[18F]FDG PET/CT Radiomics in Untreated Breast Carcinoma: A Review of the Current State and Future Directions
Abstract
1. Introduction
- The anatomic stage, which includes the characteristics of the primary tumor (T), lymph node invasion (N), and distant metastases (M),
- The prognostic stage, which incorporates the degree of tumor differentiation, hormonal receptor status, estrogen receptors (ER), progesterone receptors (PR), human epidermal growth factor receptor 2 (HER2) expression, and multigene test results alongside the anatomic stage.
1.1. Positron Emission Tomography Combined with Computed Tomography Using 18F-Fluoro-2-deoxy-D-glucose ([18F]FDG PET/CT)
1.2. Radiomics
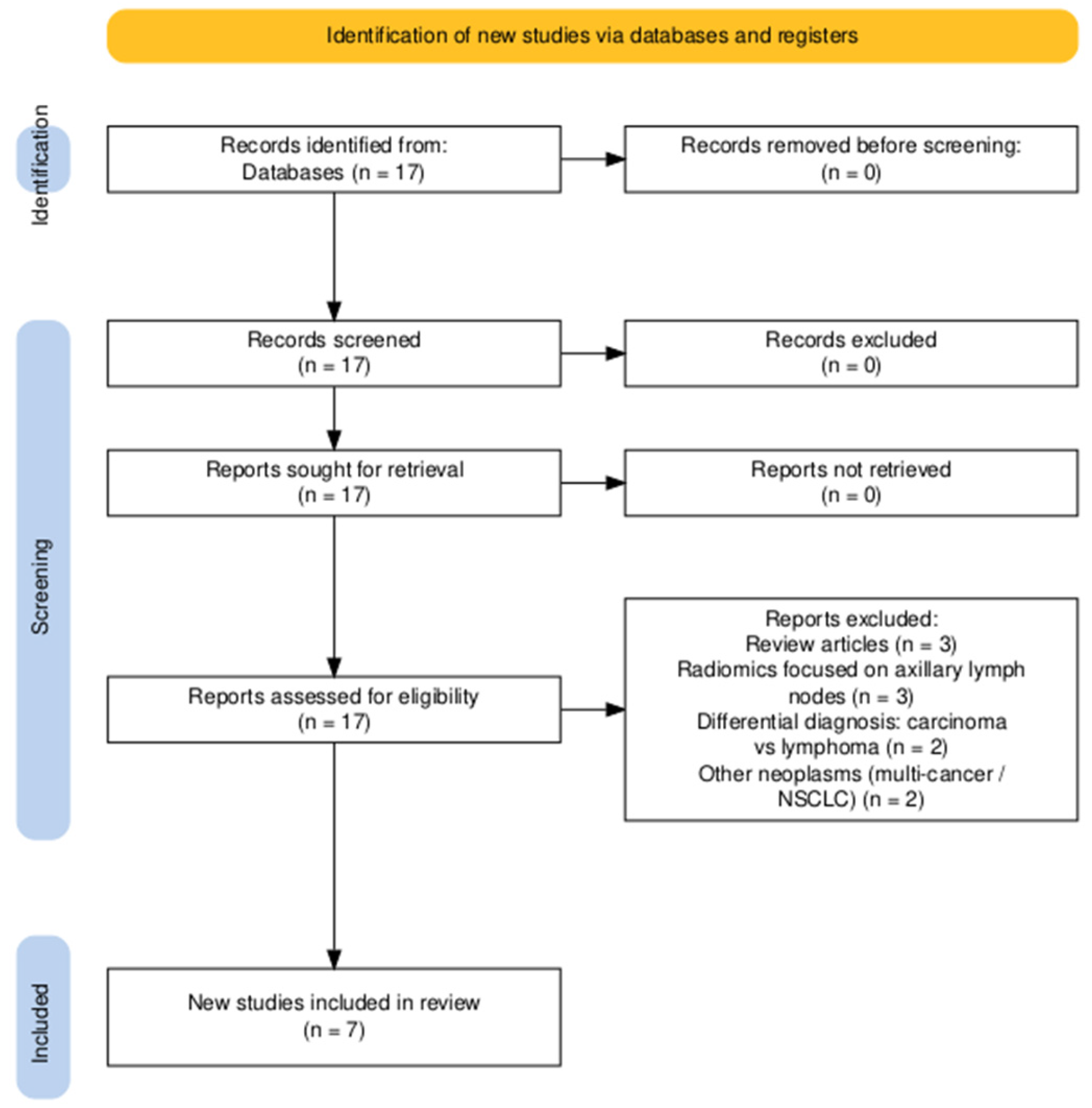
2. Materials and Methods
- highlighting correlations between radiomic data and the tumor histopathological profile (ER, PR, HER2, Ki67);
- exploring the potential of radiomics for personalizing diagnostic and treatment strategies in breast carcinoma.
3. Results
4. Discussion
4.1. Methodological Limitations of the Analyzed Studies
4.2. Prediction of pCR in NAC
4.3. Characterization of the Molecular Profile and Hormonal Receptor Status
4.4. Individual Patient Parameters, Biological and Methodological Variability
4.5. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| [18F]FDG | 18F-fluoro-2-deoxy-D-glucose |
| AJCC | American Joint Committee on Cancer |
| AR | Androgen receptor |
| AUC | Area under the ROC curve |
| BC | Breast carcinoma |
| CGITA | Chang-Gung Memorial Hospital Taiwan |
| ER | Estrogen receptor |
| GLCM | Gray-Level Co-occurrence Matrix |
| HER2 | Human epidermal growth factor receptor 2 |
| IBSI | Image Biomarker Standardisation Initiative |
| KNN | K-nearest neighbors |
| LABC | Locally advanced breast carcinoma |
| LASSO | Least absolute shrinkage and selection operator |
| LR | Logistic regression |
| MRI | Magnetic resonance imaging |
| MTV | Metabolic tumor volume |
| NCCN | American National Comprehensive Cancer Network |
| NHOCmax | The normalized distance between the voxel with SUVmax and the geometric center of the tumor |
| NHOCpeak | The normalized distance between the center of the SUVpeak volume and the geometric center of the tumor |
| NHOPmax | The normalized distance between the voxel with SUVmax and the tumor perimeter |
| NHOPpeak | The normalized distance between the SUVpeak volume center and the tumor perimeter |
| pCR | pathological complete response |
| PET/CT | Positron emission tomography combined with computed tomography |
| PR | Progesterone receptor |
| ROC | receiver operating characteristic curve |
| ROI | Region of interest |
| SHAPE | Shape-based features |
| SMOTE | Synthetic oversampling technique for the minority class |
| SULmax | Standardized uptake value normalized to lean body mass—maximum |
| SUV | Standardized uptake value |
| SUVmax | Maximum value of the standardized uptake value |
| SUVmean | Mean value of the standardized uptake value |
| SUVpeak | Average SUV within a fixed-size volume of 1 cm3 sphere |
| SVM | Support Vector Machine |
| TC | Tumor cluster |
| TI | Textural index |
| TLG | Total lesion glycolysis |
| VOI | Volume of interest |
Appendix A
| Study | Selection (Max. 4) | Comparability (Max. 2) | Outcome (Max. 3) | Total (Max. 9) |
|---|---|---|---|---|
| Ha et al., 2017 [32] | 3 points single-centre LABC cohort, consecutive patients no internal control outcome not present at start (pCR not yet occurred) | 1 point adjusted for T-stage and Ki-67 but not age or BMI | 2 points pCR from pathology follow-up median > 24 months <10% lost (not reported) | 6 points |
| Boughdad et al., 2018 [33] | 3 points consecutive normal and breast cancer patients community & tertiary-centre study explicit exclusion of prior therapy | 1 point age and menopausal status modelled subgroup by intrinsic subtype | 2 points radiomic outcomes extracted blindly (software); no longitudinal outcome so limited follow-up; | 6 points |
| Liu et al., 2021 [34] | 4 points multicentre study tissue diagnosis confirmed controls = other subtypes within cohort | 2 points multivariable model adjusts for tumour size, SUV and age | 2 points outcome = IHC subtype (gold-standard)
| 8 points |
| Araz et al., 2022 [35] | 3 points consecutive patients single centre internal controls outcome absent at baseline | 1 point only ER/PR tested vs. SUVs | 2 points HR status from IHC
| 6 points |
| Jia et al., 2023 [36] | 3 points clear inclusion and histology-proved internal controls outcome absent | 1 point model includes MTV and clinicopathological factors but missing age or grade | 2 points androgen receport status from pathology
| 6 points |
| Hou et al., 2024 [37] | 4 points prospectively maintained patient database PET protocol | 1 point adjusted for molecular subtype no sociodemographics | 2 points
| 7 points |
| Hong et al., 2024 [38] | 4 points pretreatment PET clear criteria consecutive cohort | 1 point model includes MTV, subtype but is missing age or BMI | 3 points pCR & PFS median follow-up 39 months censoring < 5% | 8 points |
| Name | Category | Modality Scope | Type/License | Notes | URL |
|---|---|---|---|---|---|
| PyRadiomics | Software (open-source/freeware) | Multimodality (CT/MR/PET/US) | Open-source (BSD-3-Clause) | Python library for radiomics feature extraction; supports DICOM SR TID 1500 output | https://github.com/AIM-Harvard/pyradiomics |
| LIFEx | Software (open-source/freeware) | PET, SPECT, MR, CT, US | Freeware (research use) | GUI application for radiomic feature calculation; published in Cancer Research (2018) | https://www.lifexsoft.org/ |
| 3D Slicer | Software (open-source/freeware) | Multimodality Via 3D Slicer | Open-source | Graphical interface to PyRadiomics inside 3D Slicer | https://github.com/AIM-Harvard/SlicerRadiomics |
| CGITA | Software (open-source/freeware) | Molecular imaging (PET/SPECT) also CT/MR | Open-source (MATLAB-based) | Texture analysis/radiomics for molecular images; original 2014 paper on PMC | https://pmc.ncbi.nlm.nih.gov/articles/PMC3976812/ |
| Author | Objective | No. of Patients | Materials and Methods | Results | Conclusions |
|---|---|---|---|---|---|
| Ha et al. (2017) [32] | Evaluation of [18F]FDG PET/CT radiomics for LABC characterization and prediction of response to NAC | 73 | Retrospective study Image analysis: CGITA ver. 1.4 109 textural features Statistical analysis: MedCalc version 14.8.1 and R version 3. 2.3 Unsupervised Clustering Pearson Correlation; Hierarchical clustering (heatmaps); Kruskal–Wallis test; Chi-square test; Univariate and multivariate logistic regression; Kaplan–Meier curve and log-rank test; Univariate and multivariate Cox regression; Bonferroni correction; | TC II: had a pCR, increased SUVmax, and high intratumoral heterogeneity. TC I: Associated with an increased risk of recurrence. Significant radiomic features: SUVmax, MTV, TLG, and features specific to intratumoral heterogeneity (NL_EntropyGLCM, NL_HomogeneityGLCM, ZPGLSZM, Skewness) Notable differences between tumor clusters for radiomic parameters | Radiomic pretreatment correlates with Ki67, can predict pCR, risk of recurrence, and response to NAC. |
| Boughdad et al. (2018) [33] | The influence of age on metabolic parameters and radiomic features in breast tumors and healthy breast tissue. Biological variability in radiomic models | 522 | Retrospective study Image analysis: LIFEx software (www.lifexsoft.org) Metabolic markers: SUVmax, SUVmean, SUVpeak, MTV, TLG 4 histogram-based indices (HBI) SkewnessH, KurtosisH, EntropyH, EnergyH 31 TIs, of which 6 indices were considered robust: Homogeneity, Entropy, Gray-Level Run Length (LRE), Neighboring Gray-Level Dependence (SRE), Gray-Level Zone Length (LGZE), High Gray-Level Zone Length (HGZE) Statistical Analysis: IBM SPSS Statistics v22. ANOVA test; Bonferroni/Hochberg tests; Spearman correlation; | Age groups: PRE (under 45 years old), PERI (45 to under 55 years old), POST (55 to under 85 years old). Significant differences were found between metabolic markers (SUVmax, SUVmean, SUVpeak) and 12 TI (including homogeneity, SRE, HGZE), p < 0.05. Metabolic markers decrease with age. Homogeneity and LRE tend to increase. TI: Significant differences between groups for homogeneity, SRE, and HGZE (p < 0.05). Significant differences were observed in triple-negative tumors between PET and TI parameters (p < 0.05). | There are significant differences (SUV and TI) depending on age, especially in triple-negative carcinoma. Age should be considered as an additional variable in radiomics studies. |
| Liu et al. (2021) [34] | Using radiomics for the classification of breast cancer molecular subtypes | 273 | Retrospective study Image analysis: ImageJ 1.50i software (National Institute of Health, Bethesda, MD, USA) MATLAB, The MathWorks Inc., Natick, MA, USA Metabolic markers: SUVmax, SUVmean, SUVpeak, MTV, TLG 1710 radiomic features (855 PET-specific and 855 CT-based, respectively) Statistical analysis: R statistical software Wilcoxon rank-sum test; inter-feature correlation coefficient (R); Cox and LASSO regression; Rad-score; AUC; 10-fold cross-validation with 10 repetitions | Mean AUC: 0.856 (Luminal vs. Non-Luminal), 0.818 (HER2+ vs. HER2−), 0.888 (TN vs. Non-TN). Average accuracy: 0.864 (Luminal vs. Non-Luminal), 0.847 (HER+ vs. HER2−), 0.893 (TN vs. Non-TN). Average sensitivity: 0.801 (Luminal vs. Non-Luminal), 0.908 (HER2+ vs. HER2−), 0.933 (TN vs. Non-TN). Average Specificity: 0.905 (Luminal vs. Non-Luminal), 0.764 (HER+ vs. HER2−), 0.839 (TN vs. Non-TN) | Radiomics-based models can provide more information than classic PET/CT metabolic markers. Based on imaging phenotypes, radiomics can predict the molecular profile of breast carcinoma. |
| Araz et al. (2022) [35] | Establishing the status of hormone receptors in breast tumors using PET/CT radiomics | 153 | Retrospective study Image analysis: LIFEx (www.lifexsoft.org) Metabolic markers: SUVmin; SUVmax; SUVpeak and SUVmax, MTV and TLG respectively 42 radiomic features: first-order parameters-skewness; kurtosis; entropy-histo; energy, SHAPE-sphericity, compactness; second-order: GLCM homogeneity, GLCM energy, GLCM contrast, GLCM entropy, GLCM dissimilarity) higher-order: GLRLM (SRE, LRE, LGRE, HGRE, SRLGE, SRHGE, LRLGE, LRHGE, GLNU, RLNU, RP) GLZLM (SZE, LZE, LGZE, HGZE, SZLGE, SZHGE, LZLGE, LZHGE, GLNU, ZLNU, ZP) Coarseness, Contrast, Busyness Statistical analysis: WEKA 3.7 and SPSS 11.5 Mann–Whitney U test; binary logistic regression analysis; ROC; Hoeffding tree; J48; multilayer perceptron; 10-fold cross-validation; accuracy; F-measure; precision; recall; area under the precision-recall curve | By applying radiomic data analysis methods (binary regression and CorrelationAttributeEval), only 7 statistically relevant features remained: SUVmean, SUVmax, SUVpeak, GLZLM LZE, TLG, MTV, and GLRLM-GLNU. No statistically significant correlations were found between the radiomic features and hormone receptor status. | Radiomic features could not predict hormone receptor status. Patients who had hormone receptors had lower SUVs. |
| Jia et al. (2023) [36] | Prediction of androgen receptor expression based on clinico-pathological and PET/CT radiomic features | 48 | Retrospective study Image analysis: LIFEx v7.0.0 (www.lifexsoft.org) 80 radiomic features Metabolic markers: SUVmin, SUVmean, SUVmax, SUVstd, UVpeak. TLG, MTV Shape: Sphericity, Compacity, Volume-ml, Volume-vx Histogram: Skewness, Kurtosis, Entropy_log10, Entropy_log2, Energy GLCM: Homogeneity, Energy, Contrast, Correlation, Entropy_log10, Entropy_log2, Dissimilarity GLRLM: SRE, LRE, LGRE, HGRE, SRLGE, SRHGE, LRLGE, LRHGE, GLNU, RLNU, RP GLZLM: SZE, LZE, LGZE, HGZE, SZLGE, SZHGE, LZLGE, LZHGE, GLNU, ZLNU, ZP NGLDM: Coarseness, Contrast, Busyness Statistical analysis: IBM SPSS statistics version 26.0, Python version 3.11 (https://www.python.org), MedCalc software (MedCalc Software, Ostend, Belgium), and R version 4.2.1 (http://www.R-project.org) The radiomic features were integrated into a multivariate logistic regression model, which was subsequently evaluated using the ROC curve, the Hosmer-Lemeshow test, and DCA. Kolmogorov–Smirnov test, Levene’s test, t-test, Mann–Whitney U test, Chi-square test, Fisher’s test, Pearson correlation, 10-fold cross-validation | MTV is significantly associated with androgen receptor expression (p < 0.05) (an increased MTV value could be associated with negative receptor expression). Based on the logistic regression model, MTV, SHAPE-sphericityCT, and GLCM-contrastCT were included in the androgen receptor prediction model. The latter being a good predictor of androgen receptor status (OR = 9, p = 0.018). The model showed an AUC ROC of 0.832, sensitivity and specificity over 75%. The Hosmer-Lemeshow test indicated good calibration (p > 0.05). | The model built based on clinico-pathological data and PET/CT radiomic features can predict the presence of the androgen receptor in patients with breast carcinoma. |
| Hou et al. (2024) [37] | Evaluation of intratumoral and peritumoral radiomic features for assessing pCR response after NAC | 190 | Retrospective study Image analysis: 3D Slicer v4.11.20200930 and PyRadiomics (IBSI) After applying the Wavelet and Laplacian-Gaussian filters, 3864 radiomic features were obtained as follows: 1932 intratumoral and 1932 peritumoral. Statistical analysis: R software 4.3.1 and Python 3.7.9 Pearson correlation, Spearman correlation, t-test, LASSO, ORC-AUC and DeLong test, χ2, SMOTE. SVM, KNN, LR, NB Classifiers | 10 radiomic features filtered through wavelet and Laplacian-Gaussian, then selected by LASSO: pet.intra.wavelet. HLH_firstorder_Skewness, ct.peri.wavelet. HHH_glrlm_LowGrayLevelRunEmphasis, ct.intra. wavelet. HLH_glcm_MCC, pet.intra.wavelet. HHL_firstorder_Skewness, pet.peri.wavelet. HHH_firstorder_10Percentile, ct.intra.wavelet. LHL_glcm_Idn, pet.peri.wavelet. HHL_glrlm_LowGrayLevelRunEmphasis, ct.peri.wavelet. LLH_firstorder_RootMeanSquared, pet.peri.wavelet. LHH_glszm_ZoneEntropy, pet.intra.wavelet. The features showed robustness to segmentation (ICC > 0.75) and were validated with SMOTE. Intratumoral and peritumoral SVM had the best prediction of pCR, with an AUC of 0.83, significantly outperforming KNN, LR, and NB. The molecular subtype models maintained high performance (AUC 0.86–0.92). | Combining intra- and peritumoral features improves performance in pCR prediction. |
| Hong et al. (2024) [38] | The main objective was to demonstrate that NHOC and NHOP can predict progression-free survival and rPC in NAC for patients with breast carcinoma. | 135 | Retrospective study Image analysis: LIFEx v. 7.6.0 (www.lifexsoft.org) according to (IBSI) Classic metabolic markers SUVmax/peak, MTV, TLG Distance-derived radiomic markers: NHOCmax/peak and NHOPmax/peak Statistical Analysis: MedCalc Statistical v. 22.021 Mann–Whitney U, Kruskal–Wallis, Spearman, ROC-AUC, logistic regression, Cox, Kaplan–Meier, log-rank test | NHOCmax AUC = 0.71 (95% CI: 0.59–0.80); NHOCpeak AUC = 0.69 significantly better than SUVmax, SUVpeak, MTV, TLG. With low NHOCmax and NHOCpeak values (“hot spot” near the center), the chance of pCR is higher. MTV and NHOCpeak demonstrated independence from the rest of the markers. NHOCpeak mic (≤0.27)—92% progression-free survival at 5 years compared to 67%. | NHOCpeak can predict pCR and response to NAC. Increased NHOCpeak is associated with a poorer prognosis. |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer. 2024. Available online: https://gco.iarc.who.int/today (accessed on 15 December 2024).
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Xu, H.; Xu, B. Breast cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2023, 35, 565–583. [Google Scholar] [CrossRef]
- Grimm, L.J.; Avery, C.S.; Hendrick, E.; Baker, J.A. Benefits and Risks of Mammography Screening in Women Ages 40 to 49 Years. J. Prim. Care Community Health 2022, 13, 21501327211058322. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Obeagu, E.I.; Obeagu, G.U. Breast cancer: A review of risk factors and diagnosis. Medicine 2024, 103, e36905. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Morciano, F.; Amodeo, S.; Gori, E.; Romanucci, G.; Belli, P.; Tommasini, O.; Fornasa, F.; Rella, R. Special Types of Breast Cancer: Clinical Behavior and Radiological Appearance. J. Imaging 2024, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- McCart Reed, A.E.; Kalinowski, L.; Simpson, P.T.; Lakhani, S.R. Invasive lobular carcinoma of the breast: The increasing importance of this special subtype. Breast Cancer Res. 2021, 23, 6. [Google Scholar] [CrossRef]
- Teichgraeber, D.C.; Guirguis, M.S.; Whitman, G.J. Breast Cancer Staging: Updates in the AJCC Cancer Staging Manual, 8th Edition, and Current Challenges for Radiologists, From the AJR Special Series on Cancer Staging. Am. J. Roentgenol. 2021, 217, 278–290. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Sennm, H.J.; Panel Members. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soliman, N.A.; Yussif, S.M. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol. Med. 2016, 13, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.-H.; Payandeh, M.; Sadeghi, M.; Motamed, H.; Sadeghi, E. The correlation between Ki-67 with other prognostic factors in breast cancer: A study in Iranian patients. Indian J. Med. Paediatr. Oncol. 2016, 37, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Hindie, E. Breast cancer: Initial workup and staging with FDG PET/CT. Clin. Transl. Imaging 2021, 9, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Hadebe, B.; Harry, L.; Ebrahim, T.; Pillay, V.; Vorster, M. The Role of PET/CT in Breast Cancer. Diagnostics 2023, 13, 597. [Google Scholar] [CrossRef]
- Ulaner, G.A. PET/CT for Patients With Breast Cancer: Where Is the Clinical Impact? Am. J. Roentgenol. 2019, 213, 254–265. [Google Scholar] [CrossRef]
- Zhang-Yin, J. State of the Art in 2022 PET/CT in Breast Cancer: A Review. J. Clin. Med. 2023, 12, 968. [Google Scholar] [CrossRef]
- Groheux, D.; Vaz, S.C.; Poortmans, P.; Mann, R.M.; Ulaner, G.A.; Cook, G.J.R.; Hindié, E.; Woll, J.P.P.; Jacene, H.; Rubio, I.T.; et al. Role of 18F-FDG PET/CT in patients with invasive breast carcinoma of no special type: Literature review and comparison between guidelines. Breast 2024, 78, 103806. [Google Scholar] [CrossRef]
- Lother, D.; Robert, M.; Elwood, E.; Smith, S.; Tunariu, N.; Johnston, S.R.D.; Parton, M.; Bhaludin, B.; Millard, T.; Downey, K.; et al. Imaging in metastatic breast cancer, CT, PET/CT, MRI, WB-DWI, CCA: Review and new perspectives. Cancer Imaging 2023, 23, 53. [Google Scholar] [CrossRef]
- Cristo Santos, J.; Henriques Abreu, M.; Seoane Santos, M.; Duarte, H.; Alpoim, T.; Próspero, I.; Sousa, S.; Abreu, P.H. Bone Metastases Detection in Patients with Breast Cancer: Does Bone Scintigraphy Add Information to PET/CT? Oncologist 2023, 28, e600–e605. [Google Scholar] [CrossRef]
- Niikura, N.; Costelloe, C.M.; Madewell, J.E.; Hayashi, N.; Yu, T.-K.; Liu, J.; Palla, S.L.; Tokuda, Y.; Theriault, R.L.; Hortobagyi, G.N.; et al. FDG-PET/CT Compared with Conventional Imaging in the Detection of Distant Metastases of Primary Breast Cancer. Oncologist 2011, 16, 1111–1119. [Google Scholar] [CrossRef]
- Djassemi, N.; Rampey, S.; Motiani, J. Examining the evolving utility of 18FDG-PET/CT in breast cancer recurrence. Transl. Cancer Res. 2020, 9, S116–S121. [Google Scholar] [CrossRef]
- Yildirim, N.; Simsek, M.; Aldemir, M.N.; Bilici, M.; Tekin, S.B. Relationship between 18-FDG-PET/CT and Clinicopathological Features and Pathological Responses in Patients with Locally Advanced Breast Cancers. Eurasian J. Med. 2019, 51, 153–158. [Google Scholar] [CrossRef]
- Idris, M.; Campos, A.; Moore, C. Radiomics. Available online: https://radiopaedia.org/articles/radiomics?lang=us (accessed on 17 December 2024).
- Tagliafico, A.S.; Piana, M.; Schenone, D.; Lai, R.; Massone, A.M.; Houssami, N. Overview of radiomics in breast cancer diagnosis and prognostication. Breast 2020, 49, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bouron, C.; Mathie, C.; Seegers, V.; Morel, O.; Jézéquel, P.; Lasla, H.; Guillerminet, C.; Girault, S.; Lacombe, M.; Sher, A.; et al. Prognostic Value of Metabolic, Volumetric and Textural Parameters of Baseline [18F]FDG PET/CT in Early Triple-Negative Breast Cancer. Cancers 2022, 14, 637. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, X.; Zhang, J.; Wang, S.; Wang, Y.; Wan, X.; Zhu, X.; Song, X.; Tong, Z.; Yang, M.; et al. Evaluation of prognostic risk factors of triple-negative breast cancer with 18F-FDG PET/CT parameters, clinical pathological features and biochemical indicators. Front. Cell Dev. Biol. 2024, 12, 1421981. [Google Scholar] [CrossRef]
- Vaz, S.C.; Woll, J.P.P.; Cardoso, F.; Groheux, D.; Cook, G.J.R.; Ulaner, G.A.; Jacene, H.; Rubio, I.T.; Schoones, J.W.; Peeters, M.-J.V.; et al. Joint EANM-SNMMI guideline on the role of 2-[18F]FDG PET/CT in no special type breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2706–2732. [Google Scholar] [CrossRef]
- Urso, L.; Manco, L.; Castello, A.; Evangelista, L.; Guidi, G.; Castellani, M.; Florimonte, L.; Cittanti, C.; Turra, A.; Panareo, S. PET-Derived Radiomics and Artificial Intelligence in Breast Cancer: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13409. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 11 July 2025).
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Ha, S.; Park, S.; Bang, J.-I.; Kim, E.-K.; Lee, H.-Y. Metabolic Radiomics for Pretreatment 18F-FDG PET/CT to Characterize Locally Advanced Breast Cancer: Histopathologic Characteristics, Response to Neoadjuvant Chemotherapy, and Prognosis. Sci. Rep. 2017, 7, 1556. [Google Scholar] [CrossRef]
- Boughdad, S.; Nioche, C.; Orlhac, F.; Jehl, L.; Champion, L.; Buvat, I. Influence of age on radiomic features in 18F-FDG PET in normal breast tissue and in breast cancer tumors. Oncotarget 2018, 9, 30855–30868. [Google Scholar] [CrossRef]
- Liu, J.; Bian, H.; Zhang, Y.; Gao, Y.; Yin, G.; Wang, Z.; Li, X.; Ma, W.; Xu, W. Molecular subtype classification of breast cancer using established radiomic signature models based on 18F-FDG PET/CT images. Front. Biosci. Landmark 2021, 26, 475–484. [Google Scholar] [CrossRef]
- Araz, M.; Soydal, Ç.; Gündüz, P.; Kırmızı, A.; Bakırarar, B.; Dizbay Sak, S.; Özkan, E. Can Radiomics Analyses in 18F-FDG PET/BT Images of Primary Breast Carcinoma Predict Hormone Receptor Status? Mol. Imaging Radionucl. Ther. 2022, 31, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Lv, Q.; Zhang, B.; Yu, C.; Sang, S.; Deng, S. Assessment of androgen receptor expression in breast cancer patients using 18F-FDG PET/CT radiomics and clinicopathological characteristics. BMC Med. Imaging 2023, 23, 93. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Chen, K.; Wan, X.; Luo, H.; Li, X.; Xu, W. Intratumoral and peritumoral radiomics for preoperative prediction of neoadjuvant chemotherapy effect in breast cancer based on 18F-FDG PET/CT. J. Cancer Res. Clin. Oncol. 2024, 150, 484. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, S.M.; Yoo, I.D.; Lee, J.E.; Han, S.W.; Kim, S.Y.; Lee, J.W. Clinical value of SUVpeak-to-tumor centroid distance on FDG PET/CT for predicting neoadjuvant chemotherapy response in patients with breast cancer. Cancer Imaging 2024, 24, 136. [Google Scholar] [CrossRef]
- Filippi, L.; Urso, L.; Ferrari, C.; Guglielmo, P.; Evangelista, L. The impact of PET imaging on triple negative breast cancer: An updated evidence-based perspective. Eur. J. Nucl. Med. Mol. Imaging 2024, 52, 263–279. [Google Scholar] [CrossRef]
- Hou, X.; Chen, K.; Luo, H.; Xu, W.; Li, X. Identification of HER2-over-expression, HER2-low-expression, and HER2-zero-expression statuses in breast cancer based on 18F-FDG PET/CT radiomics. Cancer Imaging 2025, 25, 62. [Google Scholar] [CrossRef]
- Hwang, A.; Rashid, S.; Shi, S.; Blew, C.; Levine, M.; Saha, A. 18F-FDG PET/CT Semiquantitative and Radiomic Features for Assessing Pathologic Axillary Lymph Node Status in Clinical Stage I–III Breast Cancer Patients: A Systematic Review. Curr. Oncol. 2025, 32, 300. [Google Scholar] [CrossRef]
| Author | Selection (0–4) | Comparability (0–2) | Result (0–3) | Total NOS (0–9) |
|---|---|---|---|---|
| Ha et al. [32] | 3 | 1 | 2 | 6 |
| Boughdad et al. [33] | 3 | 1 | 2 | 6 |
| Liu et al. [34] | 4 | 2 | 2 | 8 |
| Araz et al. [35] | 3 | 1 | 2 | 6 |
| Jia et al. [36] | 3 | 1 | 2 | 6 |
| Hou et al. [37] | 4 | 1 | 2 | 7 |
| Hong et al. [38] | 4 | 1 | 3 | 8 |
| Ha et al. (2017) [32] | Boughdad et al. (2018) [33] | Liu et al. (2021) [34] | Araz et al. (2022) [35] | Jia et al. (2023) [36] | Hou et al. (2024) [37] | Hong et al. (2024) [38] | |
|---|---|---|---|---|---|---|---|
| Objective | [18F]FDG PET/CT radiomics for the characterization of LABC and the prediction of pCR | Age influence on metabolic and radiomic parameters in BC and healthy breast tissue. | The use of radiomics for the classification of molecular subtypes of breast cancer | Prediction of hormonal receptor status through radiomics | Predicting AR expression using radiomic characteristics | Evaluation of intratumoral and peritumoral radiomic characteristics for assessing pCR response after NAC | NHOC and NHOP predict breast carcinoma NAC progression-free survival and rPC |
| Study type | Retrospective Monocentric | Retrospective Monocentric | Retrospective Multicentric | Retrospective Monocentric | Retrospective Monocentric | Retrospective Monocentric | Retrospective Multicentric |
| No. of patients | 73 | 522 | 273 | 153 | 48 | 190 | 135 |
| Image and radiomic analysis | CGITA version 1.4 109 TI | LIFEx software 4 indices based on histograms 31 TI | ImageJ 1.50i and MATLAB 1710 TI (855 specific to PET, respectively 855 based on CT) | LIFEx 42 TI: first, second, and higher-order parameters | LIFEx v7.0.0 80 TI | 3D Slicer software 4.11.20200930 and PyRadiomics (IBSI) 1932 intratumoral TI 1932 peritumoral TI | LIFEx v. 7.6.0 compliant (IBSI) 4 classic metabolic markers 4 radiomic markers derived from distance |
| Statistical analysis | MedCalc 14.8.1 and R version 3.2.3 Unsupervised clustering, Pearson, Kruskal–Wallis, χ2, uni/multi logistic regression, Kaplan–Meier, Cox, Bonferroni | IBM SPSS Statistics v22.0 Test ANOVA Test Bonferroni Hochberg Spearman | R 3.2.2 Wilcoxon rank-sum, coefficient inter feature R, LASSO-Cox, Rad score, ROC, 10 × 10 fold cross validated | WEKA 3.7 and SPSS 11.5 Mann–Whitney U test, binary logistic regression, ROC, Hoeffding tree, J48, multilayer perceptron, 10-fold cross- validation | IBM SPSS 26.0, Python version 3.11, MedCalc and R version 4.2.1 Kolmogorov–Smirnov tests, Levene, t-test, Mann–Whitney U, χ2, Fisher, Pearson, multivariate logistic, ROC, 10-fold cross-validated | R software 4.3.1 and Python 3.7.9 Pearson and Spearman correlation, t-test, LASSO, ROC-AUC and DeLong test, χ2, SMOTE. SVM, KNN, LR, NB classifiers | MedCalc Statistical v. 22.021 Mann–Whitney U, Kruskal–Wallis, Spearman, ROC-AUC, logistic regression, cox, Kaplan–Meier, log-rank test |
| Radiomic features | SUVmax, MTV, TLG, NL_EntropyGLCM, NL_HomogeneityGLCM, ZPGLSZM, Skewness | SUVmax SUVmean SUVpeak 12 TI including: Homogeneity SRE HGZE | LASSO reduced the TI set from 1710 to 9 (3 for each breast cancer classification). The individual TI were not listed in the main text of the study. | 7 statistically relevant TI: SUVmean, SUVmax, SUVpeak, GLZLM LZE, TLG, MTV, and GLRLM-GLNU. | MTV, SHAPE-sphericityCT, and GLCM-contrastCT; GLCM-contrastCT being the strongest predictor (OR-9, p = 0.018) | Include 10 wavelet-filtered features, Laplacian-Gaussian (5 intratumoral and 5 peritumoral) selected by LASSO. The features demonstrated robustness in segmentation (ICC > 0.75) and were validated with SMOTE. | 4 radiomic features derived from distance: NHOCmax/peak and NHOPmax/peak |
| Results | Cluster TC II (increased SUVmax values and heterogeneity) achieved pCR; Cluster TC I = high risk of recidivism. Significant differences | SUVmax/mean /peak decrease with age Homogeneity and LRE increase with age. Significant differences, especially in the TN subtype (p < 0.050) | Mean AUC 0.856 Luminal vs. Non-Luminal 0.818 HER+ vs. HER2− 0.888 TN vs. Non-TN | No radiomic feature predicted hormone receptor status | The final logistic model based on MTV, SHAPE-sphericityCT, and GLCM-contrastCT can predict androgen receptor status with an AUC of 0.83 and sensitivity and specificity > 75%. | Intratumoral and peritumoral SVM had the best prediction of pCR, with an AUC of 0.83, significantly outperforming KNN, LR, and NB. Molecular subtype models maintained high performance (AUC 0.86–0.92). | NHOCmax and NHOCpeak AUCs of 0.71 and 0.69 predict NAC pCR better than SUVmax/peak, MTV, and TLG. Low NHOCmax and NHOCpeak enhance pCR risk. Low NHOCpeak (≤0.27)—92% progression-free survival at 5 years vs. 67%. |
| Conclusions | Pre-treatment radiomics correlates with Ki-67, predicts pCR and recurrence risk. | Age influences SUV and TI, especially in triple-negative BC. Radiomic studies should include age. | Radiomic models are superior to classic [18F]FDG PET/CT parameters in identifying the molecular subtype. | Radiomics did not predict the hormonal receptor status. Patients with hormone receptors had lower SUVs | The model built on clinico-pathological data and [18F]FDG PET/CT radiomic features can predict AR presence in BC. | The combination of intra- and peritumoral features increases performance in predicting pCR. | NHOCpeak can predict pCR and the response to NAC. Increased NHOCpeak is associated with a worse prognosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitoi, A.; Mititelu, R.-M.; Medar, C.; Constantin, C.; Bolocan, V.-O.; Mateș, I.-N. [18F]FDG PET/CT Radiomics in Untreated Breast Carcinoma: A Review of the Current State and Future Directions. Diagnostics 2025, 15, 2231. https://doi.org/10.3390/diagnostics15172231
Mitoi A, Mititelu R-M, Medar C, Constantin C, Bolocan V-O, Mateș I-N. [18F]FDG PET/CT Radiomics in Untreated Breast Carcinoma: A Review of the Current State and Future Directions. Diagnostics. 2025; 15(17):2231. https://doi.org/10.3390/diagnostics15172231
Chicago/Turabian StyleMitoi, Alexandru, Raluca-Mihaela Mititelu, Cosmin Medar, Ciprian Constantin, Vlad-Octavian Bolocan, and Ioan-Nicolae Mateș. 2025. "[18F]FDG PET/CT Radiomics in Untreated Breast Carcinoma: A Review of the Current State and Future Directions" Diagnostics 15, no. 17: 2231. https://doi.org/10.3390/diagnostics15172231
APA StyleMitoi, A., Mititelu, R.-M., Medar, C., Constantin, C., Bolocan, V.-O., & Mateș, I.-N. (2025). [18F]FDG PET/CT Radiomics in Untreated Breast Carcinoma: A Review of the Current State and Future Directions. Diagnostics, 15(17), 2231. https://doi.org/10.3390/diagnostics15172231






