Diagnostic Evaluation of an Increased Risk of Developing Small Intestinal Bacterial Overgrowth Associated with Glucagon-like Peptide-1 (GLP-1) Receptor Agonists and Dual GLP-1/GIP Receptor Agonists: A Global Retrospective Multicenter Cohort Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Patient Selection
2.3. Query Diagnostic Validation
2.4. Covariates and Matching
2.5. Propensity-Matched Study Outcomes
2.6. Statistical Analysis
3. Results
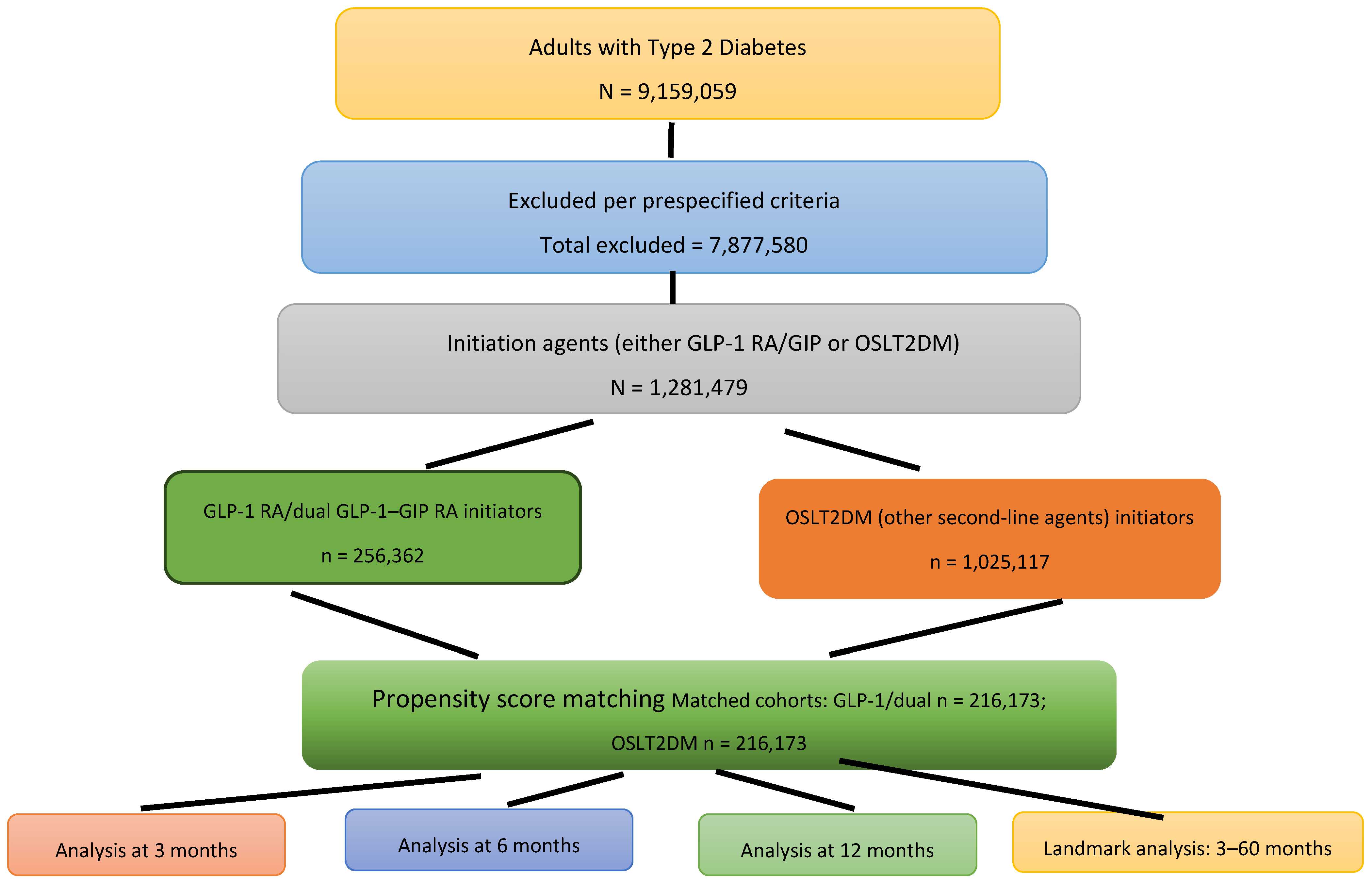
3.1. Study Population
3.2. SIBO Incidence Rate in Short-Term and Long-Term Outcome
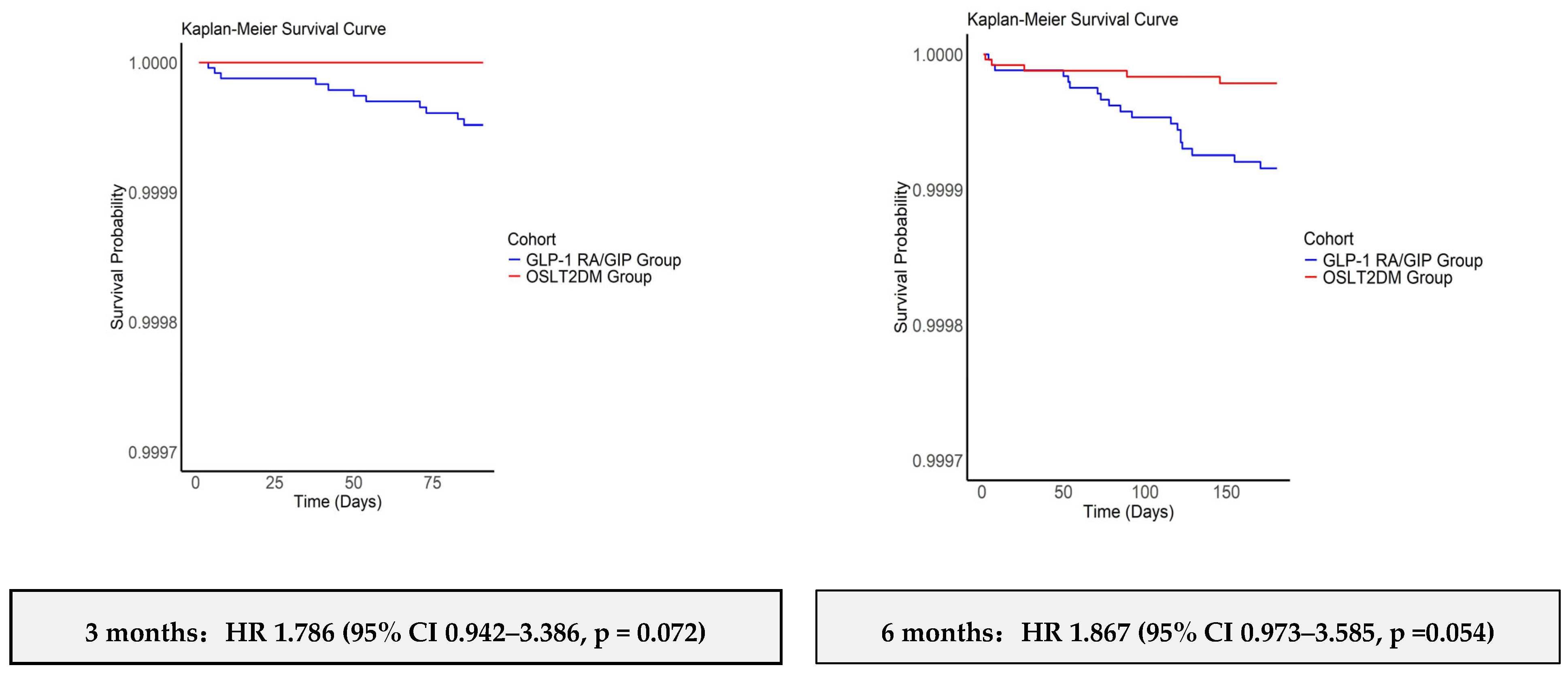
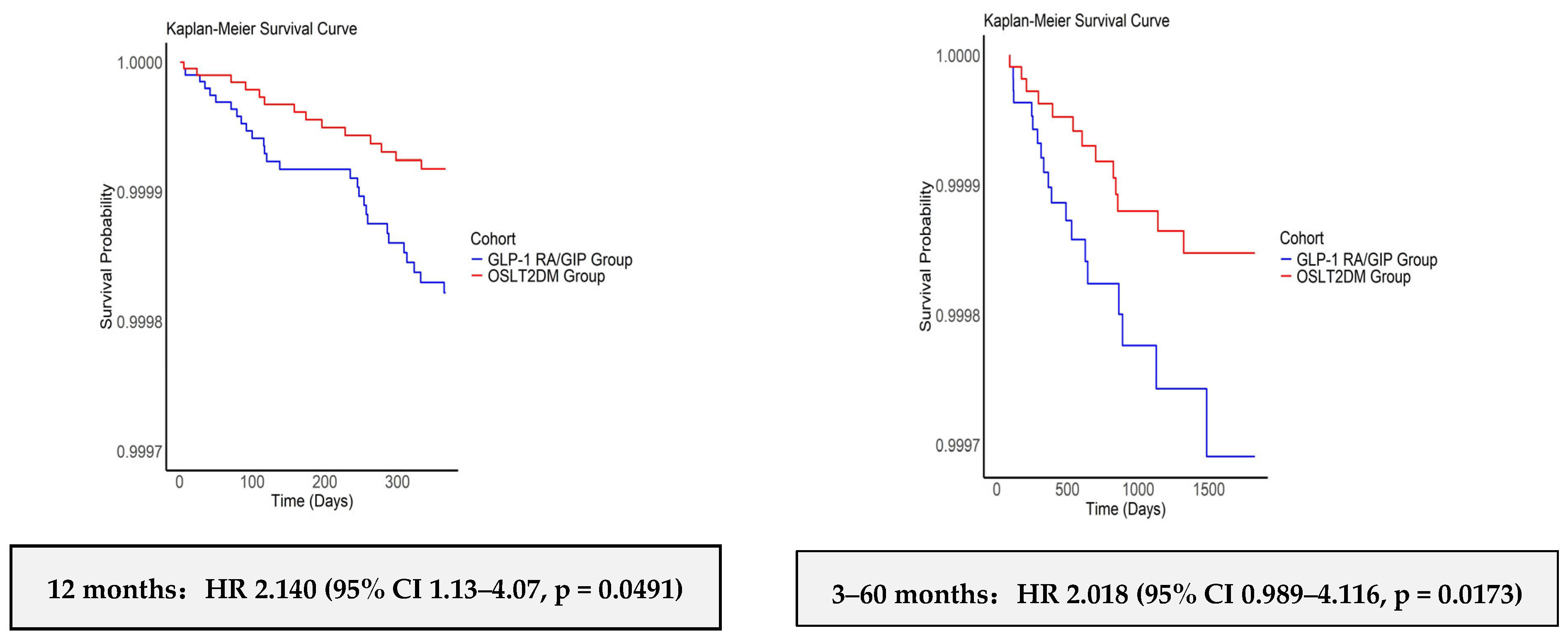
3.3. Kaplan–Meier Analyses: Univariable Cox Proportional Hazards
4. Discussion
4.1. Epidemiology and Therapeutic Context
4.2. SIBO Background and Diagnostic Approch
4.3. Study Outcomes: Interpretation and Limitations
4.4. Mechanisms Hypothesis
4.5. Study Strength and Limitations
4.6. Clinical Implicationn and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SIBO | Small intestinal bacterial overgrowth |
| GLP-1 | Glucagon-like peptide-1 (GLP-1) |
| GIP | glucose-dependent insulinotropic polypeptide |
| OSLT2DM | other second-line diabetes medications |
| SGLT2 | sodium-glucose cotransporter-2 inhib |
| TZD | thiazolidinediones |
| DPP-4 | dipeptidyl peptidase 4 inhibitors |
| T2DM | type 2 diabetes |
References
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Latif, W.; Lambrinos, K.J.; Patel, P.; Rodriguez, R. Compare and Contrast the Glucagon-Like Peptide-1 Receptor Agonists (GLP1RAs). In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Vilsboll, T.; Bain, S.C.; Leiter, L.A.; Lingvay, I.; Matthews, D.; Simo, R.; Helmark, I.C.; Wijayasinghe, N.; Larsen, M. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes. Metab. 2018, 20, 889–897. [Google Scholar] [CrossRef]
- Forzano, I.; Varzideh, F.; Avvisato, R.; Jankauskas, S.S.; Mone, P.; Santulli, G. Tirzepatide: A Systematic Update. Int. J. Mol. Sci. 2022, 23, 14631. [Google Scholar] [CrossRef]
- Clark, L. GLP-1 receptor agonists: A review of glycemic benefits and beyond. JAAPA 2024, 37, 1–4. [Google Scholar] [CrossRef]
- Collins, L.; Costello, R.A. Glucagon-Like Peptide-1 Receptor Agonists. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sodhi, M.; Rezaeianzadeh, R.; Kezouh, A.; Etminan, M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA 2023, 330, 1795–1797. [Google Scholar] [CrossRef] [PubMed]
- Carris, N.W.; Wallace, S.; DuCoin, C.G.; Mhaskar, R.; Stern, M.; Bunnell, B. Discontinuing semaglutide after weight loss: Strategy for weight maintenance and a possible new side effect. Can. J. Physiol. Pharmacol. 2024, 102, 391–395. [Google Scholar] [CrossRef]
- Beas, R.; Riva-Moscoso, A.; Montalvan-Sanchez, E.; Principe-Meneses, F.S.; Aljaras, R.; Ramirez-Rojas, M.; Izquierdo-Veraza, D.; Calderon, G. Prevalence of small intestinal bacterial overgrowth in patients with gastroparesis: A systematic review and meta-analysis. Gastroenterol. Hepatol. Bed Bench 2023, 16, 438–447. [Google Scholar] [CrossRef]
- Dukowicz, A.C.; Lacy, B.E.; Levine, G.M. Small intestinal bacterial overgrowth: A comprehensive review. Gastroenterol. Hepatol. 2007, 3, 112–122. [Google Scholar]
- Sellge, G.; Ockenga, J. Small intestinal bacterial overgrowth (SIBO)—Therapy, nutrition, microbiome. Dtsch. Med. Wochenschr. 2024, 149, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- TriNetX. Available online: https://trinetx.com (accessed on 2 December 2024).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Exenatide: Drug Information Uptodate 2023. Available online: https://www.uptodate.com/contents/exenatide-drug-information?search=bydureon&source=panel_searchresult&selectedTitle=1~35&usage_type=panel&kp_tab=drug_general&display_rank=1#F595855 (accessed on 2 December 2024).
- Haukoos, J.S.; Lewis, R.J. The Propensity Score. JAMA 2015, 314, 1637–1638. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef]
- Cannon, A.; Handelsman, Y.; Heile, M.; Shannon, M. Burden of Illness in Type 2 Diabetes Mellitus. J. Manag. Care Spec. Pharm. 2018, 24, S5–S13. [Google Scholar] [CrossRef]
- Mahase, E. GLP-1 agonists: US sees 700% increase over four years in number of patients without diabetes starting treatment. BMJ 2024, 386, q1645. [Google Scholar] [CrossRef]
- Syed, Y.Y. Tirzepatide: First Approval. Drugs 2022, 82, 1213–1220. [Google Scholar] [CrossRef]
- Ansari, H.U.H.; Qazi, S.U.; Sajid, F.; Altaf, Z.; Ghazanfar, S.; Naveed, N.; Ashfaq, A.S.; Siddiqui, A.H.; Iqbal, H.; Qazi, S. Efficacy and Safety of Glucagon-Like Peptide-1 Receptor Agonists on Body Weight and Cardiometabolic Parameters in Individuals With Obesity and Without Diabetes: A Systematic Review and Meta-Analysis. Endocr. Pract. 2024, 30, 160–171. [Google Scholar] [CrossRef]
- Iqbal, J.; Wu, H.X.; Hu, N.; Zhou, Y.H.; Li, L.; Xiao, F.; Wang, T.; Jiang, H.L.; Xu, S.N.; Huang, B.L.; et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus-a systematic review and meta-analysis of randomized control trials. Obes. Rev. 2022, 23, e13435. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Ramachandram, D.S.; Hasan, S.S.; Thiruchelvam, K. Efficacy and safety of GLP-1 receptor agonists in the management of obstructive sleep apnea in individuals without diabetes: A systematic review and meta-analysis of randomized, placebo-controlled trials. Sleep Med. 2025, 129, 40–44. [Google Scholar] [CrossRef]
- Jalil, J.E.; Gabrielli, L.; Ocaranza, M.P.; MacNab, P.; Fernandez, R.; Grassi, B.; Jofre, P.; Verdejo, H.; Acevedo, M.; Cordova, S.; et al. New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review. Int. J. Mol. Sci. 2024, 25, 4407. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.; Wang, L.; Chen, C.; Chen, L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database. Front. Endocrinol. 2022, 13, 1043789. [Google Scholar] [CrossRef]
- Alibilli, A.S.; Jain, V.; Mane, H.; Yue, X.; Ratzki-Leewing, A.; Merchant, J.S.; Criss, S.; Nguyen, Q.C.; McCoy, R.G.; Nguyen, T.T. Harnessing Facebook to Investigate Real-World Mentions of Adverse Events of Glucagon-Like Peptide-1 Receptor Agonist (GLP-1 RA) Medications: Observational Study of Facebook Posts From 2022 to 2024. JMIR Infodemiology 2025, 5, e73619. [Google Scholar] [CrossRef]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef]
- Cangemi, D.J.; Lacy, B.E.; Wise, J. Diagnosing Small Intestinal Bacterial Overgrowth: A Comparison of Lactulose Breath Tests to Small Bowel Aspirates. Dig. Dis. Sci. 2021, 66, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Cappola, J.J. GLP-1 Receptor Agonists in Diabetes and Obesity: A Case Report and Review of Bowel Obstruction Risks and Management. Cureus 2025, 17, e81891. [Google Scholar] [CrossRef] [PubMed]
- Parkman, H.P.; Rim, D.S.; Anolik, J.R.; Dadparvar, S.; Maurer, A.H. Glucagonlike Peptide-1 Receptor Agonists: The Good, the Bad, and the Ugly-Benefits for Glucose Control and Weight Loss with Side Effects of Delaying Gastric Emptying. J. Nucl. Med. Technol. 2024, 52, 3–7. [Google Scholar] [CrossRef]
- Maselli, D.; Atieh, J.; Clark, M.M.; Eckert, D.; Taylor, A.; Carlson, P.; Burton, D.D.; Busciglio, I.; Harmsen, W.S.; Vella, A.; et al. Effects of liraglutide on gastrointestinal functions and weight in obesity: A randomized clinical and pharmacogenomic trial. Obesity 2022, 30, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Kalas, M.A.; Dang, T.Q.; Galura, G.; Alvarado, L.; Dwivedi, A.K.; Deoker, A.; McCallum, R. Frequency of GLP-1 receptor agonists use in diabetic patients diagnosed with delayed gastric emptying and their demographic profile. J. Investig. Med. 2023, 71, 11–16. [Google Scholar] [CrossRef]
- Kato, S.; Sato, T.; Fujita, H.; Kawatani, M.; Yamada, Y. Effects of GLP-1 receptor agonist on changes in the gut bacterium and the underlying mechanisms. Sci. Rep. 2021, 11, 9167. [Google Scholar] [CrossRef]
- Pianka, E.R. On r- and K-Selection. Am. Nat. 1970, 104, 592–597. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Y.B. Intestinal microbiota and chronic constipation. Springerplus 2016, 5, 1130. [Google Scholar] [CrossRef]
| Search Terms | Associated Code |
|---|---|
| Surgery on the Esophagus | CPT: 43279, 43280, 43283, 43284, 43285, 43286, 43287, 43288, 43289, 1007215, 1007219, 1007295 *, 1007298, 1007341, 1020218, 1007335 ICD-10: 0DV1, 0DV2, 0DV3, 0DV4, 0DV5, 0D11, 0D51, 0D52, 0D53, 0D54, 0D55, 0D84, 0DB1, 0DB2, 0DB3, 0DB4, 0DB5, 0DC1, 0DC2, 0DC3, 0DC4, 0DC5, 0DD1, 0DD2, 0DD3, 0DD4, 0DD55, 0DF5, 0DL1, 0DL2, 0DL3, 0DL4, 0DL5, 0DT1, 0DT2, 0DT3, 0DT4, 0DT5, 0D71, 0D72, 0D73, 0D74, 0D75 |
| Surgery on the Stomach | CPT: 1007344, 1007345, 1007352, 1007372, 1007392, 1007385 ICD-10: 0DV6, 0DV7, 0DB6, 0DB7, 0DC6, 0DC7, 0DD6, 0DD7, 0DF6, 0DL6, 0DL7, 0DT6, 0DT7, 0D16 |
| Dyskinesia of Esophagus | IDC-10: K22.4 |
| Scleroderma | IDC-10: M34 |
| Radiation Therapy | TNX Curated: 1001 |
| Lupus Erythematosus | ICD-10: L93 |
| Sjörgen Syndrome | ICD-10: M35.0 |
| Acquired Absence of Other Specified Parts of Digestive Tracts | ICD-10: Z90.49 |
| Other abdominal surgeries | CPT: 1007463, 1007422, 1007570, 1007695, 1007795, 1007908, 1007952, 1007846, 1007854, 1007892, 1018154, 47579, 1007887, 47700, 47701, 47570, 47715, 1007592, 1007596, 1007620, 1007663, 1007671, 1007687, 1007692 SNOMED: 4558008, 80146002, 11466000 ICD-9: 46.2 |
| Ehlers-Danlos Syndrome | ICD-10: Q79.6 |
| Intestinal Adhesions with Obstruction | ICD-10: K56.5 |
| Unspecified Diseases of Spinal Cord | ICD-10: G95 |
| Parkinson’s Disease | ICD-10: G20 |
| Multiple Sclerosis | ICD-10: G35 |
| Amyloidosis | ICD-10: E85 |
| Crohn’s Disease | ICD-10: K50 |
| Ulcerative Colitis | ICD-10: K51 |
| Other Specified Noninfective Gastroenteritis and Colitis | ICD-10: K52.8 |
| Noninfective Gastroenteritis and Colitis, Unspecified | ICD-10: K52.9 |
| Cystic Fibrosis | ICD-10: E84 |
| Other Unspecified Intestinal Obstruction | ICD-10: K56.6 |
| Fistula of Intestine | ICD-10: K63.2 |
| Intestinal Failure | ICD-10: K90.83 |
| Type 2 Diabetes Mellitus | ICD-10: E11 |
| Gastroparesis | ICD-10: K31.84 |
| Glucagon-like Protein-1 Receptor Agonists | ATC: A10BJ RxNorm: 1440051, 1551291, 1991302, 2601723, 475968, 60548 ** |
| Sulfonylureas | ATC: A10BB |
| Dipeptidyl Peptidase 4 Inhibitors | ATC: A10BH |
| Sodium-Glucose Co-Transporter 2 Inhibitors | ATC: A10BK |
| Thiazolidinediones | ATC: A10BG |
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Cohort, No. (%) | Cohort, No. (%) | |||||
| Demographics | GLP-1 RA | Control | Std Diff. | GLP-1 RA | Control | Std Diff. |
| Total No. | 234,701 | 952,418 | 216,173 | 216,173 | ||
| Age, mean (SD), y | 55.6 ± 13.5 | 62.5 ± 12.6 | 0.5279 | 56.7 ± 13.0 | 56.7 ± 13.4 | 0.002 |
| Sex | ||||||
| Female | 130,070 (55.4%) | 394,335 (41.4%) | 0.2833 | 115,650 (53.5%) | 115,379 (53.4%) | 0.0025 |
| Male | 94,047 (40.1%) | 535,217 (56.2%) | 0.327 | 91,358 (42.3%) | 91,454 (42.3%) | 0.0009 |
| Ethnicity | ||||||
| Hispanic/LatinX | 19,273 (8.2%) | 86,609 (9.1%) | 0.0314 | 18,213 (8.4%) | 17,304 (8.0%) | 0.0153 |
| Not Hispanic/LatinX | 137,998 (58.8%) | 485,443 (51.0%) | 0.1578 | 125,595 (58.1%) | 131,526 (60.8%) | 0.0559 |
| Unknown | 77,430 (33.0%) | 380,366 (39.9%) | 0.1447 | 72,365 (33.5%) | 67,343 (31.2%) | 0.0497 |
| Race | ||||||
| Asian | 8366 (3.6%) | 79,874 (8.4%) | 0.2045 | 8231 (3.8%) | 7947 (3.7%) | 0.0069 |
| Black | 44,108 (18.8%) | 132,399 (13.9%) | 0.1326 | 39,336 (18.2%) | 39,746 (18.4%) | 0.0049 |
| White | 129,966 (55.4%) | 459,037 (48.2%) | 0.144 | 119,423 (55.2%) | 123,649 (57.2%) | 0.0394 |
| American Indian or Alaska Native | 792 (0.3%) | 2606 (0.3%) | 0.0116 | 741 (0.3%) | 577 (0.3%) | 0.0138 |
| Native Hawaiian or Other Pacific Islander | 1430 (0.6%) | 5888 (0.6%) | 0.0011 | 1332 (0.6%) | 1566 (0.7%) | 0.0133 |
| Unknown | 40,556 (17.3%) | 234,549 (24.6%) | 0.1813 | 38,337 (17.7%) | 34,059 (15.8%) | 0.053 |
| Diabetic Comorbidities | ||||||
| Primary hypertension | 159,587 (68.0%) | 640,293 (67.2%) | 0.0164 | 148,292 (68.6%) | 148,742 (68.8%) | 0.0045 |
| Hyperlipidemia | 109,315 (46.6%) | 401,625 (42.2%) | 0.0888 | 100,947 (46.7%) | 101,353 (46.9%) | 0.0038 |
| Atherosclerotic heart disease of native coronary artery | 31,441 (13.4%) | 205,487 (21.6%) | 0.2166 | 30,824 (14.3%) | 30,893 (14.3%) | 0.0009 |
| Other hypothyroidism | 35,475 (15.1%) | 91,180 (9.6%) | 0.1691 | 30,643 (14.2%) | 30,511 (14.1%) | 0.0018 |
| Type 2 diabetes mellitus with neurological complications | 28,515 (12.2%) | 95,272 (10.0% | 0.0684 | 26,598 (12.3%) | 26,714 (12.4%) | 0.0016 |
| Chronic kidney disease | 26,023 (11.1%) | 147,717 (15.5%) | 0.1305 | 25,181 (11.6%) | 25,195 (11.7%) | 0.0002 |
| Heart failure | 16,791 (7.2%) | 129,371 (13.6%) | 0.2121 | 16,593 (7.7%) | 17,064 (7.9%) | 0.0081 |
| Other functional intestinal disorders | 18,922 (8.1%) | 57,691 (6.1%) | 0.0783 | 16,232 (7.5%) | 15,598 (7.2%) | 0.0112 |
| Peripheral vascular disease, unspecified | 8504 (3.6%) | 42,087 (4.4%) | 0.0405 | 8128 (3.8%) | 7986 (3.7%) | 0.0035 |
| Atherosclerosis | 8079 (3.4%) | 40,400 (4.2%) | 0.0416 | 7757 (3.6%) | 7631 (3.5%) | 0.0031 |
| Cerebral infarction | 7801 (3.3%) | 50,448 (5.3%) | 0.0973 | 7609 (3.5%) | 7467 (3.5%) | 0.0036 |
| End-stage renal disease | 3604 (1.5%) | 17,977 (1.9%) | 0.0271 | 3414 (1.6%) | 3417 (1.6%) | 0.0001 |
| Fibrosis and cirrhosis of liver | 2979 (1.3%) | 13,675 (1.4%) | 0.0144 | 2824 (1.3%) | 2667 (1.2%) | 0.0065 |
| Other immunodeficiencies | 2369 (1.0%) | 4166 (0.4%) | 0.0675 | 1839 (0.9%) | 1984 (0.9%) | 0.0072 |
| Malnutrition | 1716 (0.7%) | 14,370 (1.5%) | 0.0739 | 1671 (0.8%) | 1505 (0.7%) | 0.009 |
| Opioid dependence | 1762 (0.8%) | 4262 (0.4%) | 0.0393 | 1515 (0.7%) | 1500 (0.7%) | 0.0008 |
| Human immunodeficiency virus (HIV) disease | 1376 (0.6%) | 3418 (0.4%) | 0.0332 | 1189 (0.6%) | 1271 (0.6%) | 0.005 |
| Drug-induced constipation | 1078 (0.5%) | 2148 (0.2%) | 0.04 | 894 (0.4%) | 875 (0.4%) | 0.0014 |
| Celiac disease | 614 (0.3%) | 658 (0.1%) | 0.0474 | 442 (0.2%) | 289 (0.1%) | 0.0172 |
| Other chronic pancreatitis | 408 (0.2%) | 3720 (0.4%) | 0.0409 | 404 (0.2%) | 467 (0.2%) | 0.0065 |
| Immunodeficiency with predominantly antibody defects | 361 (0.2%) | 812 (0.1%) | 0.0198 | 305 (0.1%) | 249 (0.1%) | 0.0072 |
| Diverticular disease of small intestine without perforation or abscess | 183 (0.1%) | 776 (0.1%) | 0.0012 | 172 (0.1%) | 154 (0.1%) | 0.003 |
| Common variable immunodeficiency | 100 (>0.1%) | 181 (>0.1%) | 0.0135 | 83 (>0.1%) | 65 (>0.1%) | 0.0045 |
| Acromegaly and pituitary gigantism | 92 (>0.1%) | 194 (>0.1%) | 0.0109 | 76 (>0.1%) | 67 (>0.1%) | 0.0023 |
| Alcohol-induced chronic pancreatitis | 42 (>0.1%) | 155 (>0.1%) | 0.0207 | 42 (>0.1%) | 92 (>0.1%) | 0.0131 |
| Achlorhydria | 39 (>0.1%) | 47 (>0.1%) | 0.0113 | 36 (>0.1%) | 17 (>0.1%) | 0.0079 |
| Myotonic muscular dystrophy | 25 (>0.1%) | 111 (>0.1%) | 0.0009 | 23 (>0.1%) | 28 (>0.1%) | 0.0021 |
| Combined immunodeficiencies | 21 (>0.1%) | 58 (>0.1%) | 0.0033 | 16 (>0.1%) | 15 (>0.1%) | 0.0005 |
| Tropical sprue | 10 * (>0.1%) | 12 (>0.1%) | 0.0057 | 10 (>0.1%) | 10 * (>0.1%) | 0.0001 |
| Gastroenteritis and colitis due to radiation | 11 (>0.1%) | 46 (>0.1%) | 0.0002 | 10 * (>0.1%) | 12 (>0.1%) | 0.0013 |
| Systemic sclerosis (scleroderma) | 0 (0.0%) | 0 (0.0%) | 0 | 0 (0.0%) | 0 (0.0%) | 0 |
| Amyloidosis | 0 (0.0%) | 0 (0.0%) | 0 | 0 (0.0%) | 0 (0.0%) | 0 |
| Gastroparesis | 0 (0.0%) | 0 (0.0%) | 0 | 0 (0.0%) | 0 (0.0%) | 0 |
| Labs | ||||||
| BMI | 37.2 ± 8.33 | 31.5 ± 7.52 | 0.7151 | 36.4 ± 7.9 | 35.9 ± 8.31 | 0.0595 |
| At least 25 kg/m2 | 168,304 (71.7%) | 492,218 (51.7%) | 0.421 | 150,141 (69.5%) | 150,743 (69.7%) | 0.0061 |
| At least 30 kg/m2 | 150,585 (64.2%) | 345,445 (36.3%) | 0.5809 | 132,446 (61.3%) | 132,557 (61.3) | 0.0011 |
| At least 35 kg/m2 | 111,793 (47.6%) | 188,694 (19.8%) | 0.6157 | 94,275 (43.6%) | 94,681 (43.8%) | 0.0038 |
| At least 40 kg/m2 | 70,047 (29.8%) | 95,351 (10.0%) | 0.5126 | 55,850 (25.8%) | 56,129 (26.0%) | 0.0029 |
| At least 45 kg/m2 | 38,925 (16.6%) | 44,772 (4.7%) | 0.3927 | 29,328 (13.6%) | 29,483 (13.6%) | 0.0021 |
| At most 50 kg/m2 | 164,607 (70.1%) | 554,881 (58.3%) | 0.2496 | 149,230 (69.0%) | 148,578 (68.7%) | 0.0065 |
| At most 45 kg/m2 | 155,179 (66.1%) | 542,021 (56.9%) | 0.1901 | 141,532 (65.5%) | 140,779 (65.1%) | 0.0073 |
| At most 40 kg/m2 | 135,445 (57.7%) | 508,701 (53.4%) | 0.0866 | 124,545 (57.6%) | 123,635 (57.2%) | 0.0085 |
| At most 35 kg/m2 | 100,515 (42.8%) | 439,347 (46.1%) | 0.0665 | 93,690 (43.3%) | 92,811 (42.9%) | 0.0082 |
| At most 30 kg/m2 | 52,960 (22.6%) | 298,935 (31.4%) | 0.1998 | 50,079 (23.2%) | 49,462 (22.9%) | 0.0068 |
| Hemoglobin—A1C | 7.79 ± 2.16 | 8.1 ± 2.18 | 0.1445 | 7.88 ± 2.17 | 7.95 ± 2.25 | 0.0313 |
| At least 7.0% | 105,322 (44.9%) | 357,521 (37.5%) | 0.1495 | 98,200 (45.4%) | 96,673 (44.7%) | 0.0142 |
| At least 7.5% | 87,847 (37.4%) | 298,785 (31.4%) | 0.1278 | 82,081 (40.0%) | 81,397 (37.7%) | 0.0065 |
| At least 8.0% | 75,221 (32.1%) | 248,228 (26.1%) | 0.1321 | 70,059 (32.4%) | 69,792 (32.3%) | 0.0026 |
| At least 8.5% | 64,362 (27.4%) | 203,207 (21.3%) | 0.1421 | 59,668 (27.6%) | 59,585 (27.6%) | 0.0009 |
| At least 9.0% | 56,002 (23.9%) | 170,295 (17.9%) | 0.1476 | 51,692 (23.9%) | 51,718 (23.9%) | 0.0003 |
| At least 9.5% | 47,943 (20.4%) | 141,157 (14.8%) | 0.1475 | 44,070 (20.4%) | 44,053 (20.4%) | 0.0002 |
| At least 10.0% | 41,338 (17.6%) | 118,895 (12.5%) | 0.1438 | 37,856 (17.5%) | 37,817 (17.5%) | 0.0005 |
| At most 11.0% | 150,745 (64.2%) | 432,191 (45.4%) | 0.3857 | 133,601 (61.8%) | 129,609 (60.0%) | 0.0378 |
| At most 10.5% | 148,443 (63.2%) | 422,823 (44.4%) | 0.3851 | 131,369 (60.8%) | 127,446 (59.0%) | 0.037 |
| At most 10.0% | 145,620 (62.0%) | 411,384 (43.2%) | 0.3845 | 128,625 (59.5%) | 124,702 (57.7%) | 0.0368 |
| At most 9.5% | 142,545 (60.7%) | 399,096 (41.9%) | 0.3836 | 125,658 (58.1%) | 121,680 (56.3%) | 0.0372 |
| At most 9.0% | 138,532 (59.0%) | 383,069 (40.2%) | 0.3829 | 121,816 (56.4%) | 117,808 (54.5%) | 0.0373 |
| At most 8.5% | 133,853 (57.0%) | 363,584 (38.2%) | 0.3845 | 117,348 (54.3%) | 113,434 (52.5%) | 0.0363 |
| At most 8.0% | 129,033 (55.0%) | 343,442 (36.1%) | 0.3869 | 112,735 (52.2%) | 108,934 (50.4%) | 0.0352 |
| Medications | ||||||
| Opiod analgesics | 108,932 (46.4%) | 381,176 (40.0%) | 0.1293 | 98,570 (45.6%) | 99,173 (45.9%) | 0.0056 |
| Proton pump inhibitors | 71,525 (30.5%) | 267,157 (28.1%) | 0.0533 | 64,820 (30.0%) | 65,383 (30.2%) | 0.0057 |
| Dicyclomine | 4813 (2.1%) | 9964 (1.0%) | 0.0814 | 3952 (1.8%) | 3864 (1.8%) | 0.0031 |
| Loperamide | 3068 (1.3%) | 11,347 (1.2%) | 0.0104 | 2749 (1.3%) | 2600 (1.2%) | 0.0062 |
| Diphenoxylate | 1123 (0.5%) | 2909 (0.3%) | 0.0277 | 945 (0.4%) | 940 (0.4%) | 0.0004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Veccia, D.; Liu, B.D.X.; Tse, W.; Fass, R.; Song, G. Diagnostic Evaluation of an Increased Risk of Developing Small Intestinal Bacterial Overgrowth Associated with Glucagon-like Peptide-1 (GLP-1) Receptor Agonists and Dual GLP-1/GIP Receptor Agonists: A Global Retrospective Multicenter Cohort Analysis. Diagnostics 2025, 15, 2264. https://doi.org/10.3390/diagnostics15172264
Sun Y, Veccia D, Liu BDX, Tse W, Fass R, Song G. Diagnostic Evaluation of an Increased Risk of Developing Small Intestinal Bacterial Overgrowth Associated with Glucagon-like Peptide-1 (GLP-1) Receptor Agonists and Dual GLP-1/GIP Receptor Agonists: A Global Retrospective Multicenter Cohort Analysis. Diagnostics. 2025; 15(17):2264. https://doi.org/10.3390/diagnostics15172264
Chicago/Turabian StyleSun, Yan, Donovan Veccia, Benjamin Douglas Xun Liu, William Tse, Ronnie Fass, and Gengqing Song. 2025. "Diagnostic Evaluation of an Increased Risk of Developing Small Intestinal Bacterial Overgrowth Associated with Glucagon-like Peptide-1 (GLP-1) Receptor Agonists and Dual GLP-1/GIP Receptor Agonists: A Global Retrospective Multicenter Cohort Analysis" Diagnostics 15, no. 17: 2264. https://doi.org/10.3390/diagnostics15172264
APA StyleSun, Y., Veccia, D., Liu, B. D. X., Tse, W., Fass, R., & Song, G. (2025). Diagnostic Evaluation of an Increased Risk of Developing Small Intestinal Bacterial Overgrowth Associated with Glucagon-like Peptide-1 (GLP-1) Receptor Agonists and Dual GLP-1/GIP Receptor Agonists: A Global Retrospective Multicenter Cohort Analysis. Diagnostics, 15(17), 2264. https://doi.org/10.3390/diagnostics15172264







