Multiparametric Quantitative Ultrasound as a Potential Imaging Biomarker for Noninvasive Detection of Nonalcoholic Steatohepatitis: A Clinical Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
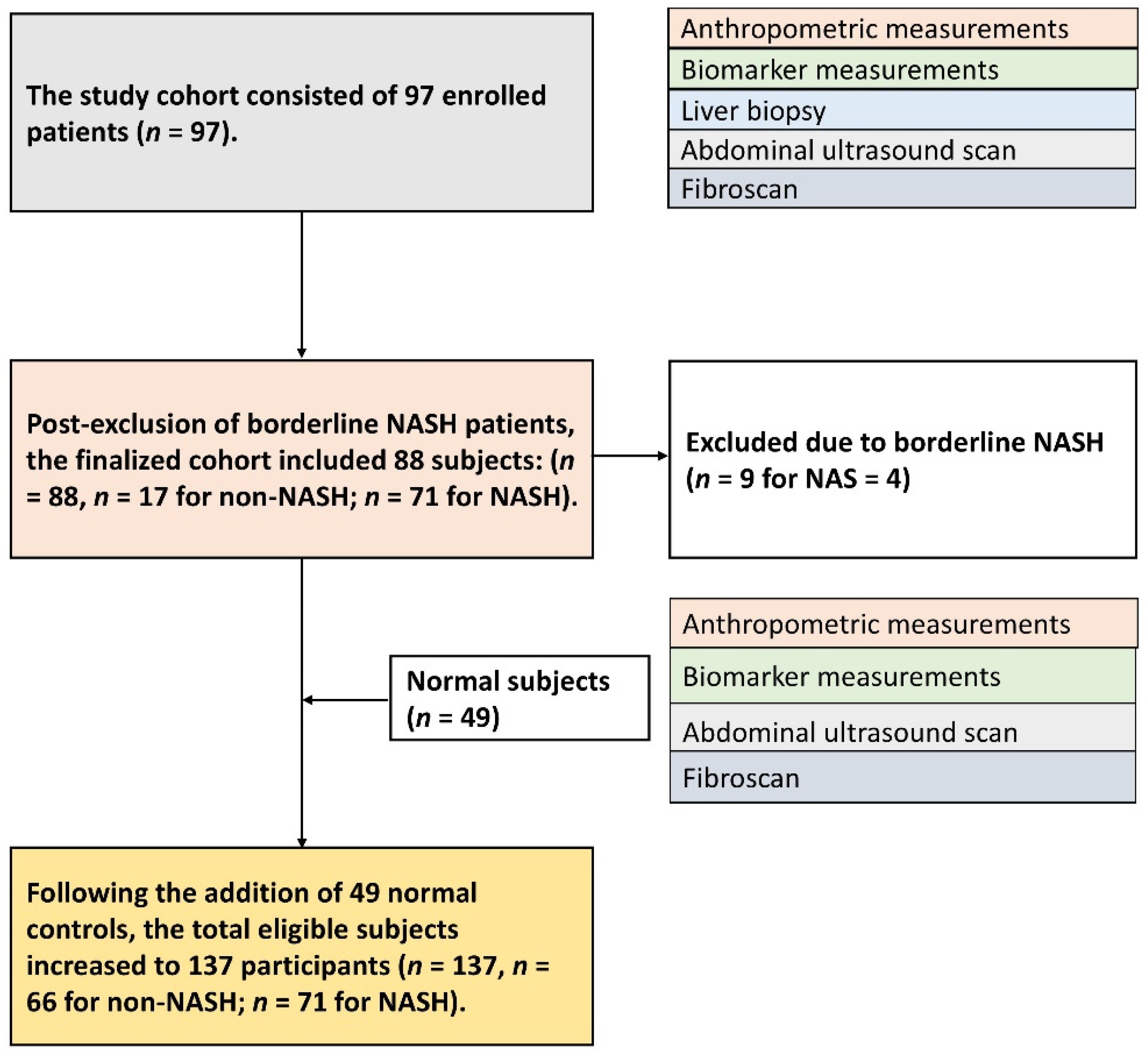
2.1. Subject Enrollment
2.2. FibroScan Measurement
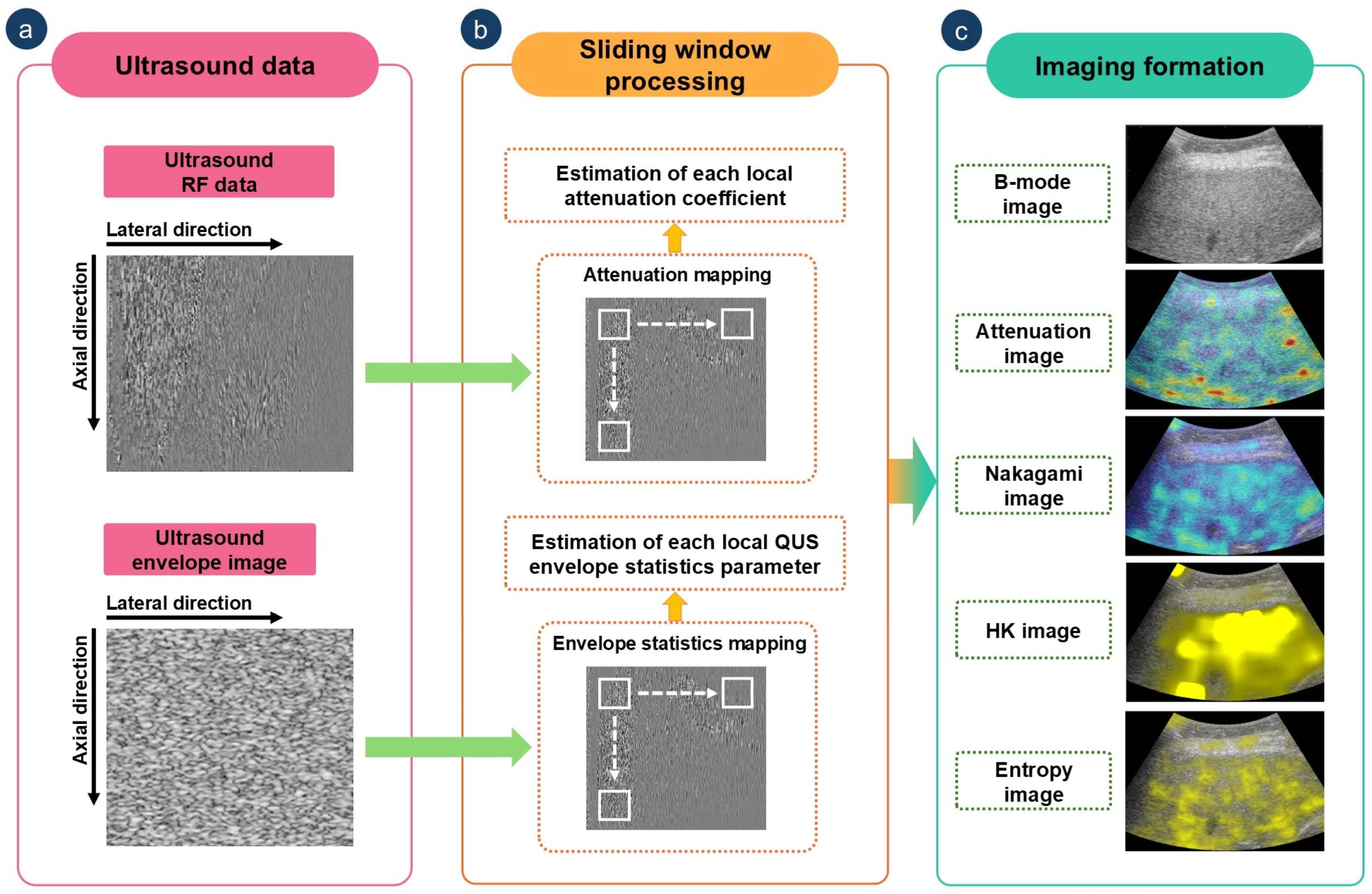
2.3. Ultrasound Backscattered Data Acquisition
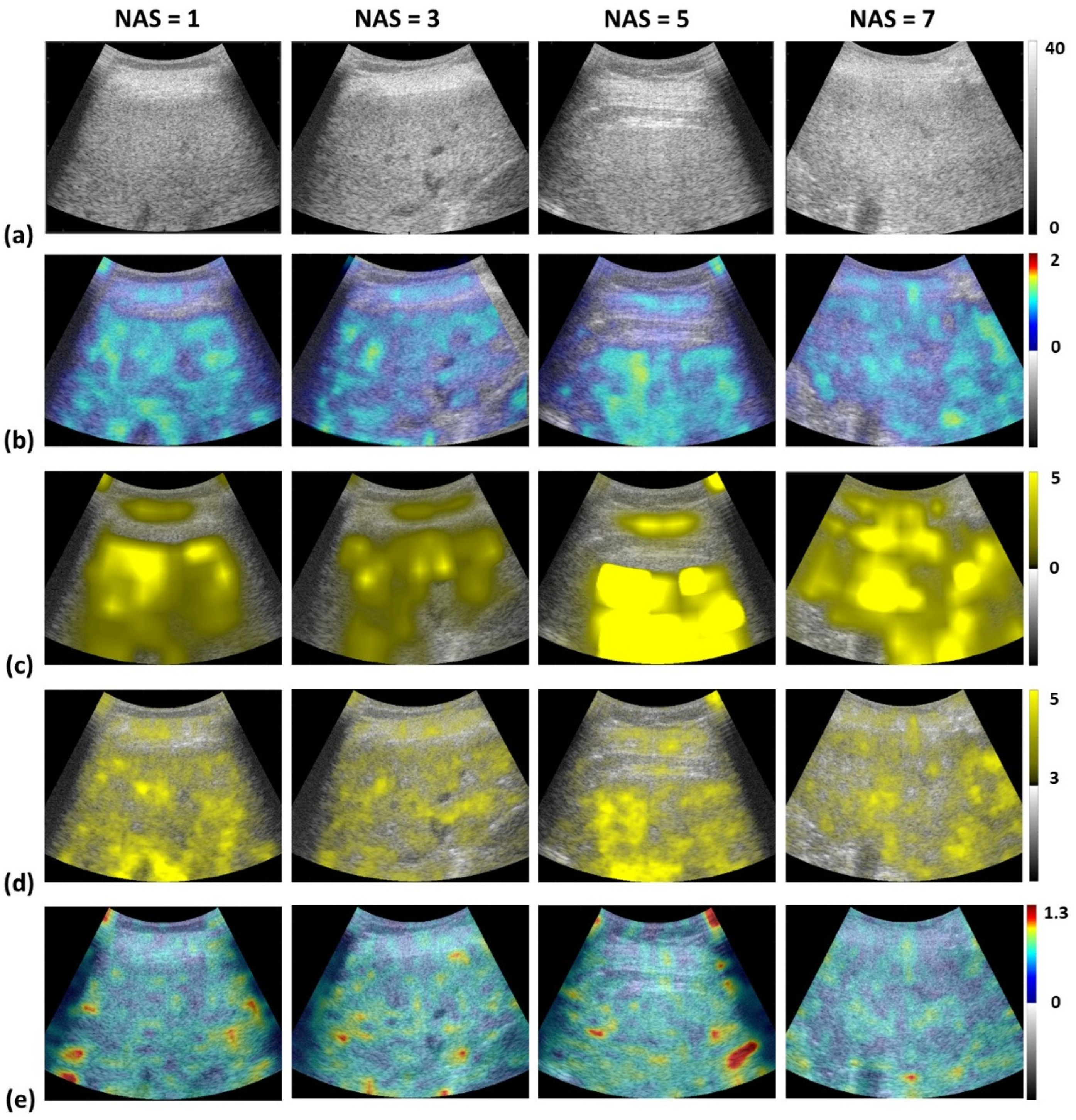
2.4. Envelope Statistics and Attenuation Imaging
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.S.; An, T.H.; Park, H.-J.; Kim, W.K.; Bae, K.-H.; Oh, K.-J. Metabolic spectrum of liver failure in type 2 diabetes and obesity: From NAFLD to NASH to HCC. Int. J. Mol. Sci. 2021, 22, 4495. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Shi, Y.W.; Fan, J.G. Surveillance of the progression and assessment of treatment endpoints for nonalcoholic steatohepatitis. Clin. Mol. Hepatol. 2023, 29, S228–S243. [Google Scholar] [CrossRef] [PubMed]
- Kadi, D.; Loomba, R.; Bashir, M.R. Diagnosis and monitoring of nonalcoholic steatohepatitis: Current state and future directions. Radiology 2024, 310, e222695. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Shankar, S.S.; Yates, K.P.; Bolognese, J.; Daly, E.; Dehn, C.A.; Neuschwander-Tetri, B.; Kowdley, K.; Vuppalanchi, R.; Behling, C.; et al. Diagnostic performance of circulating biomarkers for non-alcoholic steatohepatitis. Nat. Med. 2023, 29, 2656–2664. [Google Scholar] [CrossRef]
- Song, S.J.; Nogami, A.; Liang, L.Y.; Yoneda, M.; Leung, H.H.; Nakajima, A.; Lai, J.C.; Wong, G.L.; Shu, S.S.; Wong, V.W.; et al. Performance of continuous controlled attenuation parameter and liver stiffness measurement by the novel SmartExam in metabolic dysfunction-associated steatotic liver disease. Liver Int. 2024, 44, 1167–1175. [Google Scholar] [CrossRef]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.-K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.-H.; Wong, V.W.-S.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef]
- Ali, A.H.; Al Juboori, A.; Petroski, G.F.; Diaz-Arias, A.A.; Syed-Abdul, M.M.; Wheeler, A.A.; Ganga, R.R.; Pitt, J.B.; Spencer, N.M.; Hammoud, G.M.; et al. The utility and diagnostic accuracy of transient elastography in adults with morbid obesity: A prospective study. J. Clin. Med. 2022, 11, 1201. [Google Scholar] [CrossRef]
- Mendoza, Y.P.; Rodrigues, S.G.; Delgado, M.G.; Murgia, G.; Lange, N.F.; Schropp, J.; Montani, M.; Dufour, J.; Berzigotti, A. Inflammatory activity affects the accuracy of liver stiffness measurement by transient elastography but not by two-dimensional shear wave elastography in non-alcoholic fatty liver disease. Liver Int. 2022, 42, 102–111. [Google Scholar] [CrossRef]
- Millonig, G.; Friedrich, S.; Adolf, S.; Fonouni, H.; Golriz, M.; Mehrabi, A.; Stiefel, P.; Pöschl, G.; Büchler, M.W.; Seitz, H.K.; et al. Liver stiffness is directly influenced by central venous pressure. J. Hepatol. 2010, 52, 206–210. [Google Scholar] [CrossRef]
- Bozic, D.; Podrug, K.; Mikolasevic, I.; Grgurevic, I. Ultrasound methods for the assessment of liver steatosis: A critical appraisal. Diagnostics 2022, 12, 2287. [Google Scholar] [CrossRef]
- Bae, J.S.; Lee, D.H.; Lee, J.Y.; Kim, H.; Yu, S.J.; Lee, J.H.; Cho, E.J.; Lee, Y.B.; Han, J.K.; Choi, B.I. Assessment of hepatic steatosis by using attenuation imaging: A quantitative, easy-to-perform ultrasound technique. Eur. Radiol. 2019, 29, 6499–6507. [Google Scholar] [CrossRef]
- Ferraioli, G.; Kumar, V.; Ozturk, A.; Nam, K.; de Korte, C.L.; Barr, R.G. US attenuation for liver fat quantification: An AIUM-RSNA QIBA pulse-echo quantitative ultrasound initiative. Radiology 2022, 302, 495–506. [Google Scholar] [CrossRef]
- Pirmoazen, A.M.; Khurana, A.; El Kaffas, A.; Kamaya, A. Quantitative ultrasound approaches for diagnosis and monitoring hepatic steatosis in nonalcoholic fatty liver disease. Theranostics 2020, 10, 4277–4289. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.M.; Lee, G.; Jeon, S.K.; Joo, I. Quantitative evaluation of hepatic steatosis using advanced imaging techniques: Focusing on new quantitative ultrasound techniques. Korean J. Radiol. 2022, 23, 13–29. [Google Scholar] [CrossRef]
- Tamura, K.; Mamou, J.; Yoshida, K.; Hachiya, H.; Yamaguchi, T. Ultrasound-based lipid content quantification using double Nakagami distribution model in rat liver steatosis. Jpn. J. Appl. Phys. 2020, 59, SKKE23. [Google Scholar] [CrossRef]
- Chattopadhyay, T.; Lu, C.H.; Chao, Y.P.; Wang, C.Y.; Tai, D.I.; Lai, M.W.; Zhou, Z.; Tsui, P.H. Ultrasound detection of nonalcoholic steatohepatitis using convolutional neural networks with dual-branch global–local feature fusion architecture. Med. Biol. Eng. Comput. 2025. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A. The NAS and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Cai, J.J.; Yu, Y.; She, Z.G.; Li, H. Nonalcoholic fatty liver disease: An update on the diagnosis. Gene Expr. 2019, 19, 187–198. [Google Scholar] [CrossRef]
- Larrue, A.; Noble, J.A. Nakagami imaging with small windows. In Proceedings of the 2011 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Chicago, IL, USA, 30 March–2 April 2011; pp. 887–890. [Google Scholar]
- Muhtadi, S.; Razzaque, R.R.; Chowdhury, A.; Garra, B.S.; Kaisar Alam, S. Texture quantified from ultrasound Nakagami parametric images is diagnostically relevant for breast tumor characterization. J. Med. Imaging 2023, 10, S22410. [Google Scholar] [CrossRef]
- Zhou, Z.; Fang, J.; Cristea, A.; Lin, Y.H.; Tsai, Y.W.; Wan, Y.L.; Yeow, K.M.; Ho, M.C.; Tsui, P.H. Value of homodyned K distribution in ultrasound parametric imaging of hepatic steatosis: An animal study. Ultrasonics 2020, 101, 106001. [Google Scholar] [CrossRef]
- Mamou, J.; Oelze, M.L. (Eds.) Quantitative Ultrasound in Soft Tissues, 2nd ed.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Yan, D.; Li, Q.; Chuang, Y.; Lu, C.; Yang, A.; Lin, C.; Shieh, J.; Weng, W.; Tsui, P. Ultrasound attenuation imaging as a strategy for evaluation of early and late ambulatory functions in Duchenne muscular dystrophy. Med. Phys. 2024, 51, 8074–8086. [Google Scholar] [CrossRef]
- Su, J.Q.; Liu, J.S. Linear combinations of multiple diagnostic markers. J. Am. Stat. Assoc. 1993, 88, 1350–1355. [Google Scholar] [CrossRef]
- Ravaioli, F.; Dajti, E.; Mantovani, A.; Newsome, P.N.; Targher, G.; Colecchia, A. Diagnostic accuracy of FibroScan-AST (FAST) score for the non-invasive identification of patients with fibrotic non-alcoholic steatohepatitis: A systematic review and meta-analysis. Gut 2023, 72, 1399–1409. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Jang, J.Y.; Park, S.Y.; Lee, H.W.; Lee, C.K.; Kim, S.U. Comparison of FibroScan-aspartate aminotransferase (FAST) score and other non-invasive surrogates in predicting high-risk non-alcoholic steatohepatitis criteria. Front. Med. 2022, 9, 869190. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, X. Diagnostic accuracy of FibroScan-AST (FAST) score, non-alcoholic fatty liver fibrosis score (NFS), FibroScan, and liver fibrosis index (FIB-4) for identifying fibrotic non-alcoholic steatohepatitis in patients with chronic hepatitis B with metabolic dysfunction-associated fatty liver disease. Ann. Med. 2024, 56, 2420858. [Google Scholar] [PubMed]
- Karim, G.; Giri, D.; Wyatt, B.; Dinani, A.M. A real-world experience utilizing the FAST score to identify patients with nonalcoholic steatohepatitis fibrosis. Gastro Hep Adv. 2024, 3, 476–481. [Google Scholar] [CrossRef]
- Villani, R.; Lupo, P.; Sangineto, M.; Romano, A.D.; Serviddio, G. Liver ultrasound elastography in non-alcoholic fatty liver disease: A state-of-the-art summary. Diagnostics 2023, 13, 1236. [Google Scholar] [CrossRef]
- Trop, I.; Destrempes, F.; El Khoury, M.; Robidoux, A.; Gaboury, L.; Allard, L.; Chayer, B.; Cloutier, G. The added value of statistical modeling of backscatter properties in the management of breast lesions at US. Radiology 2015, 275, 666–674. [Google Scholar] [CrossRef]
- Fang, J.; Lai, M.W.; Cheng, H.T.; Cristea, A.; Zhou, Z.; Tsui, P.H. Imaging the effects of whole-body vibration on the progression of hepatic steatosis by quantitative ultrasound based on backscatter envelope statistics. Pharmaceutics 2022, 14, 741. [Google Scholar] [CrossRef] [PubMed]
| Normal Participants | Non-NASH | NASH | |
|---|---|---|---|
| No. of participants | 49 | 17 | 71 |
| Male/female | 15/34 | 10/7 | 39/32 |
| Demographics | |||
| Age (years) | |||
| Mean ± SD | 56.65 ± 12.28 ** | 47.12 ± 11.57 | 50.28 ± 12.77 ** |
| Median | 59.00 | 49 | 54.00 |
| Height (cm) | |||
| Mean ± SD | 160.40 ± 10.39 | 162.29 ± 7.83 | 164.01 ± 9.59 ** |
| Median | 159.00 | 163.00 | 163.00 |
| Weight (kg) | |||
| Mean ± SD | 60.70 ± 12.22 ** | 71.36 ± 11.27 | 75.39 ± 13.90 ** |
| Median | 58.10 | 71.00 | 75.00 |
| Anthropometrics | |||
| BMI (kg/cm2) | |||
| Mean ± SD | 23.32 ± 2.79 ** | 27.11 ± 4.01 | 27.90 ± 3.65 ** |
| Median | 22.90 | 26.85 | 28.00 |
| Liver function test | |||
| AST (U/L) | |||
| Mean ± SD | 19.30 ± 8.26 *, ** | 47.11 ± 31.74 * | 72.32 ± 38.35 |
| Median | 18.00 | 39.00 | 60.00 |
| ALT (U/L) | |||
| Mean ± SD | 25.04 ± 4.47 *, ** | 72.65 ± 67.90 * | 116.71 ± 73.02 |
| Median | 24.00 | 76.00 | 100.00 |
| Histology | |||
| Steatosis score | |||
| Mean ± SD | N/A | 1.12 ± 0.48 | 2.61 ± 0.56 ** |
| Median | 1.00 | 3.00 | |
| Hepatocyte ballooning score | |||
| Mean ± SD | N/A | 0.47 ± 0.51 | 1.46 ± 0.58 ** |
| Median | 0.00 | 2.00 | |
| Lobular inflammation score | |||
| Mean ± SD | N/A | 0.88 ± 0.49 | 1.67 ± 0.50 ** |
| Median | 1.00 | 2.00 | |
| FibroScan measurement | |||
| LSM (kPa) | |||
| Mean ± SD | 3.78 ± 0.55 *, ** | 6.38 ± 2.00 | 11.99 ± 10.40 ** |
| Median | 3.70 | 5.90 | 9.10 |
| CAP (dB/m) | |||
| Mean ± SD | 210.57 ± 24.69 *, ** | 287.82 ± 36.36 | 308.28 ± 45.28 ** |
| Median | 219.00 | 279.00 | 310.00 |
| Non-NASH | NASH | |
|---|---|---|
| No. of participants | 66 | 71 |
| Male/female | 25/41 | 39/32 |
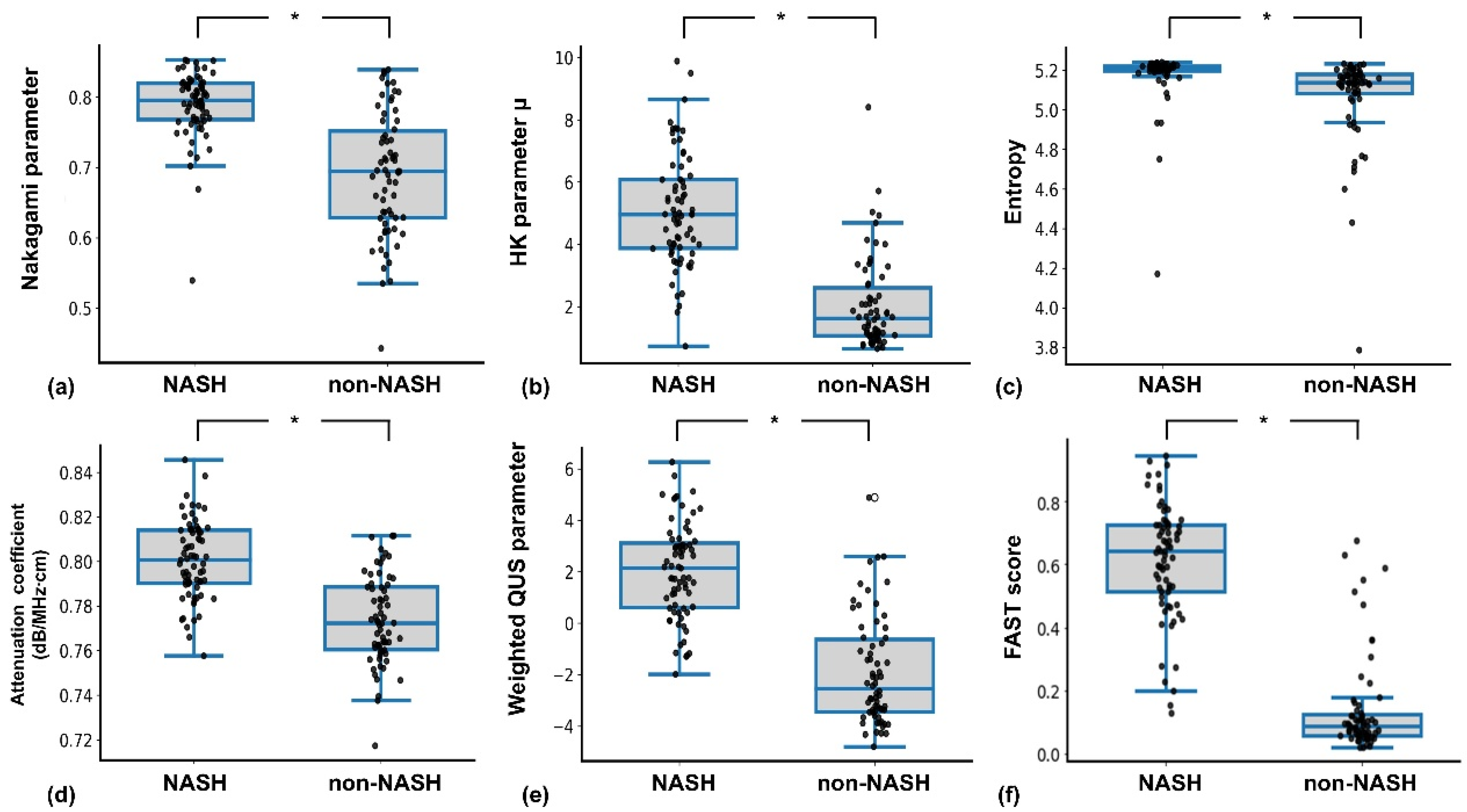
| Nakagami parameter | ||
| Mean ± SD | 0.69 ± 0.087 | 0.79 ± 0.04 * |
| Median | 0.69 | 0.79 |
| HK parameter μ | ||
| Mean ± SD | 2.06 ± 1.46 | 5.07 ± 1.81 * |
| Median | 1.62 | 4.96 |
| Entropy parameter | ||
| Mean ± SD | 5.06 ± 0.23 | 5.17 ± 0.14 * |
| Median | 5.13 | 5.21 |
| Attenuation coefficient | ||
| Mean ± SD | 0.77 ± 0.01 | 0.80 ± 0.01 * |
| Median | 0.77 | 0.80 |
| Weighted QUS parameter | ||
| Mean ± SD | −1.89 ± 2.10 | 2.04 ± 1.86 * |
| Median | −2.54 | 2.14 |
| FAST score | ||
| Mean ± SD | 0.14 ± 0.15 | 0.61 ± 0.18 * |
| Median | 0.08 | 0.64 |
| Parameter | Nakagami Parameter m | HK Parameter μ | Entropy Parameter H | Attenuation Coefficient α | LDA (m, μ, H, α) | FAST Score |
|---|---|---|---|---|---|---|
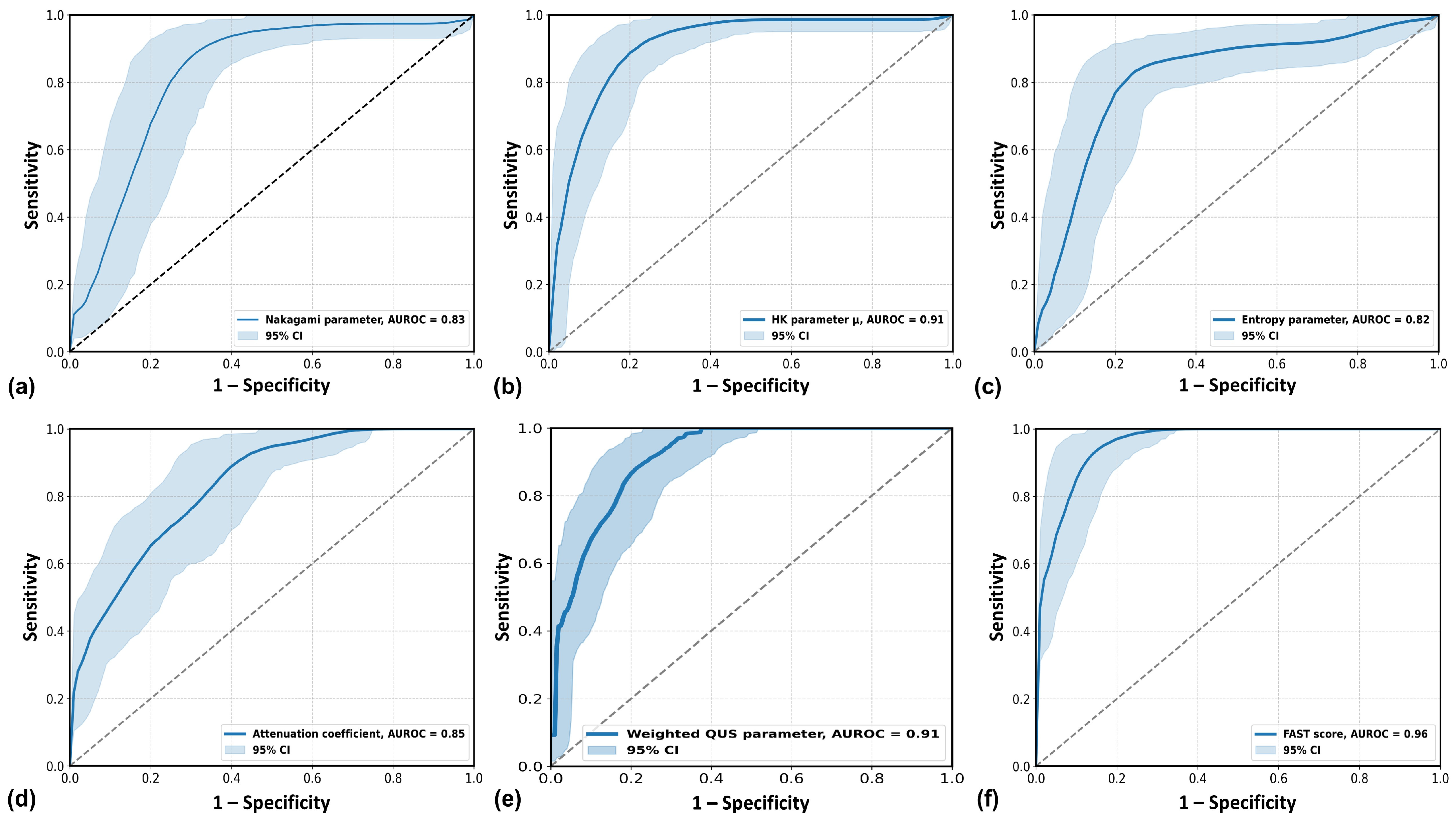
| Accuracy (%) | 81.75 | 85.40 | 81.75 | 78.10 | 84.67 | 91.24 |
| AUROC | 0.83 * | 0.91 | 0.82 * | 0.85 * | 0.91 | 0.96 |
| Cutoff value | 0.75 | 3.41 | 5.19 | 0.78 | −0.13 | 0.41 |
| Sensitivity (%) | 88.73 | 85.92 | 83.10 | 87.32 | 88.73 | 91.55 |
| Specificity (%) | 74.24 | 84.85 | 80.30 | 68.18 | 80.30 | 90.91 |
| LR+ | 3.44 | 5.67 | 4.22 | 2.74 | 4.50 | 10.07 |
| LR− | 0.15 | 0.17 | 0.21 | 0.19 | 0.14 | 0.09 |
| NPV (%) | 85.96 | 84.85 | 81.54 | 83.33 | 86.89 | 90.91 |
| PPV (%) | 78.75 | 85.92 | 81.94 | 74.70 | 82.89 | 91.55 |
| p value (DeLong test) compared to FAST score | 0.0008 | 0.105 | 0.0004 | 0.003 | 0.105 | reference |
| Parameter | Complement of Overlap Coefficient | Bhattacharyya Distance | KL Divergence | Silhouette Score |
|---|---|---|---|---|
| Nakagami parameter m | 0.66 | 0.33 | 3.79 | 0.39 |
| HK parameter μ | 0.73 | 0.43 | 10.36 | 0.48 |
| Entropy parameter H | 0.60 | 0.09 | 1.16 | 0.10 |
| Attenuation coefficient α | 0.61 | 0.26 | 3.11 | 0.34 |
| Weighted QUS parameter | 0.73 | 0.49 | 9.16 | 0.50 |
| FAST score | 0.86 | 0.97 | 11.63 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chattopadhyay, T.; Chan, H.-J.; Le, D.C.; Wang, C.-Y.; Tai, D.-I.; Zhou, Z.; Tsui, P.-H. Multiparametric Quantitative Ultrasound as a Potential Imaging Biomarker for Noninvasive Detection of Nonalcoholic Steatohepatitis: A Clinical Feasibility Study. Diagnostics 2025, 15, 2214. https://doi.org/10.3390/diagnostics15172214
Chattopadhyay T, Chan H-J, Le DC, Wang C-Y, Tai D-I, Zhou Z, Tsui P-H. Multiparametric Quantitative Ultrasound as a Potential Imaging Biomarker for Noninvasive Detection of Nonalcoholic Steatohepatitis: A Clinical Feasibility Study. Diagnostics. 2025; 15(17):2214. https://doi.org/10.3390/diagnostics15172214
Chicago/Turabian StyleChattopadhyay, Trina, Hsien-Jung Chan, Duy Chi Le, Chiao-Yin Wang, Dar-In Tai, Zhuhuang Zhou, and Po-Hsiang Tsui. 2025. "Multiparametric Quantitative Ultrasound as a Potential Imaging Biomarker for Noninvasive Detection of Nonalcoholic Steatohepatitis: A Clinical Feasibility Study" Diagnostics 15, no. 17: 2214. https://doi.org/10.3390/diagnostics15172214
APA StyleChattopadhyay, T., Chan, H.-J., Le, D. C., Wang, C.-Y., Tai, D.-I., Zhou, Z., & Tsui, P.-H. (2025). Multiparametric Quantitative Ultrasound as a Potential Imaging Biomarker for Noninvasive Detection of Nonalcoholic Steatohepatitis: A Clinical Feasibility Study. Diagnostics, 15(17), 2214. https://doi.org/10.3390/diagnostics15172214









