Liquid Biopsy’s Role in Head and Neck Tumors: Changing Paradigms in the Era of Precision Medicine
Abstract
1. Introduction
2. Head and Neck Tumors
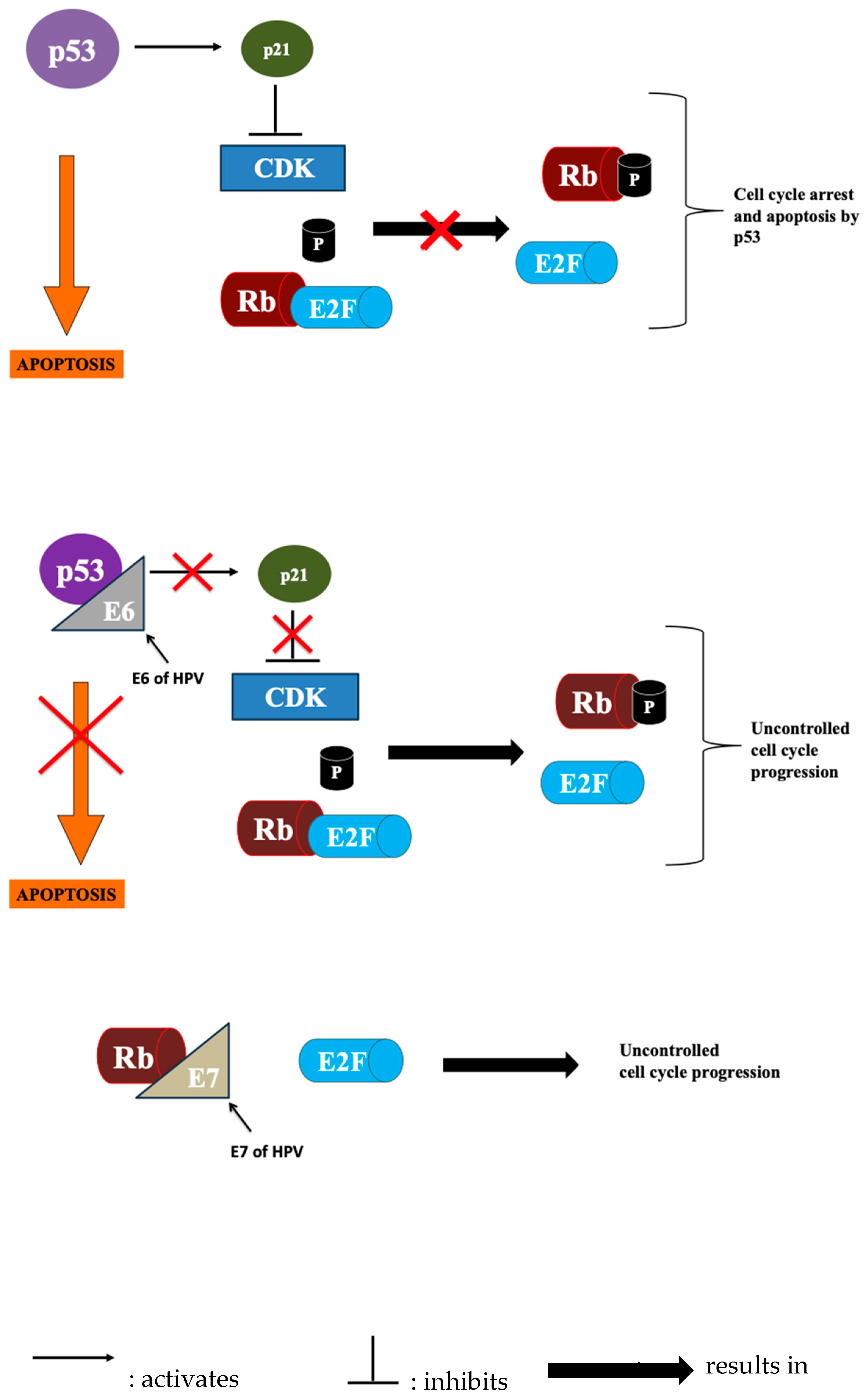
2.1. HPV-Negative HNSCC
2.2. HPV-Positive HNSCC
3. Treatment
3.1. Anti-PD-1-Axis-Targeted Therapy
3.2. Anti-CTLA-4
4. Liquid Biopsy
5. Liquid Biopsy Biomarkers in HNSCC
5.1. Circulating Tumor Cells (CTCs)
5.2. Cell-Free Tumor DNA (ctDNA)
5.3. Extracellular Vehicles (EVs)
5.4. Metabolites
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miserocchi, G.; Spadazzi, C.; Calpona, S.; De Rosa, F.; Usai, A.; De Vita, A.; Liverani, C.; Cocchi, C.; Vanni, S.; Calabrese, C.; et al. Precision Medicine in Head and Neck Cancers: Genomic and Preclinical Approaches. J. Pers. Med. 2022, 12, 854. [Google Scholar] [CrossRef] [PubMed]
- Son, E.; Panwar, A.; Mosher, C.H.; Lydiatt, D. Cancers of the Major Salivary Gland. J. Oncol. Pract. 2018, 14, 99–108. [Google Scholar] [CrossRef]
- Kong, L.; Birkeland, A.C. Liquid Biopsies in Head and Neck Cancer: Current State and Future Challenges. Cancers 2021, 13, 1874. [Google Scholar] [CrossRef]
- Oliveira, K.C.S.; Ramos, I.B.; Silva, J.M.C.; Barra, W.F.; Riggins, G.J.; Palande, V.; Pinho, C.T.; Frenkel-Morgenstern, M.; Santos, S.E.B.; Assumpcao, P.P.; et al. Current Perspectives on Circulating Tumor DNA, Precision Medicine, and Personalized Clinical Management of Cancer. Mol. Cancer Res. 2020, 18, 517–528. [Google Scholar] [CrossRef]
- Mader, S.; Pantel, K. Liquid Biopsy: Current Status and Future Perspectives. Oncol. Res. Treat. 2017, 40, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- Rodríguez, J.; Avila, J.; Rolfo, C.; Ruíz-Patiño, A.; Russo, A.; Ricaurte, L.; Ordóñez-Reyes, C.; Arrieta, O.; Zatarain-Barrón, Z.L.; Recondo, G.; et al. When Tissue is an Issue the Liquid Biopsy is Nonissue: A Review. Oncol. Ther. 2021, 9, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Arantes, L.; De Carvalho, A.C.; Melendez, M.E.; Lopes Carvalho, A. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef]
- Hanna, G.J.; Lau, C.J.; Mahmood, U.; Supplee, J.G.; Mogili, A.R.; Haddad, R.I.; Jänne, P.A.; Paweletz, C.P. Salivary HPV DNA informs locoregional disease status in advanced HPV-associated oropharyngeal cancer. Oral Oncol. 2019, 95, 120–126. [Google Scholar] [CrossRef]
- Mishra, V.; Singh, A.; Chen, X.; Rosenberg, A.J.; Pearson, A.T.; Zhavoronkov, A.; Savage, P.A.; Lingen, M.W.; Agrawal, N.; Izumchenko, E. Application of liquid biopsy as multi-functional biomarkers in head and neck cancer. Br. J. Cancer 2022, 126, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xiao, Y.; Miao, J.; Zhang, X.; Liu, M.; Zhu, L.; Liu, H.; Shen, X.; Wang, J.; Xie, B.; et al. Oxidative Stress and Inflammation: Drivers of Tumorigenesis and Therapeutic Opportunities. Antioxidants 2025, 14, 735. [Google Scholar] [CrossRef]
- Di Credico, G.; Polesel, J.; Dal Maso, L.; Pauli, F.; Torelli, N.; Luce, D.; Radoï, L.; Matsuo, K.; Serraino, D.; Brennan, P.; et al. Alcohol drinking and head and neck cancer risk: The joint effect of intensity and duration. Br. J. Cancer 2020, 123, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Diana, G.; Corica, C. Human Papilloma Virus vaccine and prevention of head and neck cancer, what is the current evidence? Oral Oncol. 2021, 115, 105168. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, D.; Jarouliya, U.; Chavda, V.; Yadav, A.K.; Chaurasia, B.; Song, M. Epidemiology, Molecular Pathogenesis, Immuno-Pathogenesis, Immune Escape Mechanisms and Vaccine Evaluation for HPV-Associated Carcinogenesis. Pathogens 2023, 12, 1380. [Google Scholar] [CrossRef]
- Riva, G.; Pecorari, G. Multimodality and Sequential Therapy in Locally Advanced Head and Neck Cancer: A Preface to the Special Issue. Cancers 2021, 13, 2609. [Google Scholar] [CrossRef]
- Barham, W.T.; Stagg, M.P.; Mualla, R.; DiLeo, M.; Kansara, S. Recurrent and Metastatic Head and Neck Cancer: Mechanisms of Treatment Failure, Treatment Paradigms, and New Horizons. Cancers 2025, 17, 144. [Google Scholar] [CrossRef]
- Grisanti, S.; Almici, C.; Consoli, F.; Buglione, M.; Verardi, R.; Bolzoni-Villaret, A.; Bianchetti, A.; Ciccarese, C.; Mangoni, M.; Ferrari, L.; et al. Circulating tumor cells in patients with recurrent or metastatic head and neck carcinoma: Prognostic and predictive significance. PLoS ONE 2014, 9, e103918. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.J.; Amdal, C.D.; Bjordal, K.; Astrup, G.L.; Herlofson, B.B.; Duprez, F.; Gama, R.R.; Jacinto, A.; Hammerlid, E.; Scricciolo, M.; et al. Serious Long-Term Effects of Head and Neck Cancer from the Survivors’ Point of View. Healthcare 2023, 11, 906. [Google Scholar] [CrossRef]
- Li, Q.; Tie, Y.; Alu, A.; Ma, X.; Shi, H. Targeted therapy for head and neck cancer: Signaling pathways and clinical studies. Signal Transduct. Target. Ther. 2023, 8, 31. [Google Scholar] [CrossRef]
- Temilola, D.O.; Adeola, H.A.; Grobbelaar, J.; Chetty, M. Liquid Biopsy in Head and Neck Cancer: Its Present State and Future Role in Africa. Cells 2023, 12, 2663. [Google Scholar] [CrossRef]
- Qian, J.M.; Schoenfeld, J.D. Radiotherapy and Immunotherapy for Head and Neck Cancer: Current Evidence and Challenges. Front. Oncol. 2020, 10, 608772. [Google Scholar] [CrossRef]
- Wongpanuwich, W.; Yodsanga, S.; Chaisuparat, R.; Amornphimoltham, P. Association Between PD-L1 and Histatin1, 3 Expression in Advanced Head and Neck Squamous Cell Carcinoma. Anticancer Res. 2022, 42, 2689–2699. [Google Scholar] [CrossRef]
- Mestiri, S.; El-Ella, D.M.A.; Fernandes, Q.; Bedhiafi, T.; Almoghrabi, S.; Akbar, S.; Inchakalody, V.; Assami, L.; Anwar, S.; Uddin, S.; et al. The dynamic role of immune checkpoint molecules in diagnosis, prognosis, and treatment of head and neck cancers. Biomed. Pharmacother. 2024, 171, 116095. [Google Scholar] [CrossRef]
- Koike, K.; Dehari, H.; Ogi, K.; Shimizu, S.; Nishiyama, K.; Sonoda, T.; Sasaki, T.; Sasaya, T.; Tsuchihashi, K.; Hasegawa, T.; et al. Prognostic value of FoxP3 and CTLA-4 expression in patients with oral squamous cell carcinoma. PLoS ONE 2020, 15, e0237465. [Google Scholar] [CrossRef]
- Martins, I.; Ribeiro, I.P.; Jorge, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Melo, J.B.; Carreira, I.M. Liquid Biopsies: Applications for Cancer Diagnosis and Monitoring. Genes 2021, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Camarero, S.; Pérez-Segura, P. Liquid Biopsy in Head and Neck Cancer: Current Evidence and Future Perspective on Squamous Cell, Salivary Gland, Paranasal Sinus and Nasopharyngeal Cancers. Cancers 2022, 14, 2858. [Google Scholar] [CrossRef] [PubMed]
- Leibetseder, A.; Preusser, M.; Berghoff, A.S. New Approaches with Precision Medicine in Adult Brain Tumors. Cancers 2022, 14, 712. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Wu, M.H.; Chang, P.H.; Lin, H.C.; Liao, C.D.; Wu, S.M.; Hung, T.M.; Lin, C.Y.; Chang, T.C.; Tzu-Tsen, Y.; et al. The change in circulating tumor cells before and during concurrent chemoradiotherapy is associated with survival in patients with locally advanced head and neck cancer. Head Neck 2019, 41, 2676–2687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Weeramange, C.E.; Hughes, B.G.M.; Vasani, S.; Liu, Z.Y.; Warkiani, M.; Hartel, G.; Ladwa, R.; Thiery, J.P.; Kenny, L.; et al. Circulating tumour cells predict recurrences and survival in head and neck squamous cell carcinoma patients. Cell. Mol. Life Sci. 2024, 81, 233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, L.; Zhang, W.; Liu, F.; Zhang, Y.; Jiang, B.; Wang, J.; Yuan, H. Circulating Tumor Cells Correlate With Prognosis in Head and Neck Squamous Cell Carcinoma. Technol. Cancer Res. Treat. 2021, 20, 1533033821990037. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hossain, M.M.; Islam, S.; Ahmed, R.; Majumder, M.; Dey, S.; Kawser, M.; Sarkar, B.; Himu, M.E.R.; Chowdhury, A.A.; et al. CTC together with Shh and Nrf2 are prospective diagnostic markers for HNSCC. BMC Mol. Cell Biol. 2024, 25, 4. [Google Scholar] [CrossRef]
- Sun, T.; Zou, K.; Yuan, Z.; Yang, C.; Lin, X.; Xiong, B. Clinicopathological and prognostic significance of circulating tumor cells in patients with head and neck cancer: A meta-analysis. Onco Targets Ther. 2017, 10, 3907–3916. [Google Scholar] [CrossRef]
- Jansson, S.; Bendahl, P.-O.; Larsson, A.-M.; Aaltonen, K.E.; Rydén, L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 2016, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Kulasinghe, A.; Schmidt, H.; Perry, C.; Whitfield, B.; Kenny, L.; Nelson, C.; Warkiani, M.E.; Punyadeera, C. A Collective Route to Head and Neck Cancer Metastasis. Sci. Rep. 2018, 8, 746. [Google Scholar] [CrossRef]
- Patel, A.; Patel, S.; Patel, P.; Tanavde, V. Saliva Based Liquid Biopsies in Head and Neck Cancer: How Far Are We From the Clinic? Front. Oncol. 2022, 12, 828434. [Google Scholar] [CrossRef]
- Stucky, A.; Viet, C.T.; Aouizerat, B.E.; Ye, Y.; Doan, C.; Mundluru, T.; Sedhiazadeh, P.; Sinha, U.K.; Chen, X.; Zhang, X.; et al. Single-Cell Molecular Profiling of Head and Neck Squamous Cell Carcinoma Reveals Five Dysregulated Signaling Pathways Associated with Circulating Tumor Cells. Cancer Control 2024, 31, 10732748241251571. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, H.; Li, G.; Wei, H.; Xie, C.; Tuo, Y.; Chen, N.; Yu, D. Temporal and spatial characteristics of tumor evolution in a mouse model of oral squamous cell carcinoma. BMC Cancer 2022, 22, 1209. [Google Scholar] [CrossRef] [PubMed]
- Kulasinghe, A.; Kenny, L.; Perry, C.; Thiery, J.-P.; Jovanovic, L.; Vela, I.; Nelson, C.; Punyadeera, C. Impact of label-free technologies in head and neck cancer circulating tumour cells. Oncotarget 2016, 7, 71223–71234. [Google Scholar] [CrossRef]
- Kaorey, N.; Dickinson, K.; Agnihotram, V.R.; Zeitouni, A.; Sadeghi, N.; Burnier, J.V. The role of ctDNA from liquid biopsy in predicting survival outcomes in HPV-negative head and neck cancer: A meta-analysis. Oral Oncol. 2025, 161, 107148. [Google Scholar] [CrossRef]
- Chikuie, N.; Urabe, Y.; Ueda, T.; Hamamoto, T.; Taruya, T.; Kono, T.; Yumii, K.; Takeno, S. Utility of plasma circulating tumor DNA and tumor DNA profiles in head and neck squamous cell carcinoma. Sci. Rep. 2022, 12, 9316. [Google Scholar] [CrossRef]
- Marret, G.; Lamy, C.; Vacher, S.; Cabel, L.; Séné, M.; Ahmanache, L.; Courtois, L.; El Beaino, Z.; Klijanienko, J.; Martinat, C.; et al. Deciphering molecular relapse and intra-tumor heterogeneity in non-metastatic resectable head and neck squamous cell carcinoma using circulating tumor DNA. Oral Oncol. 2025, 160, 107111. [Google Scholar] [CrossRef]
- Hilke, F.J.; Muyas, F.; Admard, J.; Kootz, B.; Nann, D.; Welz, S.; Rieß, O.; Zips, D.; Ossowski, S.; Schroeder, C.; et al. Dynamics of cell-free tumour DNA correlate with treatment response of head and neck cancer patients receiving radiochemotherapy. Radiother. Oncol. 2020, 151, 182–189. [Google Scholar] [CrossRef]
- Silvoniemi, A.; Laine, J.; Aro, K.; Nissi, L.; Bäck, L.; Schildt, J.; Hirvonen, J.; Hagström, J.; Irjala, H.; Aaltonen, L.M.; et al. Circulating Tumor DNA in Head and Neck Squamous Cell Carcinoma: Association with Metabolic Tumor Burden Determined with FDG-PET/CT. Cancers 2023, 15, 3970. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Lin, X.; Yin, X.; Cui, Y.; Ma, L. Prognostic value of MTV and TLG of 18 F-FDG PET in patients with head and neck squamous cell carcinoma: A meta-analysis. Medicine 2022, 101, e30798. [Google Scholar] [CrossRef]
- Lele, S.J.; Adilbay, D.; Lewis, E.; Pang, J.; Asarkar, A.A.; Nathan, C.O. ctDNA as an Adjunct to Posttreatment PET for Head and Neck Cancer Recurrence Risk Assessment. Otolaryngol. Head Neck Surg. 2024, 171, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Rapado-González, Ó.; Rodríguez-Ces, A.M.; López-López, R.; Suárez-Cunqueiro, M.M. Liquid biopsies based on cell-free DNA as a potential biomarker in head and neck cancer. Jpn. Dent. Sci. Rev. 2023, 59, 289–302. [Google Scholar] [CrossRef]
- Payne, K.F.B.; Brotherwood, P.; Suriyanarayanan, H.; Brooks, J.M.; Batis, N.; Beggs, A.D.; Gendoo, D.M.A.; Mehanna, H.; Nankivell, P. Circulating tumour DNA detects somatic variants contributing to spatial and temporal intra-tumoural heterogeneity in head and neck squamous cell carcinoma. Front. Oncol. 2024, 14, 1374816. [Google Scholar] [CrossRef]
- Schwaederle, M.; Chattopadhyay, R.; Kato, S.; Fanta, P.T.; Banks, K.C.; Choi, I.S.; Piccioni, D.E.; Ikeda, S.; Talasaz, A.; Lanman, R.B.; et al. Genomic Alterations in Circulating Tumor DNA from Diverse Cancer Patients Identified by Next-Generation Sequencing. Cancer Res. 2017, 77, 5419–5427. [Google Scholar] [CrossRef]
- Wilson, H.L.; D’Agostino, R.B., Jr.; Meegalla, N.; Petro, R.; Commander, S.; Topaloglu, U.; Zhang, W.; Porosnicu, M. The Prognostic and Therapeutic Value of the Mutational Profile of Blood and Tumor Tissue in Head and Neck Squamous Cell Carcinoma. Oncologist 2021, 26, e279–e289. [Google Scholar] [CrossRef] [PubMed]
- Damerla, R.R.; Lee, N.Y.; You, D.; Soni, R.; Shah, R.; Reyngold, M.; Katabi, N.; Wu, V.; McBride, S.M.; Tsai, C.J.; et al. Detection of Early Human Papillomavirus-Associated Cancers by Liquid Biopsy. JCO Precis. Oncol. 2019, 3, PO.18.00276. [Google Scholar] [CrossRef] [PubMed]
- Chera, B.S.; Kumar, S.; Shen, C.; Amdur, R.; Dagan, R.; Green, R.; Goldman, E.; Weiss, J.; Grilley-Olson, J.; Patel, S.; et al. Plasma Circulating Tumor HPV DNA for the Surveillance of Cancer Recurrence in HPV-Associated Oropharyngeal Cancer. J. Clin. Oncol. 2020, 38, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Chundury, A.; Kim, S. Radiation Dose De-Escalation in HPV-Positive Oropharynx Cancer: When Will It Be an Acceptable Standard of Care? J. Clin. Oncol. 2021, 39, 947–949. [Google Scholar] [CrossRef]
- Alami, I.E.; Gihbid, A.; Charoute, H.; Khaali, W.; Brahim, S.M.; Tawfiq, N.; Cadi, R.; Belghmi, K.; El Mzibri, M.; Khyatti, M. Prognostic value of Epstein-Barr virus DNA load in nasopharyngeal carcinoma: A meta-analysis. Pan Afr. Med J. 2022, 41, 6. [Google Scholar] [CrossRef]
- Chang, W.H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. Methods Mol. Biol. 2021, 2174, 143–170. [Google Scholar] [CrossRef]
- Qu, X.; Li, J.W.; Chan, J.; Meehan, K. Extracellular Vesicles in Head and Neck Cancer: A Potential New Trend in Diagnosis, Prognosis, and Treatment. Int. J. Mol. Sci. 2020, 21, 8260. [Google Scholar] [CrossRef]
- Sanesi, L.; Mori, G.; Troiano, G.; Ballini, A.; Valzano, F.; Dioguardi, M.; Muzio, L.L.; Magalhaes, M.; Caponio, V.C.A. Salivary exosomal microRNA profile as biomonitoring tool for diagnosis and prognosis of patients with head and neck squamous cell carcinoma: A systematic review. Arch. Oral Biol. 2024, 165, 106012. [Google Scholar] [CrossRef]
- Hofmann, L.; Ludwig, S.; Vahl, J.M.; Brunner, C.; Hoffmann, T.K.; Theodoraki, M.-N. The Emerging Role of Exosomes in Diagnosis, Prognosis, and Therapy in Head and Neck Cancer. Int. J. Mol. Sci. 2020, 21, 4072. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Song, F.; Zheng, Y.L.; Lv, J.; Wang, Q.F.; Xu, N. Exosomes in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 894. [Google Scholar] [CrossRef]
- Li, X.; Cao, Y.; Gong, X.; Li, H. Long noncoding RNAs in head and neck cancer. Oncotarget 2017, 8, 10726–10740. [Google Scholar] [CrossRef]
- Chaudhary, R.; Wang, X.; Cao, B.; De La Iglesia, J.; Masannat, J.; Song, F.; Hernandez-Prera, J.C.; Gimbrone, N.T.; Slebos, R.J.; Chung, C.H. Long noncoding RNA, LINC00460, as a prognostic biomarker in head and neck squamous cell carcinoma (HNSCC). Am. J. Transl. Res. 2020, 12, 684–696. [Google Scholar]
- Minami, S.; Chikazu, D.; Ochiya, T.; Yoshioka, Y. Extracellular vesicle-based liquid biopsies in cancer: Future biomarkers for oral cancer. Transl. Oncol. 2023, 38, 101786. [Google Scholar] [CrossRef]
- Ludwig, S.; Floros, T.; Theodoraki, M.N.; Hong, C.S.; Jackson, E.K.; Lang, S.; Whiteside, T.L. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 4843–4854. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef]
- Teng, Y.; Gao, L.; Loveless, R.; Rodrigo, J.P.; Strojan, P.; Willems, S.M.; Nathan, C.-A.; Mäkitie, A.A.; Saba, N.F.; Ferlito, A. The Hidden Link of Exosomes to Head and Neck Cancer. Cancers 2021, 13, 5802. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Zhu, G. Extracellular vesicle-based biomarker in head and neck cancer: Prospects and challenges. Malig. Spectr. 2024, 1, 75–90. [Google Scholar] [CrossRef]
- Mohd Faizal, N.F.; Vincent-Chong, V.K.; Ramanathan, A.; Paterson, I.C.; Karen-Ng, L.P.; Zaini, Z.M. Metabolomic Profiling of Oral Potentially Malignant Disorders and Its Clinical Values. Biomedicines 2024, 12, 2899. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kamarajan, P.; Cho, A.; Wang, S.; Hung, G.C.; Najarzadegan, F.; Wong, D.T.; Ton-That, H.; Wang, C.Y.; Kapila, Y.L. Biological biomarkers of oral cancer. Periodontology 2000 2024, 96, 250–280. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Zhang, R.; Song, Y.; Cao, J.; Bi, N.; Wang, J.; He, J.; Bai, J.; Dong, L.; et al. Global and targeted metabolomics of esophageal squamous cell carcinoma discovers potential diagnostic and therapeutic biomarkers. Mol. Cell. Proteom. 2013, 12, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
| Disadvantages of Tissue Biopsy | Advantages of Liquid Biopsy |
|---|---|
| Sampling error: May fail to capture the most representative part of the tumor | Offers a more comprehensive molecular profile by capturing circulating tumor DNA (ctDNA) from multiple tumor sites |
| Limited to localized and easily accessible tumors, with difficulty in identifying metastasis | Powerful in detecting tumors distant from primary site via analysis of bodily fluids (e.g., blood) |
| Poor detection of tumoral heterogeneity | Potential to exhibit the presence of all the existing subclones |
| Pain and discomfort caused by its invasive nature | Minimally invasive and easy-to-collect nature |
| Non-real-time monitoring: incapable of performing the procedure consecutively on patients due to invasive nature | Repeatability and serial sample collection over time allows for evaluation and assessment of disease progression |
| Costly and time-consuming | Low cost and time required for collecting samples |
| Advantages | Disadvantages | |
|---|---|---|
| CTCs | Provides a complete and necessary overview (genomics and proteomics); Enables testing on living cells, both in vitro and in vivo; Presence of immune checkpoint markers on CTCs allows for immunotherapeutic studies | Few found in circulation and in other fluids; Contamination with healthy neighboring cells, such as leukocytes; Requires rapid processing of fresh blood samples; Loss of epithelial markers (Ep-CAM-negative CTCs) during EMT and misses mesenchymal-type CTCs; Low sensitivity in early-stage and HPV+ disease; Some methods (e.g., filtration and centrifugation) can damage fragile CTCs, preventing downstream culture or functional assays |
| CtDNA | Isolated from different sources and many different biofluids; Less biofluid volume is necessary (when compared to CTCs); Associated with many cancer types; Allows for the detection of many genetic mutations; Its short half-life allows for tracking of tumor load changes | A small amount of material is shed in the circulation, especially in early-stage and locally advanced HNCs; Release of DNA from normal cells dying from apoptosis or other processes may interfere with tumor ctDNA detection |
| EVs | Provide comprehensive information from many biomolecules (DNA/RNA/proteins); Present in large amounts in several bodily fluids; miRNA in EV cargo is relatively stable and not easily degraded and, hence, readily stored | Lack of standardization for detection and isolation; Difficulty in specifically isolating tumor-derived EVs from the total EV population; Biological properties must be validated: EV size and content |
| Metabolites | Specific signatures (such as high lactic acid and ketone body levels) are correlated with tumor burden, cancer stage, and treatment resistance in pilot studies. Help to understand the tumor microenvironment (e.g., hypoxia and Warburg effect) | Low tumor specificity and confounded by systemic factors; Require complex computational models to identify meaningful patterns; Large prospective validation studies are lacking Pre-analytical stability is a major concern |
| Disadvantages of Liquid Biopsy |
|---|
| Detection of both ctDNA and CTC is not achievable to the same extent at all tumoral sites within the same patient, even within the same tumoral entity. |
| Large bodily fluid samples are required |
| Optimal sensitivities and specificities of different biomarkers have not yet been definitively established. **** |
| Standardization gap: No universal protocol across institutions: |
|
| Anatomical and biological considerations: |
|
| Operational barriers: |
|
| Parameter | CTCs | ctDNA | EVs | Metabolites |
|---|---|---|---|---|
| Sensitivity | Moderate: ~40–70% | High (with NGS/ddPCR): ~75–95% in metastatic HNSCC, ~50–70% in localized disease | Sensitivity for tumor-derived EVs is not yet well quantified because although plasma contains billions of EVs/mL, the tumor-specific signal is diluted by the background from normal cells. | Moderate–High: Sensitive platforms can detect pM-nM concentrations. Specific tumor-derived metabolic signatures are still being defined. |
| Specificity | High: Relies on positive selection for epithelial (EpCAM) and/or tumor-specific markers and negative selection for CD45 (leukocytes). | Very high for mutation-specific assays (e.g., detecting a known TP53 mutation or HPV-DNA) | Moderate: Challenging to distinguish tumor-derived EVs from host EVs. Specificity depends on identifying unique surface markers or cargo (e.g., EGFRvIII and HPV-RNA). | Low–Moderate: Metabolic changes are highly influenced by non-tumor factors (diet, inflammation, exercise, and comorbidities). |
| Key Clinical Applications | - Prognostic stratification - Pharmacodynamic biomarker for therapy monitoring - Functional studies (e.g., cultures and xenografts) to study metastasis | - “Molecular Residual Disease” (MRD) detection - Real-time therapy response monitoring - Identifying actionable/targetable genomic alterations - Early relapse detection | - Early detection and diagnosis - Monitoring immune checkpoint expression (such as PD-L1+ EVs) - Intercellular communication research - Potential as drug delivery vehicles | - Identification of metabolic therapeutic targets - Adjunct to imaging for diagnosis and monitoring - Understanding the tumor microenvironment (hypoxia and Warburg effect) |
| Translational Readiness Level for HNSCC | Intermediate: An FDA-cleared platform (CellSearch®) exists but is primarily prognostic. Not yet the standard of care | High (Rapidly Advancing): ctDNA for MRD and genotyping is entering clinical guidelines via NCCN for other cancers; HNSCC-specific validation is ongoing | Low–Intermediate (Preclinical/Early Clinical): Active area of research. No standardized clinical assays exist yet | Low (Preclinical): Primarily in the research domain; not yet ready for clinical decision making |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalzal, R.N.; Fakhruddin, N.; Mahfouz, R. Liquid Biopsy’s Role in Head and Neck Tumors: Changing Paradigms in the Era of Precision Medicine. Diagnostics 2025, 15, 2262. https://doi.org/10.3390/diagnostics15172262
Zalzal RN, Fakhruddin N, Mahfouz R. Liquid Biopsy’s Role in Head and Neck Tumors: Changing Paradigms in the Era of Precision Medicine. Diagnostics. 2025; 15(17):2262. https://doi.org/10.3390/diagnostics15172262
Chicago/Turabian StyleZalzal, Rudy N., Najla Fakhruddin, and Rami Mahfouz. 2025. "Liquid Biopsy’s Role in Head and Neck Tumors: Changing Paradigms in the Era of Precision Medicine" Diagnostics 15, no. 17: 2262. https://doi.org/10.3390/diagnostics15172262
APA StyleZalzal, R. N., Fakhruddin, N., & Mahfouz, R. (2025). Liquid Biopsy’s Role in Head and Neck Tumors: Changing Paradigms in the Era of Precision Medicine. Diagnostics, 15(17), 2262. https://doi.org/10.3390/diagnostics15172262






