C-Reactive Protein to Albumin Ratio and Prognostic Nutrition Index as a Predictor of Periprosthetic Joint Infection and Early Postoperative Wound Complications in Patients Undergoing Primary Total Hip and Knee Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. Data Collection
2.4. Surgical Procedure and Prevention of Infection
2.5. Statistical Analysis
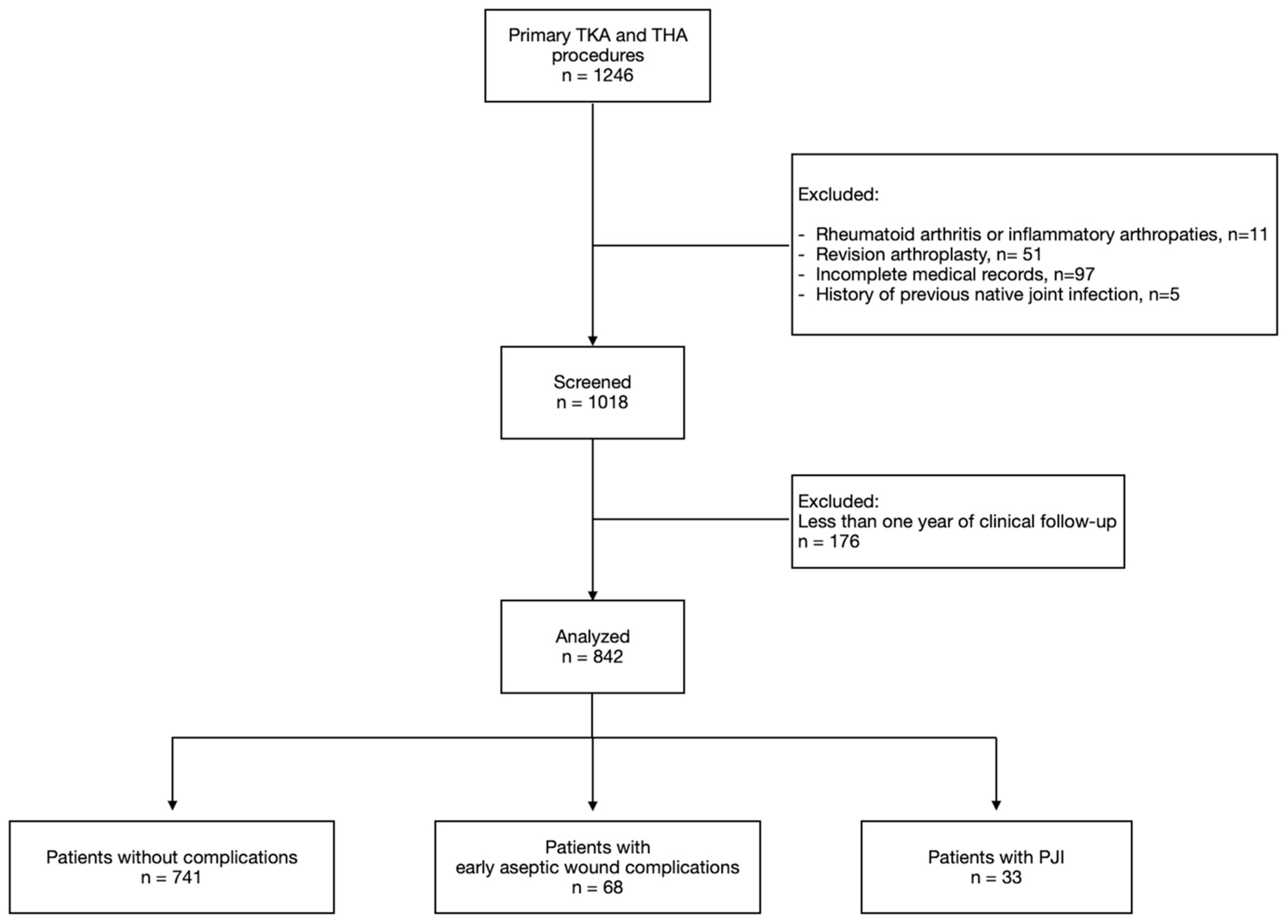
3. Results
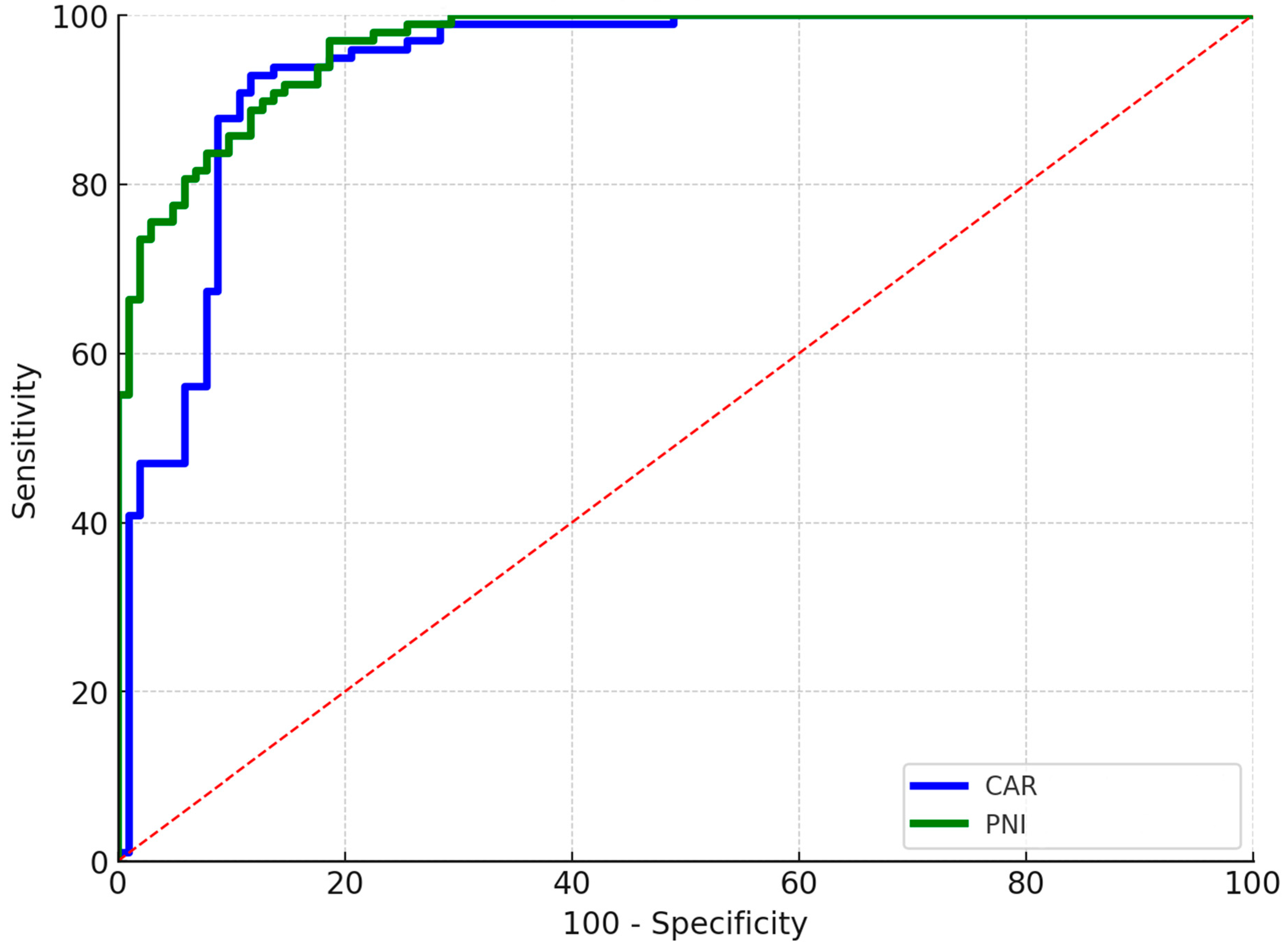
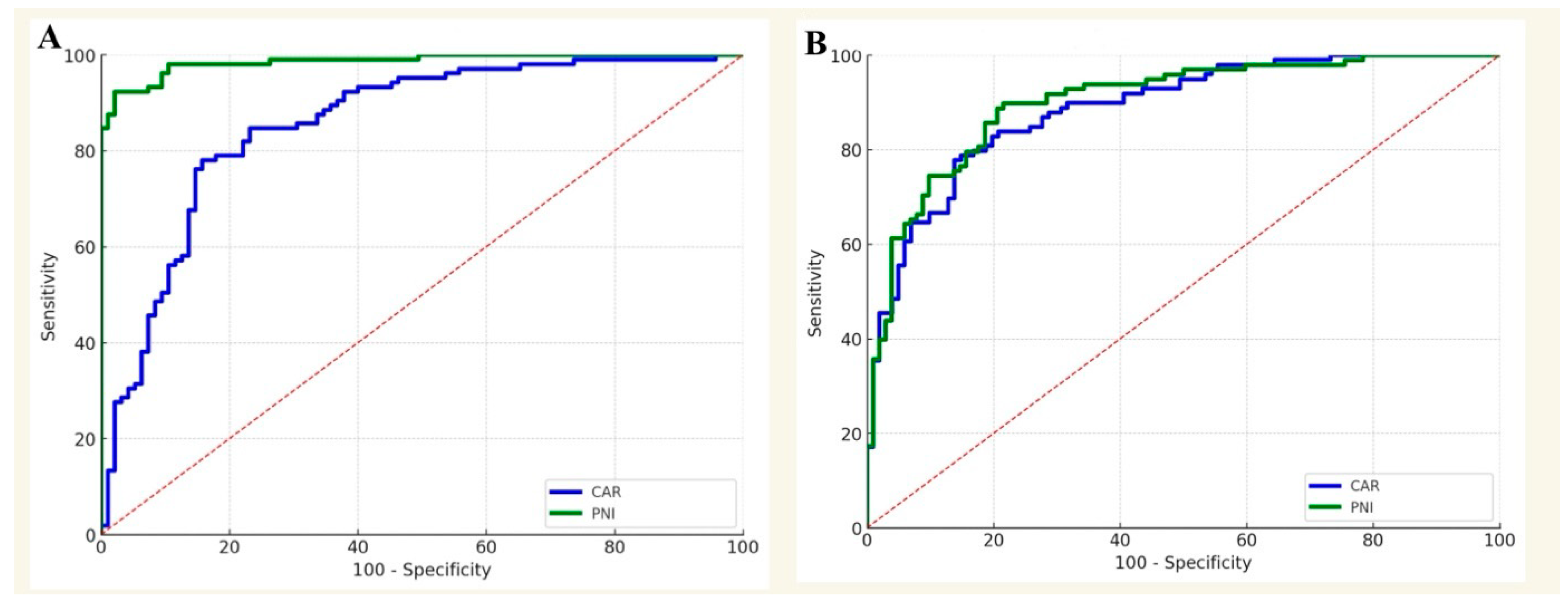
3.1. Periprosthetic Joint Infection
3.2. Procedure-Specific Analysis for PJI
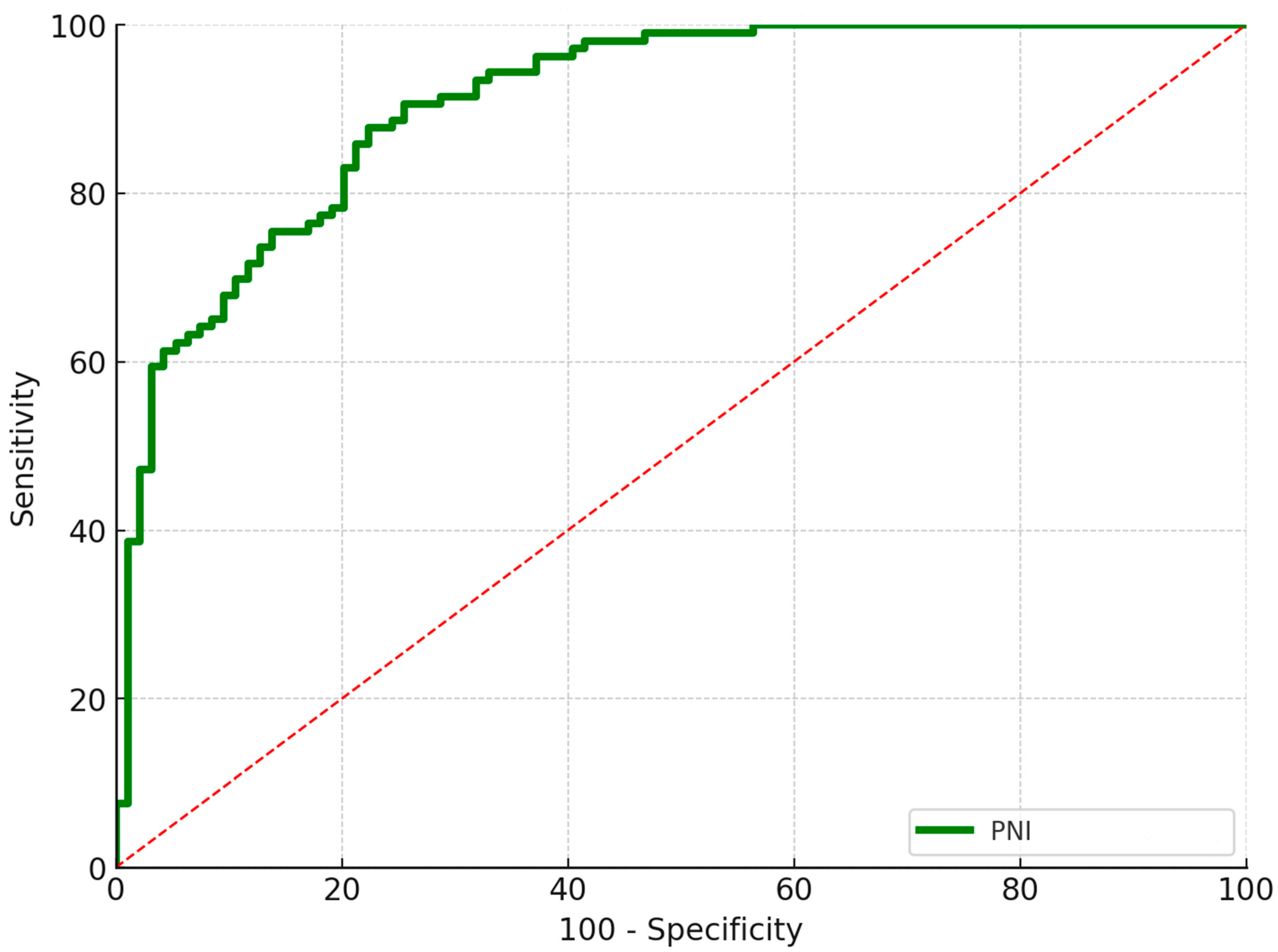
3.3. Aseptic Wound Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carr, A.J.; Robertsson, O.; Graves, S.; Price, A.J.; Arden, N.K.; Judge, A.; Beard, D.J. Knee Replacement. Lancet 2012, 379, 1331–1340. [Google Scholar] [CrossRef]
- Learmonth, I.D.; Young, C.; Rorabeck, C. The Operation of the Century: Total Hip Replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Klug, A.; Gramlich, Y.; Rudert, M.; Drees, P.; Hoffmann, R.; Weißenberger, M.; Kutzner, K.P. The Projected Volume of Primary and Revision Total Knee Arthroplasty Will Place an Immense Burden on Future Health Care Systems over the next 30 Years. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 3287–3298. [Google Scholar] [CrossRef]
- Simons, M.J.; Amin, N.H.; Scuderi, G.R. Acute Wound Complications After Total Knee Arthroplasty: Prevention and Management. J. Am. Acad. Orthop. Surg. 2017, 25, 547–555. [Google Scholar] [CrossRef]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e3. [Google Scholar] [CrossRef] [PubMed]
- Galat, D.D.; McGovern, S.C.; Larson, D.R.; Harrington, J.R.; Hanssen, A.D.; Clarke, H.D. Surgical Treatment of Early Wound Complications Following Primary Total Knee Arthroplasty. J. Bone Jt. Surg. Am. Vol. 2009, 91, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Galat, D.D.; McGovern, S.C.; Hanssen, A.D.; Larson, D.R.; Harrington, J.R.; Clarke, H.D. Early Return to Surgery for Evacuation of a Postoperative Hematoma After Primary Total Knee Arthroplasty. J. Bone Jt. Surg. Am. Vol. 2008, 90, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Adelani, M.A.; Johnson, S.R.; Keeney, J.A.; Nunley, R.M.; Barrack, R.L. Clinical Outcomes Following Re-Admission for Non-Infectious Wound Complications after Primary Total Knee Replacement. Bone Jt. J. 2014, 96–B, 619–621. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Hansen, P.; Zmistowski, B.; Kane, P.W.; Restrepo, C.; Parvizi, J. Hematoma Following Primary Total Hip Arthroplasty: A Grave Complication. J. Arthroplast. 2013, 28, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Wixted, C.M.; Charalambous, L.T.; Kim, B.I.; Case, A.; Hendershot, E.F.; Seidelman, J.L.; Seyler, T.M.; Jiranek, W.A. D-Dimer, Erythrocyte Sedimentation Rate, and C-Reactive Protein Sensitivities for Periprosthetic Joint Infection Diagnosis. J. Arthroplast. 2023, 38, 914–917. [Google Scholar] [CrossRef]
- Telang, S.S.; Palmer, R.C.; Cooperman, W.S.; Dobitsch, A.; Lieberman, J.R.; Heckmann, N.D. Albumin as a Predictor of Periprosthetic Joint Infection Following Total Joint Arthroplasty: Identifying a New Data-Driven Threshold Utilizing a Continuous Variable Analysis. J. Arthroplast. 2025, 40, 2117–2125.e4. [Google Scholar] [CrossRef]
- Shi, W.; Jiang, Y.; Tian, H.; Wang, Y.; Zhang, Y.; Yu, T.; Li, T. C-Reactive Protein-to-Albumin Ratio (CAR) and C-Reactive Protein-to-Lymphocyte Ratio (CLR) Are Valuable Inflammatory Biomarker Combination for the Accurate Prediction of Periprosthetic Joint Infection. Infect. Drug Resist. 2023, 16, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wu, G.; Chen, J.; Chen, H. Preoperative C-Reactive Protein/Albumin Ratio, a Risk Factor for Postoperative Delirium in Elderly Patients After Total Joint Arthroplasty. J. Arthroplast. 2019, 34, 2601–2605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, Y.; Luo, Y.; Lin, X.; Song, M.; Li, J.; Zhao, J.; Li, M.; Jiang, Y.; Yin, P.; et al. Prognostic Nutritional Index with Postoperative Complications and 2-Year Mortality in Hip Fracture Patients: An Observational Cohort Study. Int. J. Surg. 2023, 109, 3395–3406. [Google Scholar] [CrossRef]
- Ushirozako, H.; Hasegawa, T.; Yamato, Y.; Yoshida, G.; Yasuda, T.; Banno, T.; Arima, H.; Oe, S.; Mihara, Y.; Yamada, T.; et al. Does Preoperative Prognostic Nutrition Index Predict Surgical Site Infection after Spine Surgery? Eur. Spine J. 2021, 30, 1765–1773. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef]
- Parvizi, J.; Gehrke, T.; Chen, A.F. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Jt. J. 2013, 95-B, 1450–1452. [Google Scholar] [CrossRef]
- Wagenaar, F.-C.B.M.; Löwik, C.A.M.; Zahar, A.; Jutte, P.C.; Gehrke, T.; Parvizi, J. Persistent Wound Drainage After Total Joint Arthroplasty: A Narrative Review. J. Arthroplast. 2019, 34, 175–182. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic Nutritional Index in Gastrointestinal Surgery of Malnourished Cancer Patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Shichman, I.; Roof, M.; Askew, N.; Nherera, L.; Rozell, J.C.; Seyler, T.M.; Schwarzkopf, R. Projections and Epidemiology of Primary Hip and Knee Arthroplasty in Medicare Patients to 2040–2060. JBJS Open Access 2023, 8, e22. [Google Scholar] [CrossRef]
- Domecky, P.; Rejman Patkova, A.; Mala-Ladova, K.; Maly, J. Inflammatory Blood Parameters as Prognostic Factors for Surgical Site Infection after Primary Hip or Knee Arthroplasty: A Systematic Review Protocol. BMJ Open 2021, 11, e046027. [Google Scholar] [CrossRef]
- Jämsen, E.; Huhtala, H.; Puolakka, T.; Moilanen, T. Risk Factors for Infection After Knee Arthroplasty. J. Bone Jt. Surg. Am. Vol. 2009, 91, 38–47. [Google Scholar] [CrossRef]
- Parvizi, J.; Ghanem, E.; Joshi, A.; Sharkey, P.F.; Hozack, W.J.; Rothman, R.H. Does “Excessive” Anticoagulation Predispose to Periprosthetic Infection? J. Arthroplast. 2007, 22, 24–28. [Google Scholar] [CrossRef]
- Kim, S.; McClave, S.A.; Martindale, R.G.; Miller, K.R.; Hurt, R.T. Hypoalbuminemia and Clinical Outcomes: What Is the Mechanism behind the Relationship? Am. Surg. 2017, 83, 1220–1227. [Google Scholar] [CrossRef]
- Kim, B.-G.; Lee, Y.-K.; Park, H.-P.; Sohn, H.-M.; Oh, A.-Y.; Jeon, Y.-T.; Koo, K.-H. C-Reactive Protein Is an Independent Predictor for 1-Year Mortality in Elderly Patients Undergoing Hip Fracture Surgery. Medicine 2016, 95, e5152. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Cacciola, G.; Mancino, F.; Holzer, L.A.; De Meo, F.; De Martino, I.; Bruschetta, A.; Risitano, S.; Sabatini, L.; Cavaliere, P. Predictive Value of the C-Reactive Protein to Albumin Ratio in 30-Day Mortality after Hip Fracture in Elderly Population: A Retrospective Observational Cohort Study. J. Clin. Med. 2023, 12, 4544. [Google Scholar] [CrossRef]
- Wilson, J.M.; Boissonneault, A.R.; Schwartz, A.M.; Staley, C.A.; Schenker, M.L. Frailty and Malnutrition Are Associated With Inpatient Postoperative Complications and Mortality in Hip Fracture Patients. J. Orthop. Trauma. 2019, 33, 143–148. [Google Scholar] [CrossRef]
- Hanada, M.; Hotta, K.; Matsuyama, Y. Prognostic Nutritional Index as a Risk Factor for Aseptic Wound Complications after Total Knee Arthroplasty. J. Orthop. Sci. 2021, 26, 827–830. [Google Scholar] [CrossRef]
- Cross, M.B.; Yi, P.H.; Thomas, C.F.; Garcia, J.; Della Valle, C.J. Evaluation of Malnutrition in Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. 2014, 22, 193–199. [Google Scholar] [CrossRef]
- Man, S.L.-C.; Chau, W.-W.; Chung, K.-Y.; Ho, K.K.W. Hypoalbuminemia and Obesity Class II Are Reliable Predictors of Peri-Prosthetic Joint Infection in Patient Undergoing Elective Total Knee Arthroplasty. Knee Surg. Relat. Res. 2020, 32, 21. [Google Scholar] [CrossRef]
- Fury, M.S.; Klemt, C.; Barghi, A.; Tirumala, V.; van den Kieboom, J.; Kwon, Y.-M. Preoperative Serum C-Reactive Protein/Albumin Ratio Is a Predictor of Complications After Single-Stage Revision for the Treatment of Periprosthetic Joint Infection. J. Am. Acad. Orthop. Surg. 2021, 29, e1013–e1024. [Google Scholar] [CrossRef]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.-F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The Systemic Immune Response to Trauma: An Overview of Pathophysiology and Treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Vulliamy, P.E.; Perkins, Z.B.; Brohi, K.; Manson, J. Persistent Lymphopenia Is an Independent Predictor of Mortality in Critically Ill Emergency General Surgical Patients. Eur. J. Trauma Emerg. Surg. 2016, 42, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Faust, L.M.; Lerchenberger, M.; Gleich, J.; Linhart, C.; Keppler, A.M.; Schmidmaier, R.; Böcker, W.; Neuerburg, C.; Zhang, Y. Predictive Value of Prognostic Nutritional Index for Early Postoperative Mobility in Elderly Patients with Pertrochanteric Fracture Treated with Intramedullary Nail Osteosynthesis. J. Clin. Med. 2023, 12, 1792. [Google Scholar] [CrossRef] [PubMed]
- Tunçez, M.; Bulut, T.; Süner, U.; Önder, Y.; Kazımoğlu, C. Prognostic Nutritional Index (PNI) Is an Independent Risk Factor for the Postoperative Mortality in Geriatric Patients Undergoing Hip Arthroplasty for Femoral Neck Fracture? A Prospective Controlled Study. Arch. Orthop. Trauma. Surg. 2024, 144, 1289–1295. [Google Scholar] [CrossRef]
- Ji, C.; Cheng, J.; Su, H.; Zhu, Y.; Zou, M. Relationship between High-Sensitivity C-Reactive Protein to Lymphocyte Ratio (Hs-CLR) and Incision Complications Following Medial Opening-Wedge High Tibial Osteotomy for Knee Osteoarthritis. BMC Surg. 2025, 25, 230. [Google Scholar] [CrossRef]
| 842 Patients (568 Knees, 274 Hips) | |
|---|---|
| Age (years) | 67.2 ± 7.8 |
| Sex | |
| Female (n, %) | 602 (71.5%) |
| Male (n, %) | 240 (28.5%) |
| BMI (kg/m2) | 29.4 ± 4.5 |
| ASA score | |
| ASA II (n, %) | 749 (88.9%) |
| ASA III (n, %) | 93 (11.1%) |
| CCI | 3.59 ± 1.2 |
| Length of stay (days) | 7.58 ± 3.2 |
| Serum CRP | 3.72 ± 1.43 |
| Serum Albumin (g/L) | 4.57 ± 0.5 |
| Total lymphocyte count (/mm3) | 1754 ± 522 |
| CAR | 2.15 ± 0.7 |
| PNI | 54.5 ± 5.5 |
| Patients with PJI (n = 33) | Patients Without PJI (n = 809) | p | |
|---|---|---|---|
| Age (years) | 69.4 ± 7.8 | 68.5 ± 6.9 | 0.320 |
| Sex (male/female) | 9/24 | 219/590 | 0.842 |
| Types of operation (THA/TKA) | 13/20 | 232/577 | 0.440 |
| BMI (kg/m2) | 30.4 ± 5.4 | 29.6 ± 3.2 | 0.720 |
| ASA score (ASA II/ASA III) | 29/4 | 711/98 | 0.885 |
| CCI | 3.7 ± 1.8 | 3.6 ± 1.2 | 0.422 |
| Length of stay (days) | 8.9 ± 3.4 | 8.4 ± 2.8 | 0.502 |
| Serum CRP | 4.7 ± 3.3 | 4.1 ± 2.8 | 0.120 |
| Serum Albumin (g/L) | 3.9 ± 0.7 | 4.6 ± 0.4 | 0.015 * |
| Total lymphocyte count (/mm3) | 1432 ± 623 | 1654 ± 633 | 0.085 |
| CAR | 3.1 ± 0.6 | 2.1 ± 0.8 | <0.001 * |
| PNI | 44.1 ± 4.2 | 55.4 ± 4.8 | <0.001 * |
| Postoperative aseptic wound problems within 2 weeks | 9 (27.2%) | 59 (7.3%) | <0.001 * |
| Patients with Aseptic Operative Wound Problems (n = 68) | Patients Without Aseptic Operative Wound Problems (n = 774) | p | |
|---|---|---|---|
| Age (years) | 69.1 ± 8.9 | 68.3 ± 7.6 | 0.240 |
| Sex (male/female) | 29/39 | 215/559 | 0.442 |
| Types of operation (THA/TKA) | 30/38 | 244/530 | 0.350 |
| BMI (kg/m2) | 31.1 ± 5.4 | 29.2 ± 4.4 | 0.030 * |
| ASA score (ASA II/ASA III) | 60/8 | 681/93 | 0.805 |
| CCI | 3.9 ± 1.4 | 3.7 ± 1.6 | 0.121 |
| Length of stay (days) | 8.7 ± 5.0 | 8.6 ± 3.3 | 0.705 |
| Serum CRP | 4.3 ± 3.3 | 3.3 ± 2.8 | 0.230 |
| Serum Albumin (g/L) | 4.3 ± 0.5 | 4.6 ± 0.4 | 0.090 |
| Total lymphocyte count (/mm3) | 1591 ± 751 | 1796 ± 542 | 0.072 |
| CAR | 2.7 ± 0.7 | 2.1 ± 0.5 | 0.062 |
| PNI | 45.9 ± 3.9 | 57.3 ± 3.9 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlidag, T.; Bingol, O.; Keskin, O.H.; Durgal, A.; Yagbasan, B.; Ozdemir, G. C-Reactive Protein to Albumin Ratio and Prognostic Nutrition Index as a Predictor of Periprosthetic Joint Infection and Early Postoperative Wound Complications in Patients Undergoing Primary Total Hip and Knee Arthroplasty. Diagnostics 2025, 15, 2230. https://doi.org/10.3390/diagnostics15172230
Karlidag T, Bingol O, Keskin OH, Durgal A, Yagbasan B, Ozdemir G. C-Reactive Protein to Albumin Ratio and Prognostic Nutrition Index as a Predictor of Periprosthetic Joint Infection and Early Postoperative Wound Complications in Patients Undergoing Primary Total Hip and Knee Arthroplasty. Diagnostics. 2025; 15(17):2230. https://doi.org/10.3390/diagnostics15172230
Chicago/Turabian StyleKarlidag, Taner, Olgun Bingol, Omer Halit Keskin, Atahan Durgal, Baris Yagbasan, and Guzelali Ozdemir. 2025. "C-Reactive Protein to Albumin Ratio and Prognostic Nutrition Index as a Predictor of Periprosthetic Joint Infection and Early Postoperative Wound Complications in Patients Undergoing Primary Total Hip and Knee Arthroplasty" Diagnostics 15, no. 17: 2230. https://doi.org/10.3390/diagnostics15172230
APA StyleKarlidag, T., Bingol, O., Keskin, O. H., Durgal, A., Yagbasan, B., & Ozdemir, G. (2025). C-Reactive Protein to Albumin Ratio and Prognostic Nutrition Index as a Predictor of Periprosthetic Joint Infection and Early Postoperative Wound Complications in Patients Undergoing Primary Total Hip and Knee Arthroplasty. Diagnostics, 15(17), 2230. https://doi.org/10.3390/diagnostics15172230






