Pulmonary Fungal Infections in a Tertiary Cancer Center: A Morphological Correlation of 160 Cases with CT and PET Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Variable Classification
2.3. Histological Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Features
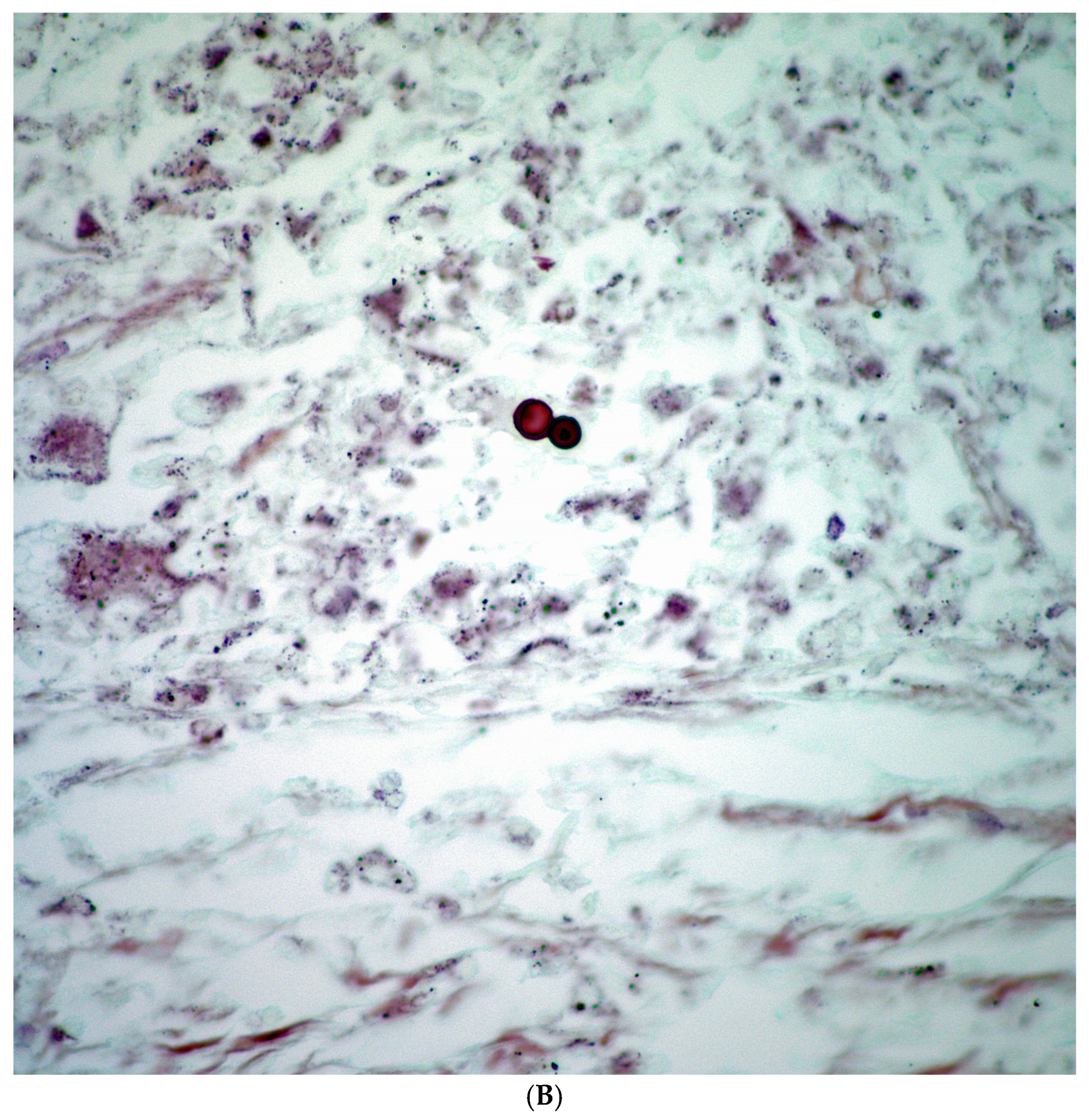
3.2. Histopathological Features
4. Discussion
5. Conclusions
- By imaging, the distinction between an infectious process and neoplasia remains a diagnostic challenge.
- Even though tissue cultures and antigen testing in plasma and/or urine are important, often these are not necessarily available, but should be part of the overall assessment of patients with pulmonary nodules.
- Careful assessment of surgical material becomes highly important in identifying a fungal organism.
- Special histochemical stains (GMS) are recommended, and when needed, PCR studies are recommended.
- Morphological description of the fungal organism is an important role of the pathologist.
- In a small proportion of cases, the infectious process may also be associated with a neoplastic process.
- Clinicians should maintain a low threshold for fungal testing in patients with pulmonary nodules.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azar, M.M.; Hage, C.A.; Kraft, C.S. Laboratory Diagnostics for Histoplasmosis. J. Clin. Microbiol. 2017, 55, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J.; Azar, M.M.; Bahr, N.C.; Spec, A.; Relich, R.F.; Hage, C. Histoplasmosis. Infect. Dis. Clin. N. Am. 2016, 30, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, S.; Nordin, A.J.; Noraini, A.R.; Rossetti, C. PET/CT in nononcological lung diseases: Current applications and future perspectives. Eur. Respir. Rev. 2016, 25, 247–258. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Wu, K.-C.; Tseng, N.-C.; Chen, Y.-J.; Chang, C.-J.; Yen, K.-Y.; Kao, C.-H. Differentiation Between Malignant and Benign Pulmonary Nodules by Using Automated Three-Dimensional High-Resolution Representation Learning With Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography. Front. Med. 2022, 9, 773041. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S. Histoplasma capsulatum: More Widespread than Previously Thought. Am. J. Trop. Med. Hyg. 2014, 90, 982–983. [Google Scholar] [CrossRef]
- Song, K.D.; Lee, K.S.; Chung, M.P.; Kwon, O.J.; Kim, T.S.; Yi, C.A.; Chung, M.J. Pulmonary Cryptococcosis: Imaging Findings in 23 Non-AIDS Patients. Korean J. Radiol. 2010, 11, 407–416. [Google Scholar] [CrossRef]
- Azar, M.M.; Bahr, N.; Loyd, J.; Hage, C.A.; Wheat, L.J. Histoplasmosis: Up-to-Date Evidence-Based Approach to Diagnosis and Management. Semin. Respir. Crit. Care Med. 2015, 36, 729–745. [Google Scholar] [CrossRef]
- Gazzoni, F.F.; Severo, L.C.; Marchiori, E.; Irion, K.L.; Guimarães, M.D.; Godoy, M.C.; Sartori, A.P.G.; Hochhegger, B. Fungal diseases mimicking primary lung cancer: Radiologic–pathologic correlation. Mycoses 2013, 57, 197–208. [Google Scholar] [CrossRef]
- Soubani, A.O.; Chandrasekar, P.H. The Clinical Spectrum of Pulmonary Aspergillosis. Chest 2002, 121, 1988–1999. [Google Scholar] [CrossRef]
- Park, M.; Ho, D.Y.; Wakelee, H.A.; Neal, J.W. Opportunistic Invasive Fungal Infections Mimicking Progression of Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2021, 22, e193–e200. [Google Scholar] [CrossRef]
- Nagarajappa, P.; Mylavarapu, M.; Gurumurthy, S.; Tschen, J.A.; Tschen, J.A. Cutaneous Coccidioidomycosis and Basal Cell Carcinoma: Case Report on a Diagnostic Dilemma. Cureus 2023, 15, e43374. [Google Scholar] [CrossRef]
- Qurashi, S.; Saleem, T.; Kovalenko, I.; Golubykh, K.; Holleran, L. Cryptococcus neoformans Presenting as a Lung Mass in an Immunocompromised Patient. Am. J. Case Rep. 2022, 23, e936968-1–e936968-6. [Google Scholar] [CrossRef]
- Sassi, C.; Stanzani, M.; Lewis, R.E.; Facchini, G.; Bazzocchi, A.; Cavo, M.; Battista, G. The utility of contrast-enhanced hypodense sign for the diagnosis of pulmonary invasive mould disease in patients with haematological malignancies. Br. J. Radiol. 2018, 91, 20170220. [Google Scholar] [CrossRef]
- Godoy, M.C.B.; Viswanathan, C.; Marchiori, E.; Truong, M.T.; Benveniste, M.F.; Rossi, S.; Marom, E.M. The reversed halo sign: Update and differential diagnosis. Br. J. Radiol. 2012, 85, 1226–1235. [Google Scholar] [CrossRef]
- Pria, H.R.F.D.; Pria, O.A.F.D.; Ahuja, J.; Agrawal, R.; Strange, C.D.; Price, M.C.; Truong, M.T. PET/CT: Interpretative Pitfalls in the Thorax. Radiol. Clin. N. Am. 2025, 63, 553–563. [Google Scholar] [CrossRef]
- Bryant, A.S.; Cerfolio, R.J. The Maximum Standardized Uptake Values on Integrated FDG-PET/CT Is Useful in Differentiating Benign From Malignant Pulmonary Nodules. Ann. Thorac. Surg. 2006, 82, 1016–1020. [Google Scholar] [CrossRef]
- Deppen, S.; Putnam, J.B., Jr.; Andrade, G.; Speroff, T.; Nesbitt, J.C.; Lambright, E.S.; Massion, P.P.; Walker, R.; Grogan, E.L. Accuracy of FDG-PET to Diagnose Lung Cancer in a Region of Endemic Granulomatous Disease. Ann. Thorac. Surg. 2011, 92, 428–433. [Google Scholar] [CrossRef]
- Yasuda, M.; Nagashima, A.; Haro, A.; Saitoh, G. Aspergilloma mimicking a lung cancer. Int. J. Surg. Case Rep. 2013, 4, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, M.; Zhang, F.; Lou, X. Clinical Diagnosis, Treatment, and Laboratory Detection of 50 Cases of Pulmonary Cryptococcosis. Comput. Math. Methods Med. 2022, 2022, 7981472. [Google Scholar] [CrossRef] [PubMed]
- Salhab, K.F.; Baram, D.; Bilfinger, T.V. Growing PET positive nodule in a patient with histoplasmosis: Case report. J. Cardiothorac. Surg. 2006, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, M.K.; Polk, J.W.; Reddy, P.; Mohanty, S.K. Surgical aspects of pulmonary histoplasmosis: A series of 110 cases. Thorax 1970, 25, 31–40. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, A.; Tammemagi, M.C.; Mayo, J.R.; Roberts, H.; Liu, G.; Soghrati, K.; Yasufuku, K.; Martel, S.; Laberge, F.; Gingras, M.; et al. Probability of Cancer in Pulmonary Nodules Detected on First Screening CT. N. Engl. J. Med. 2013, 369, 910–919. [Google Scholar] [CrossRef]
- Erasmus, J.J.; Connolly, J.E.; McAdams, H.P.; Roggli, V.L. Solitary Pulmonary Nodules: Part I. Morphologic Evaluation for Differentiation of Benign and Malignant Lesions. RadioGraphics 2000, 20, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e93S–e120S. [Google Scholar] [CrossRef] [PubMed]
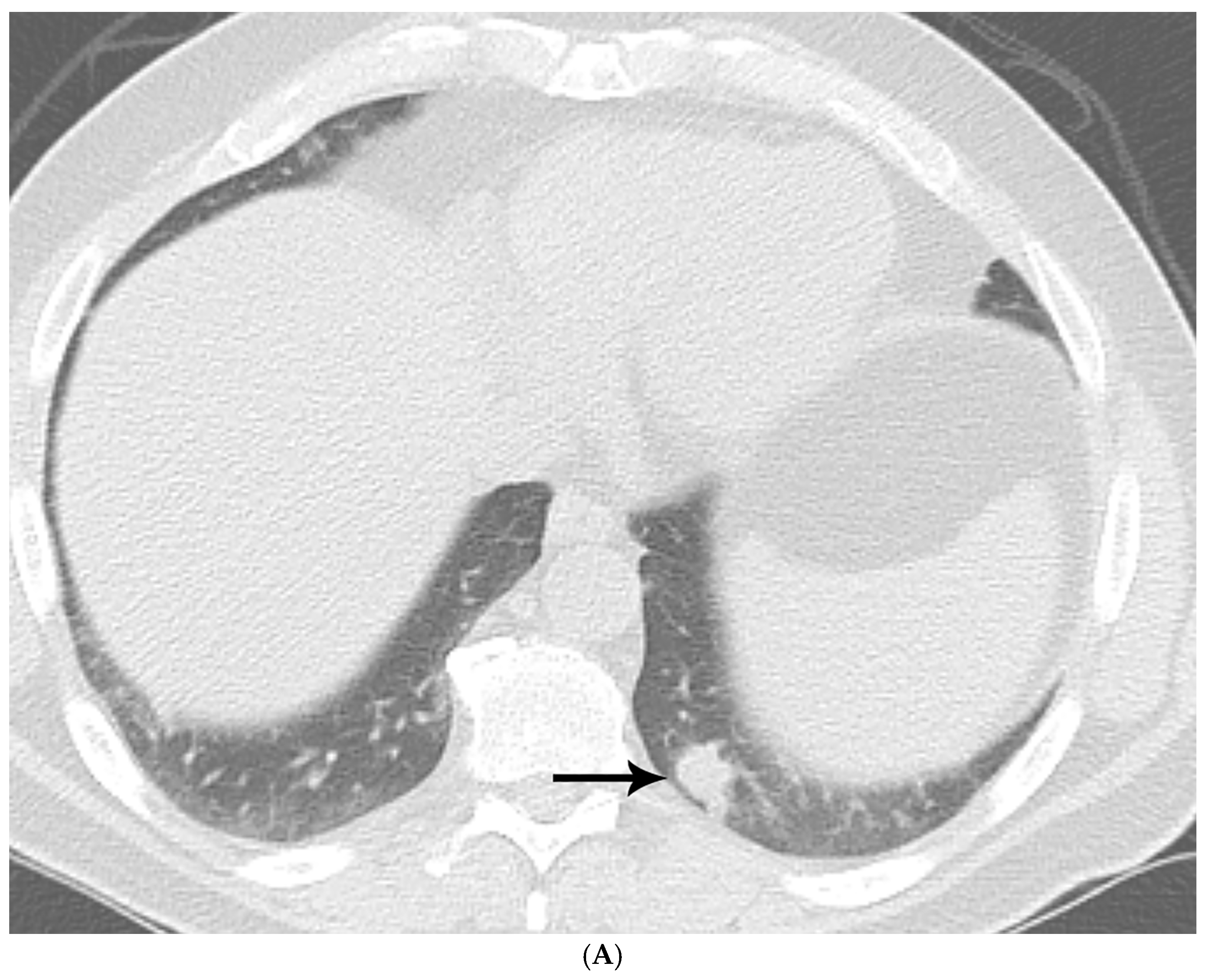
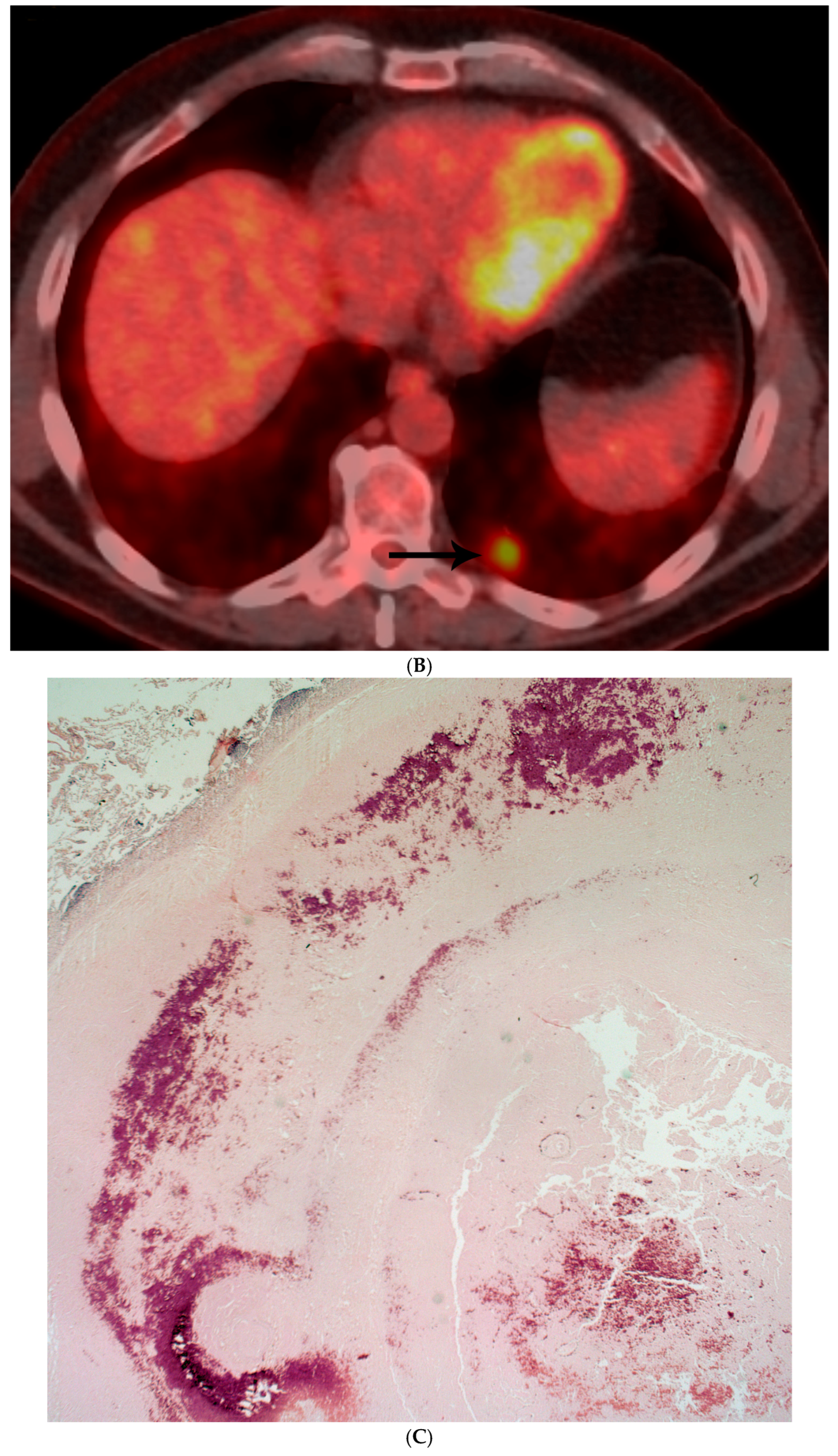

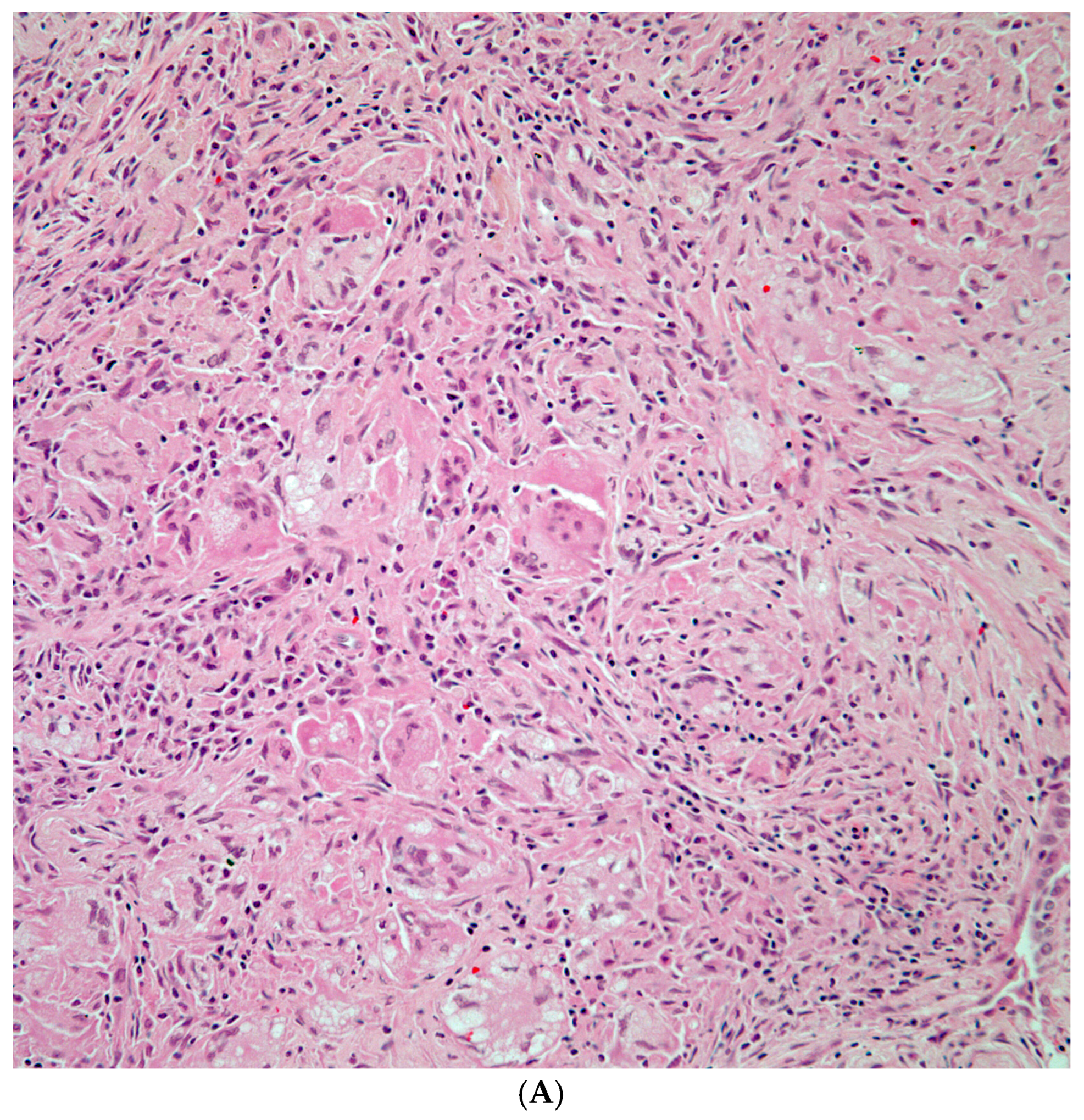
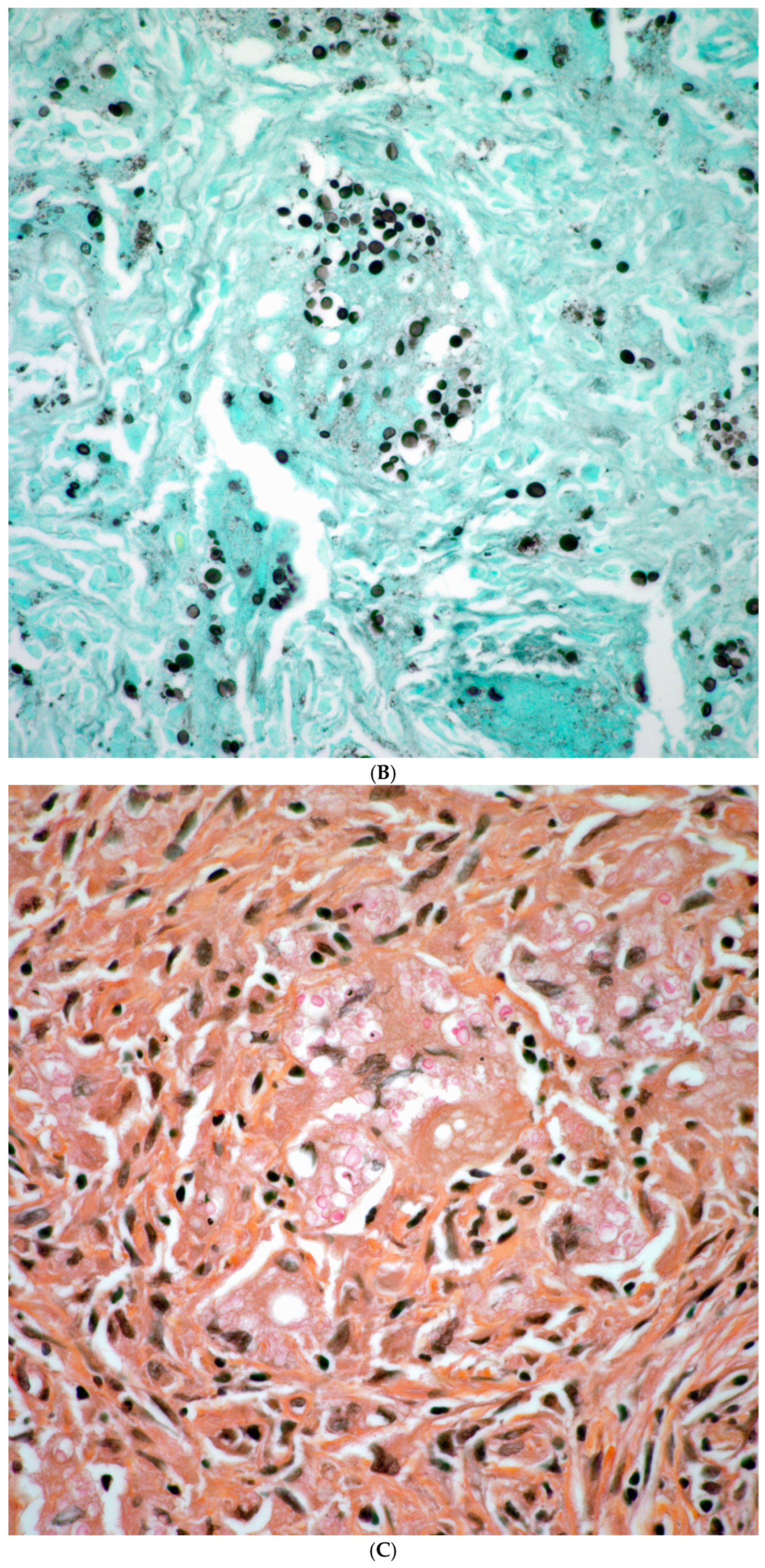





| Fungal Species | Patients with Prior Malignancy (n) | % of Species Cohort |
|---|---|---|
| Histoplasma spp. | 21 | 31.3% |
| Cryptococcus spp. | 15 | 37.5% |
| Aspergillus spp. | 19 | 55.9% |
| Coccidioides spp. | 4 | 28.6% |
| Blastomyces spp. | 2 | 50.0% |
| Mucor spp. | 0 | 0% |
| Total | 61 | 38.1% of all cases |
| Fungal Species | Cases (n) | Percentage (%) | ||
| Histoplasma spp. | 67 | 41.9% | ||
| Cryptococcus spp. | 40 | 25.0% | ||
| Aspergillus spp. | 34 | 21.3% | ||
| Coccidioides spp. | 14 | 8.8% | ||
| Blastomyces spp. | 4 | 2.5% | ||
| Mucor spp. | 1 | 0.6% | ||
| Fungal Species | Total Cases (n) | With Malignancy (n) | Fungal Only (n) | % with Malignancy |
| Histoplasma spp. | 67 | 5 | 62 | 7.5% |
| Cryptococcus spp. | 40 | 7 | 33 | 17.5% |
| Aspergillus spp. | 34 | 6 | 28 | 17.6% |
| Coccidioides spp. | 14 | 4 | 10 | 28.6% |
| Blastomyces spp. | 4 | 2 | 2 | 50.0% |
| Mucor spp. | 1 | 0 | 1 | 0% |
| Total | 160 | 24 | 136 | 15.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyos, S.; Truong, M.T.; Moran, C.A. Pulmonary Fungal Infections in a Tertiary Cancer Center: A Morphological Correlation of 160 Cases with CT and PET Imaging. Diagnostics 2025, 15, 2238. https://doi.org/10.3390/diagnostics15172238
Lyos S, Truong MT, Moran CA. Pulmonary Fungal Infections in a Tertiary Cancer Center: A Morphological Correlation of 160 Cases with CT and PET Imaging. Diagnostics. 2025; 15(17):2238. https://doi.org/10.3390/diagnostics15172238
Chicago/Turabian StyleLyos, Sebastian, Mylene T. Truong, and Cesar A. Moran. 2025. "Pulmonary Fungal Infections in a Tertiary Cancer Center: A Morphological Correlation of 160 Cases with CT and PET Imaging" Diagnostics 15, no. 17: 2238. https://doi.org/10.3390/diagnostics15172238
APA StyleLyos, S., Truong, M. T., & Moran, C. A. (2025). Pulmonary Fungal Infections in a Tertiary Cancer Center: A Morphological Correlation of 160 Cases with CT and PET Imaging. Diagnostics, 15(17), 2238. https://doi.org/10.3390/diagnostics15172238








