, red nucleus, pallidum | Phlebotomies, clonazepam | No improvement with phlebotomies, improvement with clonazepam |
| Haba-Rubio et al. 2004 [28] Case 2 | Male | 56 | Homozygous C282Y | Restless legs | Apathy, fatigue, lack of libido | no | Iron overload in liver biopsy | Reduced R2’ values (reduced iron level) in the substantia nigra, red nucleus, pallidum | Phlebotomies | Deterioration |
| Shaughnessy et al. 2005 [36] 10 patients | 7 Male | 53 (41–76) | Mainly C282Y homozygous | Restless legs | n/a | Yes (5 out of 10) | Low level ferritin, no diabetes, rest n/a | n/a | Phlebotomies | one improved, three worsened, one no significant change, rest n/a |
| Rutgers, 2007 [11] | Female | 72 | Homozygous C282Y | Ataxia | no | yes | Bronze skin pigmentation, hepatic siderosis on abdominal CT | Decreased signal in putamen and dentate nuclei on GRE | Phlebotomies | stable |
| Rosana, 2007 [10] | Male | 60 | Homozygous C282Y | Limb tremor, bilateral parkinsonism | no | no | Elevated ferritin and transferrin saturation, iron accumulation on liver biopsy, arthritis, skin hyperpigmentation | Bilateral globus pallidum and putamen (increased T1, low T2 signal) | Phlebotomies Medication (Levodopa, selegiline, paroxetine, clonazepam, mirtazapine, alprazolam) DBS of right ventral intermediate nucleus | no significant effect No effect of medication Marked improvement after DBS |
| Di Filippo, 2010 [12] | Male | 44 | Homozygous H63D | Ataxia | Dysautonomia, brisk reflexes | yes | Elevated ferritin levels, mild hepatomegaly, liver iron deposition | Olivopontocerebellar atrophy, “hot cross bun” sign in pons, no iron deposition on brain MRI | n/a | n/a |
| Chang et al. 2011 [29] | Female | 49 | n/a | Bilateral parkinsonism | no | yes | Skin hyperpigmentation, arthropathy, increased serum iron and ferritin | Decreased signal on T2* in bilateral globus pallidus, striatum, thalamus, substantia nigra and dentate nucleus | Chelation therapy (deferoxamine) Levodopa | Partial response to levodopa |
| Williams, 2013 [13] | Female | 60 | Homozygous H63D | Right-sided rest tremor/parkinsonism | Postural hypotension, daytime somnolence | yes | Mildly raised ALT | Normal MRI | Dopamine agonists, levodopa | No effect, progression |
| Ragunathan et al., 2014 [22] | Female | 57 | Homozygous C282Y | Orofacial and cervical dystonia | Depression, anxiety | yes | Increased ferritn, iron deposition in liver biopsy | Hemosiderin deposition in the basal ganglia and dentate nuclei | Phlebotomies | No improvement |
| Kumar et al. 2016 [24] | Male | 61 | Homozygous C282Y | Rest and action tremor of left upper limb, left-sided parkinsonism | no | No | Increased ferritin and transferrin saturation | symmetrical T1 hyperintensity in the lentiform nuclei and substantia nigra bilaterally | Phlebotomies Levodopa-carbidopa | Improvement of parkinsonism with levodopa |
| Kumar et al. 2016 [24] | Male | 64 | C282Y homozygous mutation in HFE gene, heterozygous mutation c.212G>A (p.Gly71Asp) in hepcidin gene | Left-sided choreiform movements of the extremities | no | no | Increased serum ferritin and transferrin saturation | T2 hypointensity with low signal on SWI in both caudate, lentiform nuclei, dorsolateral thalami and dentate nuclei consistent with iron deposition | Phlebotomies Tetrabenazine | chorea improved on tetrabenazine |
| Kumar et al. 2016 [24] | Female | 61 | Homozygous C282Y | Postural and action tremor | no | no | Increased serum ferritin, transferrin saturation, iron overload in liver biopsy | low signal on SWI in the dentate, red nuclei and substantia nigra | Phlebotomies Propranolol | tremor improved with propranolol |
| Girotra, 2017 [25] | Male | 41 | Compound heterozygous (C282Y, H63D) | Bilateral hand rest and action tremor, right-sided parkinsonism, restless legs | Depression, fatigue, anosmia | yes | n/a | Normal MRI | Levodopa | Improvement of tremor |
| Fatima 2019 [37] | Male | 60 | Homozygous C282Y | RLS, periodic limb movement disorder (when ferritin dropped to 57 ng/mL after phlebotomy) | n/a | no | Increased ferritin prior phlebotomy (>9000 ng/mL) | n/a | Phlebotomies Dopamine agonists (pramipexole, rotigotine), gabapentin, codeine or combinations | No response to medication Improvement with iron therapy when ferritin dropped |
| Scarlini et al. 2020 [38] | Male | 59 | Homozygous C282Y | Right-sided parkinsonism | headache | no | DM II, autoimmune thyroiditis, chondrocalcinosis, liver and heart iron deposition, increased ferritin | T2* hypointensities/calcifications within globus pallidus, substantia nigra, dentate nucleus, left thalamus | Phlebotomies | n/a |
| Banta, 2023 [26] | Male | 55 | Compound heterozygous (C282Y, H63D) | Right-sided hand tremor, parkinsonism | no | yes | Skin hyperpigmentation, DM II, arthralgia | Loss of hyperintensity of right substantia nigra on T2 | Primidone, pramipexole, ropinirole Phlebotomies | No improvement |
| Sharma et al., 2021 [21] 35 patients | n/a | 61.33 ± 12.66 | Most C282Y homozygous | Action tremor, parkinsonism, bilateral dystonic tremor | n/a | no | n/a (no liver involvement) | Increased SWI (iron deposition) in thalamus contralateral to limb with dystonic tremor | Phlebotomies, botulinum toxin, propranolol, primidone, levodopa/carbidopa | n/a excellent response of hand dystonia to botulinum toxin |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chanprasert, S.; Scaglia, F. Adult liver disorders caused by inborn errors of metabolism: Review and update. Mol. Genet. Metab. 2015, 114, 1–10. [Google Scholar] [CrossRef]
- Kim, Y.; Connor, J.R. The roles of iron and HFE genotype in neurological diseases. Mol. Asp. Med. 2020, 75, 100867. [Google Scholar] [CrossRef]
- Hollerer, I.; Bachmann, A.; Muckenthaler, M.U. Pathophysiological consequences and benefits of HFE mutations: 20 years of research. Haematologica 2017, 102, 809–817. [Google Scholar] [CrossRef]
- Pietrangelo, A. Hereditary hemochromatosis: Pathogenesis, diagnosis, and treatment. Gastroenterology 2010, 139, 393–408.e2. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.W.; Seckington, R.C.; Deugnier, Y. Haemochromatosis. Lancet 2016, 388, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Costello, D.J.; Walsh, S.L.; Harrington, H.J.; Walsh, C.H. Concurrent hereditary haemochromatosis and idiopathic Parkinson’s disease: A case report series. J. Neurol. Neurosurg. Psychiatry 2004, 75, 631–633. [Google Scholar] [CrossRef]
- Demarquay, G.; Setiey, A.; Morel, Y.; Trepo, C.; Chazot, G.; Broussolle, E. Clinical report of three patients with hereditary hemochromatosis and movement disorders. Mov. Disord. 2000, 15, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.R., Jr.; Hedley-Whyte, E.T. Idiopathic hemochromatosis (IHC): Dementia and ataxia as presenting signs. Neurology 1983, 33, 1479–1483. [Google Scholar] [CrossRef]
- Nielsen, J.E.; Jensen, L.N.; Krabbe, K. Hereditary haemochromatosis: A case of iron accumulation in the basal ganglia associated with a parkinsonian syndrome. J. Neurol. Neurosurg. Psychiatry 1995, 59, 318–321. [Google Scholar] [CrossRef]
- Rosana, A.; La Rosa, L. A case of hereditary haemochromatosis in a patient with extrapyramidal syndrome. Blood Transfus. 2007, 5, 241–243. [Google Scholar] [CrossRef]
- Rutgers, M.P.; Pielen, A.; Gille, M. Chronic cerebellar ataxia and hereditary hemochromatosis: Causal or coincidental association? J. Neurol. 2007, 254, 1296–1297. [Google Scholar] [CrossRef]
- Di Filippo, M.; Floridi, P.; Rossi, V.; Mattucci, E.; Rossi, A.; Calabresi, P.; Tambasco, N. A young patient with type C multiple system atrophy and hereditary hemochromatosis. J. Neurol. 2010, 257, 294–295. [Google Scholar] [CrossRef]
- Williams, S.; Vinjam, M.R.; Ismail, A.; Hassan, A. A parkinsonian movement disorder with brain iron deposition and a haemochromatosis mutation. J. Neurol. 2013, 260, 2170–2171. [Google Scholar] [CrossRef]
- Steinberg, K.K.; Cogswell, M.E.; Chang, J.C.; Caudill, S.P.; McQuillan, G.M.; Bowman, B.A.; Grummer-Strawn, L.M.; Sampson, E.J.; Khoury, M.J.; Gallagher, M.L. Prevalence of C282Y and H63D mutations in the hemochromatosis (HFE) gene in the United States. JAMA 2001, 285, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, D.E.; Kaminska, M.; Tomlin, S.; Lin, J.P. Medication use in childhood dystonia. Eur. J. Paediatr. Neurol. 2016, 20, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.C.; Edwards, C.Q.; Acton, R.T. HFE gene: Structure, function, mutations, and associated iron abnormalities. Gene 2015, 574, 179–192. [Google Scholar] [CrossRef]
- Tavtigian, S.V.; Greenblatt, M.S.; Lesueur, F.; Byrnes, G.B. In silico analysis of missense substitutions using sequence-alignment based methods. Hum. Mutat. 2008, 29, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Riley, G.R.; Jang, W.; Rubinstein, W.S.; Church, D.M.; Maglott, D.R. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014, 42, D980–D985. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.C. Hemochromatosis and iron overload: From bench to clinic. Am. J. Med. Sci. 2013, 346, 403–412. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Sharma, S.; Sethi, S.K.; Reese, D.; Gharabaghi, S.; Yerramsetty, K.K.; Palutla, V.K.; Chen, Y.; Haacke, E.M.; Jog, M.S. Brain iron deposition and movement disorders in hereditary haemochromatosis without liver failure: A cross-sectional study. Eur. J. Neurol. 2022, 29, 1417–1426. [Google Scholar] [CrossRef]
- Ragunathan, K.; Kalvakuri, K.; Dhillon, S. Chronic Dystonia as Presenting Symptom of Hereditary Hemochromatosis. Am. J. Gastroenterol. 2014, 109, S385. [Google Scholar] [CrossRef]
- Russo, N.; Edwards, M.; Andrews, T.; O’Brien, M.; Bhatia, K.P. Hereditary haemochromatosis is unlikely to cause movement disorders--a critical review. J. Neurol. 2004, 251, 849–852. [Google Scholar] [CrossRef]
- Kumar, N.; Rizek, P.; Sadikovic, B.; Adams, P.C.; Jog, M. Movement Disorders Associated With Hemochromatosis. Can. J. Neurol. Sci. 2016, 43, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Girotra, T.; Mahajan, A.; Sidiropoulos, C. Levodopa Responsive Parkinsonism in Patients with Hemochromatosis: Case Presentation and Literature Review. Case Rep. Neurol. Med. 2017, 2017, 5146723. [Google Scholar] [CrossRef]
- Banta, C.W.; Zonna, X.; Lott, R.; Jaisawal, P.; Elsisi, A. Drug-Resistant Parkinson’s Disease in a Patient with Hereditary Hemochromatosis: A Case Report. Cureus 2023, 15, e44530. [Google Scholar] [CrossRef]
- Dethy, S.; Caroyer, J.-M. Reversible parkinsonism associated with hemochromatosis. Mov. Disord. 2004, 19, 327. [Google Scholar]
- Haba-Rubio, J.; Staner, L.; Petiau, C.; Erb, G.; Schunck, T.; Macher, J.P. Restless legs syndrome and low brain iron levels in patients with haemochromatosis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-Y.; Lan, M.-Y.; Wang, M.-C.; Chen, Y.-F.; Liu, J.-S. Diffuse intracerebral iron accumulation in a patient with hereditary hemochromatosis and subacute parkinsonism. Mov. Disord. 2011, 26 (Suppl. 2), S333. [Google Scholar]
- Lucas, M.R.; Atkins, J.L.; Pilling, L.C.; Shearman, J.D.; Melzer, D. HFE genotypes, haemochromatosis diagnosis and clinical outcomes at age 80 years: A prospective cohort study in the UK Biobank. BMJ Open 2024, 14, e081926. [Google Scholar] [CrossRef]
- Duan, C.; Wang, M.; Zhang, Y.; Wei, X.; Huang, Y.; Zhang, H.; Cheng, L.; Gai, Z. C282Y and H63D Polymorphisms in Hemochromatosis Gene and Risk of Parkinson’s Disease: A Meta-Analysis. Am. J. Alzheimers Dis. Other Demen. 2016, 31, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Xu, H.; Jiang, H.; Xie, J. The association between the C282Y and H63D polymorphisms of HFE gene and the risk of Parkinson’s disease: A meta-analysis. Neurosci. Lett. 2015, 595, 99–103. [Google Scholar] [CrossRef]
- Ygland, E.; Taroni, F.; Gellera, C.; Caldarazzo, S.; Duno, M.; Soller, M.; Puschmann, A. Atypical Friedreich ataxia in patients with FXN p.R165P point mutation or comorbid hemochromatosis. Park. Relat. Disord. 2014, 20, 919–923. [Google Scholar] [CrossRef]
- Delatycki, M.B.; Tai, G.; Corben, L.; Yiu, E.M.; Evans-Galea, M.V.; Stephenson, S.E.; Gurrin, L.; Allen, K.J.; Lynch, D.; Lockhart, P.J. HFE p.C282Y heterozygosity is associated with earlier disease onset in Friedreich ataxia. Mov. Disord. 2014, 29, 940–943. [Google Scholar] [CrossRef]
- Walker, F.O. Does hereditary hemochromatosis influence the age of onset of Huntington’s disease? Mov. Disord. 2010, 25, 946. [Google Scholar] [CrossRef]
- Shaughnessy, P.; Lee, J.; O’Keeffe, S.T. Restless legs syndrome in patients with hereditary hemochromatosis. Neurology 2005, 64, 2158. [Google Scholar] [CrossRef] [PubMed]
- Fatima, Z. WS: Restless legs syndrome in a patient with hereditary hemochromatosis [abstract]. Mov. Disord. 2019, 34, 2151. [Google Scholar]
- Scarlini, S.; Cavallieri, F.; Fiorini, M.; Menozzi, E.; Ferrara, F.; Cavalleri, F.; Reale, C.; Garavaglia, B.; Pietrangelo, A.; Valzania, F.; et al. Idiopathic brain calcification in a patient with hereditary hemochromatosis. BMC Neurol. 2020, 20, 113. [Google Scholar] [CrossRef]
- Levi, S.; Ripamonti, M.; Moro, A.S.; Cozzi, A. Iron imbalance in neurodegeneration. Mol. Psychiatry 2024, 29, 1139–1152. [Google Scholar] [CrossRef]
- Zecca, L.; Youdim, M.B.; Riederer, P.; Connor, J.R.; Crichton, R.R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004, 5, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Bartzokis, G.; Tishler, T.A.; Lu, P.H.; Villablanca, P.; Altshuler, L.L.; Carter, M.; Huang, D.; Edwards, N.; Mintz, J. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol. Aging 2007, 28, 414–423. [Google Scholar] [CrossRef]
- Zucca, F.A.; Bellei, C.; Giannelli, S.; Terreni, M.R.; Gallorini, M.; Rizzio, E.; Pezzoli, G.; Albertini, A.; Zecca, L. Neuromelanin and iron in human locus coeruleus and substantia nigra during aging: Consequences for neuronal vulnerability. J. Neural Transm. 2006, 113, 757–767. [Google Scholar] [CrossRef]
- Loughnan, R.; Ahern, J.; Tompkins, C.; Palmer, C.E.; Iversen, J.; Thompson, W.K.; Andreassen, O.; Jernigan, T.; Sugrue, L.; Dale, A.; et al. Association of Genetic Variant Linked to Hemochromatosis with Brain Magnetic Resonance Imaging Measures of Iron and Movement Disorders. JAMA Neurol. 2022, 79, 919–928. [Google Scholar] [CrossRef]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef]
- Kelley, M.; Joshi, N.; Xie, Y.; Borgaonkar, M. Iron overload is rare in patients homozygous for the H63D mutation. Can. J. Gastroenterol. Hepatol. 2014, 28, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Felitti, V.J.; Koziol, J.A.; Ho, N.J.; Gelbart, T. Penetrance of 845G→A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet 2002, 359, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Gochee, P.A.; Powell, L.W.; Cullen, D.J.; Du Sart, D.; Rossi, E.; Olynyk, J.K. A population-based study of the biochemical and clinical expression of the H63D hemochromatosis mutation. Gastroenterology 2002, 122, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Li, X.; Zhang, W.; Zhao, X.; Ou, X.; Huang, J. Recent advance in the molecular genetics of Wilson disease and hereditary hemochromatosis. Eur. J. Med. Genet. 2016, 59, 532–539. [Google Scholar] [CrossRef]
- Branco, C.C.; Gomes, C.T.; De Fez, L.; Bulhões, S.; Brilhante, M.J.; Pereirinha, T.; Cabral, R.; Rego, A.C.; Fraga, C.; Miguel, A.G.; et al. Carriers of the Complex Allele HFE c.[187C>G;340+4T>C] Have Increased Risk of Iron Overload in São Miguel Island Population (Azores, Portugal). PLoS ONE 2015, 10, e0140228. [Google Scholar] [CrossRef][Green Version]
- Benyamin, B.; Esko, T.; Ried, J.S.; Radhakrishnan, A.; Vermeulen, S.H.; Traglia, M.; Gögele, M.; Anderson, D.; Broer, L.; Podmore, C.; et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat. Commun. 2014, 5, 4926. [Google Scholar] [CrossRef]
- Benyamin, B.; Ferreira, M.A.; Willemsen, G.; Gordon, S.; Middelberg, R.P.; McEvoy, B.P.; Hottenga, J.J.; Henders, A.K.; Campbell, M.J.; Wallace, L.; et al. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat. Genet. 2009, 41, 1173–1175. [Google Scholar] [CrossRef]
- de Tayrac, M.; Roth, M.P.; Jouanolle, A.M.; Coppin, H.; le Gac, G.; Piperno, A.; Férec, C.; Pelucchi, S.; Scotet, V.; Bardou-Jacquet, E.; et al. Genome-wide association study identifies TF as a significant modifier gene of iron metabolism in HFE hemochromatosis. J. Hepatol. 2015, 62, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Milet, J.; Dehais, V.; Bourgain, C.; Jouanolle, A.M.; Mosser, A.; Perrin, M.; Morcet, J.; Brissot, P.; David, V.; Deugnier, Y.; et al. Common variants in the BMP2, BMP4, and HJV genes of the hepcidin regulation pathway modulate HFE hemochromatosis penetrance. Am. J. Hum. Genet. 2007, 81, 799–807. [Google Scholar] [CrossRef]
- McLaren, C.E.; Emond, M.J.; Subramaniam, V.N.; Phatak, P.D.; Barton, J.C.; Adams, P.C.; Goh, J.B.; McDonald, C.J.; Powell, L.W.; Gurrin, L.C.; et al. Exome sequencing in HFE C282Y homozygous men with extreme phenotypes identifies a GNPAT variant associated with severe iron overload. Hepatology 2015, 62, 429–439. [Google Scholar] [CrossRef]
- Pelucchi, S.; Mariani, R.; Calza, S.; Fracanzani, A.L.; Modignani, G.L.; Bertola, F.; Busti, F.; Trombini, P.; Fraquelli, M.; Forni, G.L.; et al. CYBRD1 as a modifier gene that modulates iron phenotype in HFE p.C282Y homozygous patients. Haematologica 2012, 97, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Tambasco, N.; Nigro, P.; Chiappiniello, A.; Paolini Paoletti, F.; Scialpi, S.; Simoni, S.; Chiarini, P.; Parnetti, L. An Updated Overview of the Magnetic Resonance Imaging of Brain Iron in Movement Disorders. Behav. Neurol. 2022, 2022, 3972173. [Google Scholar] [CrossRef]
- Baringer, S.L.; Simpson, I.A.; Connor, J.R. Brain iron acquisition: An overview of homeostatic regulation and disease dysregulation. J. Neurochem. 2023, 165, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Hagemeier, J.; Ramanathan, M.; Schweser, F.; Dwyer, M.G.; Lin, F.; Bergsland, N.; Weinstock-Guttman, B.; Zivadinov, R. Iron-related gene variants and brain iron in multiple sclerosis and healthy individuals. Neuroimage Clin. 2018, 17, 530–540. [Google Scholar] [CrossRef]
- Berg, D.; Hoggenmüller, U.; Hofmann, E.; Fischer, R.; Kraus, M.; Scheurlen, M.; Becker, G. The basal ganglia in haemochromatosis. Neuroradiology 2000, 42, 9–13. [Google Scholar] [CrossRef]
- Atkins, J.L.; Pilling, L.C.; Heales, C.J.; Savage, S.; Kuo, C.L.; Kuchel, G.A.; Steffens, D.C.; Melzer, D. Hemochromatosis Mutations, Brain Iron Imaging, and Dementia in the UK Biobank Cohort. J. Alzheimer’s Dis. 2021, 79, 1203–1211. [Google Scholar] [CrossRef]
- Aquino, D.; Bizzi, A.; Grisoli, M.; Garavaglia, B.; Bruzzone, M.G.; Nardocci, N.; Savoiardo, M.; Chiapparini, L. Age-related iron deposition in the basal ganglia: Quantitative analysis in healthy subjects. Radiology 2009, 252, 165–172. [Google Scholar] [CrossRef]
- Rouault, T.A. Iron metabolism in the CNS: Implications for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.M.; Fitzpatrick, P.F. Mechanisms of tryptophan and tyrosine hydroxylase. IUBMB Life 2013, 65, 350–357. [Google Scholar] [CrossRef]
- Muñoz, Y.; Carrasco, C.M.; Campos, J.D.; Aguirre, P.; Núñez, M.T. Parkinson’s Disease: The Mitochondria-Iron Link. Park. Dis. 2016, 2016, 7049108. [Google Scholar] [CrossRef] [PubMed]
- Loughnan, R.; Ahern, J.; Boyle, M.; Jernigan, T.L.; Hagler, D.J., Jr.; Iversen, J.R.; Frei, O.; Smith, D.M.; Andreassen, O.; Zaitlen, N.; et al. Hemochromatosis neural archetype reveals iron disruption in motor circuits. Sci. Adv. 2024, 10, eadp4431. [Google Scholar] [CrossRef]
- Ryan, S.K.; Zelic, M.; Han, Y.; Teeple, E.; Chen, L.; Sadeghi, M.; Shankara, S.; Guo, L.; Li, C.; Pontarelli, F.; et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 2023, 26, 12–26. [Google Scholar] [CrossRef]
- Karimi, A.; Mohammadi, S.; Salehi, M.A.; Dager, S.R. Brain microstructural abnormalities in patients with Wilson’s disease: A systematic review of diffusion tenor imaging studies. Brain Imaging Behav. 2022, 16, 2809–2840. [Google Scholar] [CrossRef] [PubMed]
- Litwin, T.; Rędzia-Ogrodnik, B.; Antos, A.; Przybyłkowski, A.; Członkowska, A.; Bembenek, J.P. Brain Magnetic Resonance Imaging in Wilson’s Disease-Significance and Practical Aspects-A Narrative Review. Brain Sci. 2024, 14, 727. [Google Scholar] [CrossRef]
- Vayssiere, N.; van der Gaag, N.; Cif, L.; Hemm, S.; Verdier, R.; Frerebeau, P.; Coubes, P. Deep brain stimulation for dystonia confirming a somatotopic organization in the globus pallidus internus. J. Neurosurg. 2004, 101, 181–188. [Google Scholar] [CrossRef]
- Nambu, A. Somatotopic organization of the primate Basal Ganglia. Front. Neuroanat. 2011, 5, 26. [Google Scholar] [CrossRef]
- Kalita, J.; Misra, U.K. Markedly severe dystonia in Japanese encephalitis. Mov. Disord. 2000, 15, 1168–1172. [Google Scholar] [CrossRef]
- Walusinski, O. A history of oculogyric crises during the encephalitis lethargica pandemic. Rev. Neurol. 2022, 178, 878–885. [Google Scholar] [CrossRef]
- Matsumura, K.; Sakuta, M. Oculogyric crisis in acute herpetic brainstem encephalitis. J. Neurol. Neurosurg. Psychiatry 1987, 50, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Desmarais, J.E.; Beauclair, L.; Annable, L.; Bélanger, M.C.; Kolivakis, T.T.; Margolese, H.C. Effects of discontinuing anticholinergic treatment on movement disorders, cognition and psychopathology in patients with schizophrenia. Ther. Adv. Psychopharmacol. 2014, 4, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Antos, A.; Członkowska, A.; Smolinski, L.; Bembenek, J.; Przybyłkowski, A.; Skowrońska, M.; Kurkowska-Jastrzębska, I.; Litwin, T. Early neurological deterioration in Wilson’s disease: A systematic literature review and meta-analysis. Neurol. Sci. 2023, 44, 3443–3455. [Google Scholar] [CrossRef] [PubMed]
- Medici, V.; Weiss, K.H. Genetic and environmental modifiers of Wilson disease. Handb. Clin. Neurol. 2017, 142, 35–41. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalampokini, S.; Plaitakis, A.; Spanaki, C.; Xiromerisiou, G. Isolated Non-Progressive Hemidystonia in a Patient Homozygous for H63D Variant of Hereditary Hemochromatosis: A Case Report and Systematic Literature Review of Movement Disorders in Hereditary Hemochromatosis. Diagnostics 2025, 15, 2275. https://doi.org/10.3390/diagnostics15172275
Kalampokini S, Plaitakis A, Spanaki C, Xiromerisiou G. Isolated Non-Progressive Hemidystonia in a Patient Homozygous for H63D Variant of Hereditary Hemochromatosis: A Case Report and Systematic Literature Review of Movement Disorders in Hereditary Hemochromatosis. Diagnostics. 2025; 15(17):2275. https://doi.org/10.3390/diagnostics15172275
Chicago/Turabian StyleKalampokini, Stefania, Andreas Plaitakis, Cleanthe Spanaki, and Georgia Xiromerisiou. 2025. "Isolated Non-Progressive Hemidystonia in a Patient Homozygous for H63D Variant of Hereditary Hemochromatosis: A Case Report and Systematic Literature Review of Movement Disorders in Hereditary Hemochromatosis" Diagnostics 15, no. 17: 2275. https://doi.org/10.3390/diagnostics15172275
APA StyleKalampokini, S., Plaitakis, A., Spanaki, C., & Xiromerisiou, G. (2025). Isolated Non-Progressive Hemidystonia in a Patient Homozygous for H63D Variant of Hereditary Hemochromatosis: A Case Report and Systematic Literature Review of Movement Disorders in Hereditary Hemochromatosis. Diagnostics, 15(17), 2275. https://doi.org/10.3390/diagnostics15172275







