AI-Based HRCT Quantification in Connective Tissue Disease-Associated Interstitial Lung Disease
Abstract
1. Introduction
Unmet Clinical Needs and Rationale
2. Materials and Methods
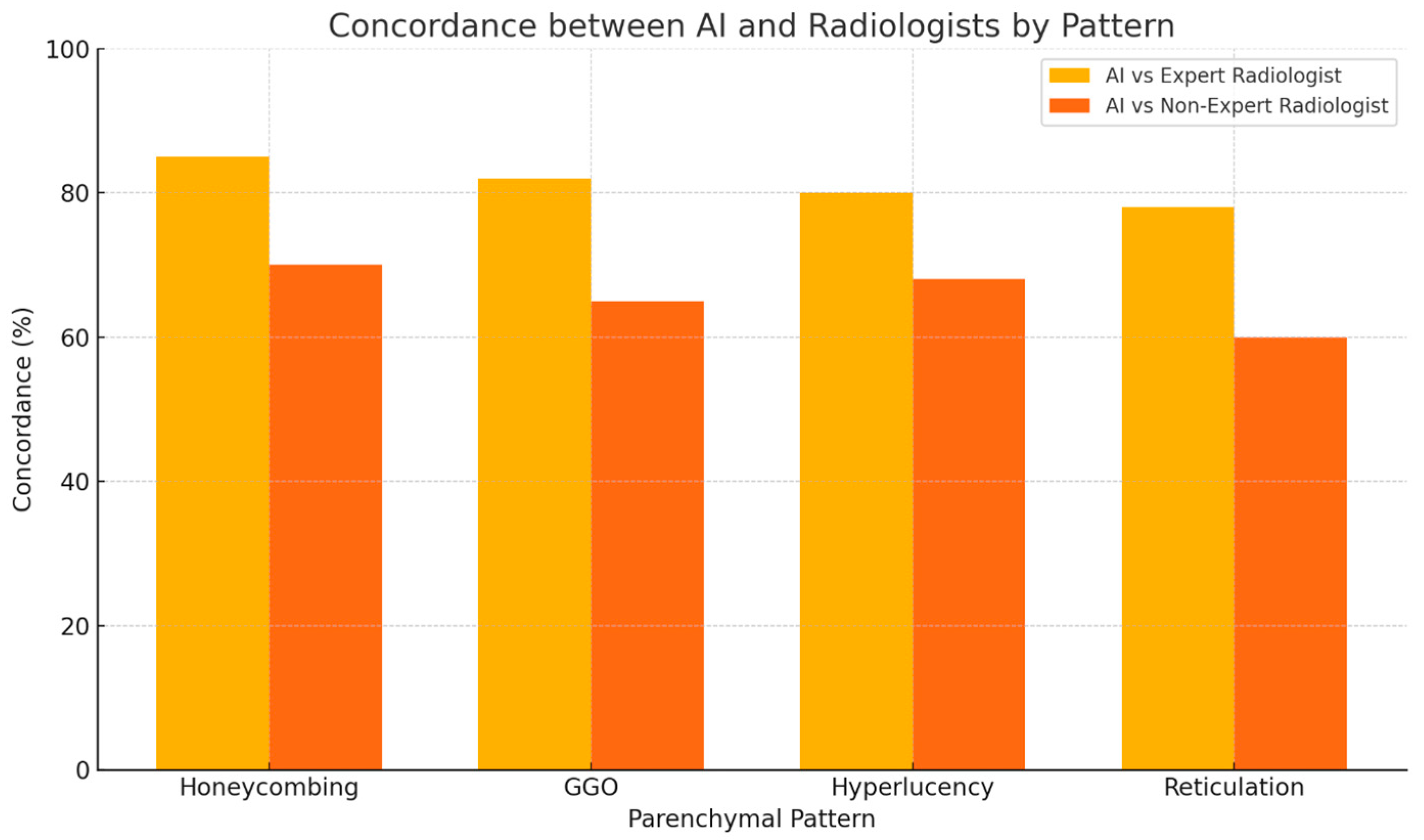
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAIDPI | Artificial Intelligence |
| CTD | Connective Tissue Disease |
| DLCO | Diffusing Capacity of the Lung for Carbon Monoxide |
| FVC | Forced Vital Capacity |
| GGO | Ground Glass Opacity |
| HRCT | High-Resolution Computed Tomography |
| ILD | Interstitial Lung Disease |
| LTA | Lung Texture Analysis |
| PF-ILD | Progressive Fibrosing Interstitial Lung Disease |
| RA | Rheumatoid Arthritis |
| SSc | Systemic Sclerosis |
References
- Nagaraja, V.; Mira-Avendano, I.; Diaz-Arumir, A.; Gotway, M.; Zamora, A.C. Interstitial lung disease in autoimmune diseases. Rev. Colomb. Reumatol. 2024, 31, S139–S153. [Google Scholar]
- Yoo, H.; Hino, T.; Han, J.; Franks, T.J.; Im, Y.; Hatabu, H.; Chung, M.P.; Lee, K.S. Connective tissue disease-related interstitial lung disease (CTD-ILD) and interstitial lung abnormality (ILA): Evolving concept of CT findings, pathology and management. Eur. J. Radiol. Open 2022, 9, 100419. [Google Scholar] [CrossRef]
- Joy, G.M.; Arbiv, O.A.; Wong, C.K.; Lok, S.D.; Adderley, N.A.; Dobosz, K.M.; Johannson, K.A.; Ryerson, C.J. Prevalence, imaging patterns and risk factors of interstitial lung disease in connective tissue disease: A systematic review and meta-analysis. Eur. Respir. Rev. 2023, 32, 220210. [Google Scholar] [CrossRef]
- Gutsche, M.; Rosen, G.D.; Swigris, J.J. Connective Tissue Disease-associated Interstitial Lung Disease: A review. Curr. Respir. Care Rep. 2012, 1, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Mulcaire-Jones, E.; Pugashetti, J.V.; Oldham, J.M.; Khanna, D. Novel Therapeutic Approaches in Connective Tissue Disease-Associated Interstitial Lung Disease. Semin. Respir. Crit. Care Med. 2024, 45, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Dellaripa, P.F. Interstitial lung disease in the connective tissue diseases; a paradigm shift in diagnosis and treatment. Clin. Immunol. 2018, 186, 71–73. [Google Scholar] [CrossRef]
- Morais, A.; Duarte, A.C.; Fernandes, M.O.; Borba, A.; Ruano, C.; Marques, I.D.; Calha, J.; Branco, J.C.; Pereira, J.M.; Salvador, M.J.; et al. Early detection of interstitial lung disease in rheumatic diseases: A joint statement from the Portuguese Pulmonology Society, the Portuguese Rheumatology Society, and the Portuguese Radiology and Nuclear Medicine Society. Pulmonology 2024, 31, 2416840. [Google Scholar] [CrossRef]
- Connerton, Á.; McCarthy, E.; Durcan, L. Connective tissue disease-associated interstitial lung disease: A rheumatologist’s perspective. Breathe 2025, 21, 240166. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.K.; Cottin, V.; Dhar, R.; Danoff, S.; Flaherty, K.R.; Brown, K.K.; Mohan, A.; Renzoni, E.; Mohan, M.; Udwadia, Z.; et al. Progressive pulmonary fibrosis: An expert group consensus statement. Eur. Respir. J. 2023, 61, 2103187. [Google Scholar] [CrossRef]
- Kwon, B.S.; Choe, J.; Chae, E.J.; Hwang, H.S.; Kim, Y.G.; Song, J.W. Progressive fibrosing interstitial lung disease: Prevalence and clinical outcome. Respir. Res. 2021, 22, 282. [Google Scholar] [CrossRef]
- McCarthy, C.; Keane, M.P. Contemporary Concise Review 2021: Interstitial lung disease. Respirology 2022, 27, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Martinez, F.J.; Smith, V.; Walsh, S.L.F. Multidisciplinary teams in the clinical care of fibrotic interstitial lung disease: Current perspectives. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2022, 31, 220003. [Google Scholar] [CrossRef]
- Thattaamuriyil Padmakumari, L.; Guido, G.; Caruso, D.; Nacci, I.; Del Gaudio, A.; Zerunian, M.; Polici, M.; Gopalakrishnan, R.; Sayed Mohamed, A.K.; De Santis, D.; et al. The Role of Chest CT Radiomics in Diagnosis of Lung Cancer or Tuberculosis: A Pilot Study. Diagnostics 2022, 12, 739. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; Patanè, V.; Sangiovanni, A.; Grassi, R.; Russo, A.; Correale, P.; Giordano, D.S.; Zaccaria, C.; Belfiore, M.P.; et al. Prognostic Value of Sarcopenia in Elderly Patients with Metastatic Non-Small-Cell Lung Cancer Undergoing Radiotherapy. Curr. Oncol. 2024, 31, 6673–6685. [Google Scholar] [CrossRef]
- Nardone, V.; Belfiore, M.P.; De Chiara, M.; De Marco, G.; Patanè, V.; Balestrucci, G.; Buono, M.; Salvarezza, M.; Di Guida, G.; D’Angiolella, D.; et al. CARdioimaging in Lung Cancer PatiEnts Undergoing Radical RadioTherapy: CARE-RT Trial. Diagnostics 2023, 13, 1717. [Google Scholar] [CrossRef]
- Insa, A.; Martín-Martorell, P.; Di Liello, R.; Fasano, M.; Martini, G.; Napolitano, S.; Vicidomini, G.; Cappabianca, S.; Franco, R.; Morgillo, F.; et al. Which treatment after first line therapy in NSCLC patients without genetic alterations in the era of immunotherapy? Crit. Rev. Oncol. Hematol. 2022, 169, 103538. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Grassi, R.; Feragalli, B.; Belfiore, M.P.; Montanelli, A.; Patelli, G.; La Porta, M.; Urraro, F.; Fusco, R.; Granata, V.; et al. Coronavirus Disease 2019 (COVID-19) in Italy: Double Reading of Chest CT Examination. Biology 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Grassi, R.; Belfiore, M.P.; Montanelli, A.; Patelli, G.; Urraro, F.; Giacobbe, G.; Fusco, R.; Granata, V.; Petrillo, A.; Sacco, P.; et al. COVID-19 pneumonia: Computer-aided quantification of healthy lung parenchyma, emphysema, ground glass and consolidation on chest computed tomography (CT). Radiol. Med. 2021, 126, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Sharp, M.; Mustafa, A.M.; Farah, N.; Bonham, C.A. Interstitial Lung Disease and Sarcoidosis. Clin. Chest Med. 2023, 44, 575–584. [Google Scholar] [CrossRef]
- Russo, A.; Viglione, V.; Franzese, L.; Patanè, V.; Valente, T.; Perrotta, F.; Reginelli, A. Unveiling the radiological odyssey: Navigating the interstitial with artificial intelligence. Respirol. Case Rep. 2024, 12, e01413. [Google Scholar] [CrossRef]
- Rea, G.; Sverzellati, N.; Bocchino, M.; Lieto, R.; Milanese, G.; D’Alto, M.; Bocchini, G.; Maniscalco, M.; Valente, T.; Sica, G. Beyond Visual Interpretation: Quantitative Analysis and Artificial Intelligence in Interstitial Lung Disease Diagnosis “Expanding Horizons in Radiology”. Diagnostics 2023, 13, 2333. [Google Scholar] [CrossRef]
- Reginelli, A.; Nardone, V.; Giacobbe, G.; Belfiore, M.P.; Grassi, R.; Schettino, F.; Del Canto, M.; Grassi, R.; Cappabianca, S. Radiomics as a New Frontier of Imaging for Cancer Prognosis: A Narrative Review. Diagnostics 2021, 11, 1796. [Google Scholar] [CrossRef] [PubMed]
- Tschalèr, L.; Jordan, S.; Aaløkken, T.M.; Becker, M.; Brunborg, C.; Bruni, C.; Clarenbach, C.; Dobrota, R.; Durheim, M.T.; Elhai, M.; et al. Validation of a semi-quantitative method to assess interstitial lung disease severity and progression in systemic sclerosis by standard and low-dose HRCT scans. RMD Open 2025, 11, e004938. [Google Scholar] [CrossRef]
- Lammi, M.R.; Baughman, R.P.; Birring, S.S.; Russell, A.M.; Ryu, J.H.; Scholand, M.; Distler, O.; LeSage, D.; Sarver, C.; Antoniou, K.; et al. Outcome Measures for Clinical Trials in Interstitial Lung Diseases. Curr. Respir. Med. Rev. 2015, 11, 163–174. [Google Scholar] [CrossRef]
- Guiot, J.; Miedema, J.; Cordeiro, A.; De Vries-Bouwstra, J.K.; Dimitroulas, T.; Søndergaard, K.; Tzouvelekis, A.; Smith, V. Practical guidance for the early recognition and follow-up of patients with connective tissue disease-related interstitial lung disease. Autoimmun. Rev. 2024, 23, 103582. [Google Scholar] [CrossRef]
- Goldin, J.; Cascella, M. Diffusing Capacity of the Lungs for Carbon Monoxide. [Updated 6 October 2024]; StatPearls Publishing: Treasure Island, FL, USA, 2025; Volume 1, p. 1. [Google Scholar]
- Ni, Y.; Yu, Y.; Dai, R.; Shi, G. Diffusing capacity in chronic obstructive pulmonary disease assessment: A meta-analysis. Chronic Respir. Dis. 2021, 18, 14799731211056340. [Google Scholar] [CrossRef]
- Devalla, L.; Ghewade, B.; Jadhav, U.; Annareddy, S. Resolving the Complexity: A Comprehensive Review on Carbon Monoxide Diffusion Capacity in Chronic Obstructive Pulmonary Disease Patients. Cureus 2024, 16, e53492. [Google Scholar] [CrossRef]
- Sahin, H.; Naz, I.; Varol, Y.; Aksel, N.; Tuksavul, F.; Ozsoz, A. COPD patients with severe diffusion defect in carbon monoxide diffusing capacity predict a better outcome for pulmonary rehabilitation. Pulmonology 2016, 22, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Vongvivitpatana, T.S.; Nambiar, A.M. A Low Forced Vital Capacity (FVC)/Diffusing Capacity of the Lung for Carbon Monoxide (DLCO) Ratio Increases Clinical Suspicion for Fibrotic Hypersensitivity Pneumonitis (FHP) Over Idiopathic Pulmonary Fibrosis (IPF). Cureus 2024, 16, e73008. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Mohan, R.; Greeshma, M.V.; Benny, D.; Patil, V.; Madhunapantula, S.V.; Jayaraj, B.S.; Chaya, S.K.; Khan, S.A.; Lokesh, K.S.; et al. Artificial Intelligence Unveils the Unseen: Mapping Novel Lung Patterns in Bronchiectasis via Texture Analysis. Diagnostics 2024, 14, 2883. [Google Scholar] [CrossRef]
- Reginelli, A.; Belfiore, M.P.; Monti, R.; Cozzolino, I.; Costa, M.; Vicidomini, G.; Grassi, R.; Morgillo, F.; Urraro, F.; Nardone, V.; et al. The texture analysis as a predictive method in the assessment of the cytological specimen of CT-guided FNAC of the lung cancer. Med. Oncol. 2020, 37, 54. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, I.; Ronchi, A.; Messina, G.; Montella, M.; Morgillo, F.; Vicidomini, G.; Tirino, V.; Grimaldi, A.; Marino, F.Z.; Santini, M.; et al. Adequacy of Cytologic Samples by Ultrasound-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology of Peripheral Pulmonary Nodules for Morphologic Diagnosis and Molecular Evaluations: Comparison With Computed Tomography-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology. Arch. Pathol. Lab. Med. 2020, 144, 361–369. [Google Scholar]
- Nardone, V.; Reginelli, A.; Grassi, R.; Boldrini, L.; Vacca, G.; D’Ippolito, E.; Annunziata, S.; Farchione, A.; Belfiore, M.P.; Desideri, I.; et al. Delta radiomics: A systematic review. Radiol. Med. 2021, 126, 1571–1583. [Google Scholar] [CrossRef]
- Zaffaroni, G.; Mannucci, A.; Koskenvuo, L.; de Lacy, B.; Maffioli, A.; Bisseling, T.; Half, E.; Cavestro, G.M.; Valle, L.; Ryan, N.; et al. Updated European guidelines for clinical management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP), gastric adenocarcinoma, proximal polyposis of the stomach (GAPPS) and other rare adenomatous polyposis syndromes: A joint EHTG-ESCP revision. Br. J. Surg. 2024, 111, znae070. [Google Scholar]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Nardone, V.; Reginelli, A.; Rubini, D.; Gagliardi, F.; Del Tufo, S.; Belfiore, M.P.; Boldrini, L.; Desideri, I.; Cappabianca, S. Delta radiomics: An updated systematic review. Radiol. Med. 2024, 129, 1197–1214. [Google Scholar] [CrossRef]
- Colombi, D.; Marvisi, M.; Ramponi, S.; Balzarini, L.; Mancini, C.; Milanese, G.; Silva, M.; Sverzellati, N.; Uccelli, M.; Ferrozzi, F. Computer-Aided Evaluation of Interstitial Lung Diseases. Diagnostics 2025, 15, 943. [Google Scholar] [CrossRef]
- Li, X.; Xu, N.; Meng, X.; Dai, C.; Qiu, X.; Ding, H.; Lv, H.; Zeng, R.; Xie, J.; Zhao, P.; et al. Dual-phase contrast-enhanced CT evaluation of dural arteriovenous fistula in patients with pulsatile tinnitus as an initial symptom. Eur. J. Radiol. 2022, 148, 110137. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W. Predictive Biomarkers and Novel Treatments for the Progressive Fibrosing Phenotype in Interstitial Lung Disease Associated with Connective Tissue Disease. Biomedicines 2025, 13, 1463. [Google Scholar] [CrossRef]
- Alsomali, H.; Palmer, E.; Aujayeb, A.; Funston, W. Early Diagnosis and Treatment of Idiopathic Pulmonary Fibrosis: A Narrative Review. Pulm. Ther. 2023, 9, 177–193. [Google Scholar] [CrossRef]
- Jee, A.S.; Sheehy, R.; Hopkins, P.; Corte, T.J.; Grainge, C.; Troy, L.K.; Symons, K.; Spencer, L.M.; Reynolds, P.N.; Chapman, S.; et al. Diagnosis and management of connective tissue disease-associated interstitial lung disease in Australia and New Zealand: A position statement from the Thoracic Society of Australia and New Zealand. Respirology 2021, 26, 23–51. [Google Scholar] [CrossRef]
- Tomassetti, S.; Poletti, V.; Ravaglia, C.; Sverzellati, N.; Piciucchi, S.; Cozzi, D.; Luzzi, V.; Comin, C.; Wells, A.U. Incidental discovery of interstitial lung disease: Diagnostic approach, surveillance and perspectives. Eur. Respir. Rev. 2022, 31, 210206. [Google Scholar] [CrossRef]
- Shao, T.; Shi, X.; Yang, S.; Zhang, W.; Li, X.; Shu, J.; Alqalyoobi, S.; Zeki, A.A.; Leung, P.S.; Shuai, Z. Interstitial Lung Disease in Connective Tissue Disease: A Common Lesion with Heterogeneous Mechanisms and Treatment Considerations. Front. Immunol. 2021, 12, 684699. [Google Scholar] [CrossRef] [PubMed]
- Ledda, R.E.; Milanese, G.; Milone, F.; Leo, L.; Balbi, M.; Silva, M.; Sverzellati, N. Interstitial lung abnormalities: New insights between theory and clinical practice. Insights Into Imaging 2022, 13, 6. [Google Scholar] [CrossRef]
- Larici, A.R.; Biederer, J.; Cicchetti, G.; Franquet Casas, T.; Screaton, N.; Remy-Jardin, M.; Parkar, A.; Prosch, H.; Schaefer-Prokop, C.; Frauenfelder, T.; et al. ESR Essentials: Imaging in fibrotic lung diseases-practice recommendations by the European Society of Thoracic Imaging. Eur. Radiol. 2025, 35, 2245–2255. [Google Scholar] [CrossRef]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. Ethical and regulatory challenges of AI technologies in healthcare: A narrative review. Heliyon 2024, 10, e26297. [Google Scholar] [CrossRef]
- Zhao, A.P.; Li, S.; Cao, Z.; Hu, P.J.-H.; Wang, J.; Xiang, Y.; Xie, D.; Lu, X. AI for science: Predicting infectious diseases. J. Saf. Sci. Resil. 2024, 5, 130–146. [Google Scholar] [CrossRef]
- Olawade, D.B.; Wada, O.J.; David-Olawade, A.C.; Kunonga, E.; Abaire, O.; Ling, J. Using artificial intelligence to improve public health: A narrative review. Front. Public Health 2023, 11, 1196397. [Google Scholar] [CrossRef] [PubMed]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Grassi, F.; Sirica, R.; Genito, E.; Ciani, G.; Patanè, V.; Monti, R.; Belfiore, M.P.; Urraro, F.; Santorsola, M.; et al. Associations between Radiomics and Genomics in Non-Small Cell Lung Cancer Utilizing Computed Tomography and Next-Generation Sequencing: An Exploratory Study. Genes 2024, 15, 803. [Google Scholar] [CrossRef]
- Baratella, E.; Borghesi, A.; Calandriello, L.; Cortese, G.; Della Casa, G.; Giraudo, C.; Grassedonio, E.; Larici, A.R.; Palmucci, S.; Romei, C.; et al. Quantification of progressive pulmonary fibrosis by visual scoring of HRCT images: Recommendations from Italian chest radiology experts. Radiol. Med. 2025, 130, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Glenn, L.M.; Troy, L.K.; Corte, T.J. Novel diagnostic techniques in interstitial lung disease. Front. Med. 2023, 10, 1174443. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, C.J.; Corte, T.J.; Myers, J.L.; Walsh, S.L.F.; Guler, S.A. A contemporary practical approach to the multidisciplinary management of unclassifiable interstitial lung disease. Eur. Respir. J. 2021, 58, 2100276. [Google Scholar] [CrossRef]
- Höppner, J.; Wollsching-Strobel, M.; Schumacher, F.; Windisch, W.; Berger, M. Progressive pulmonary fibrosis in patients with connective tissue disease-associated interstitial lung disease: An explorative study. Arch. Rheumatol. 2024, 39, 46–51. [Google Scholar] [CrossRef]
- Althobiani, M.A.; Russell, A.-M.; Jacob, J.; Ranjan, Y.; Folarin, A.A.; Hurst, J.R.; Porter, J.C. Interstitial lung disease: A review of classification, etiology, epidemiology, clinical diagnosis, pharmacological and non-pharmacological treatment. Front. Med. 2024, 11, 1296890. [Google Scholar] [CrossRef] [PubMed]

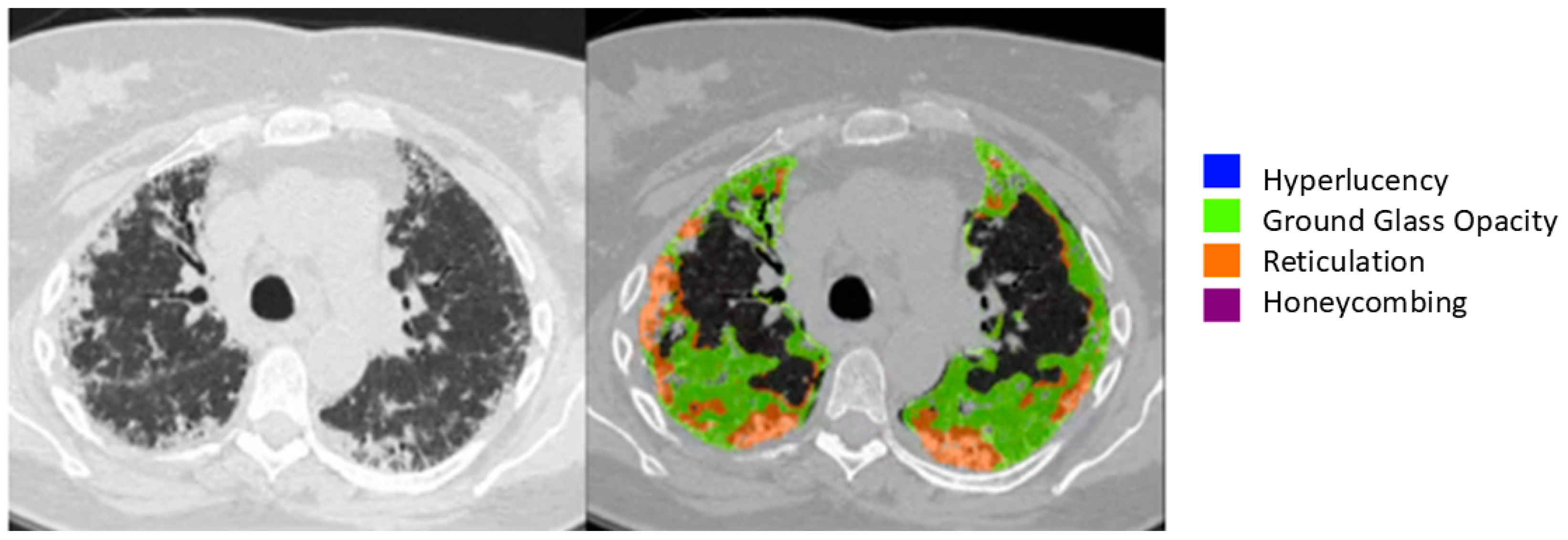
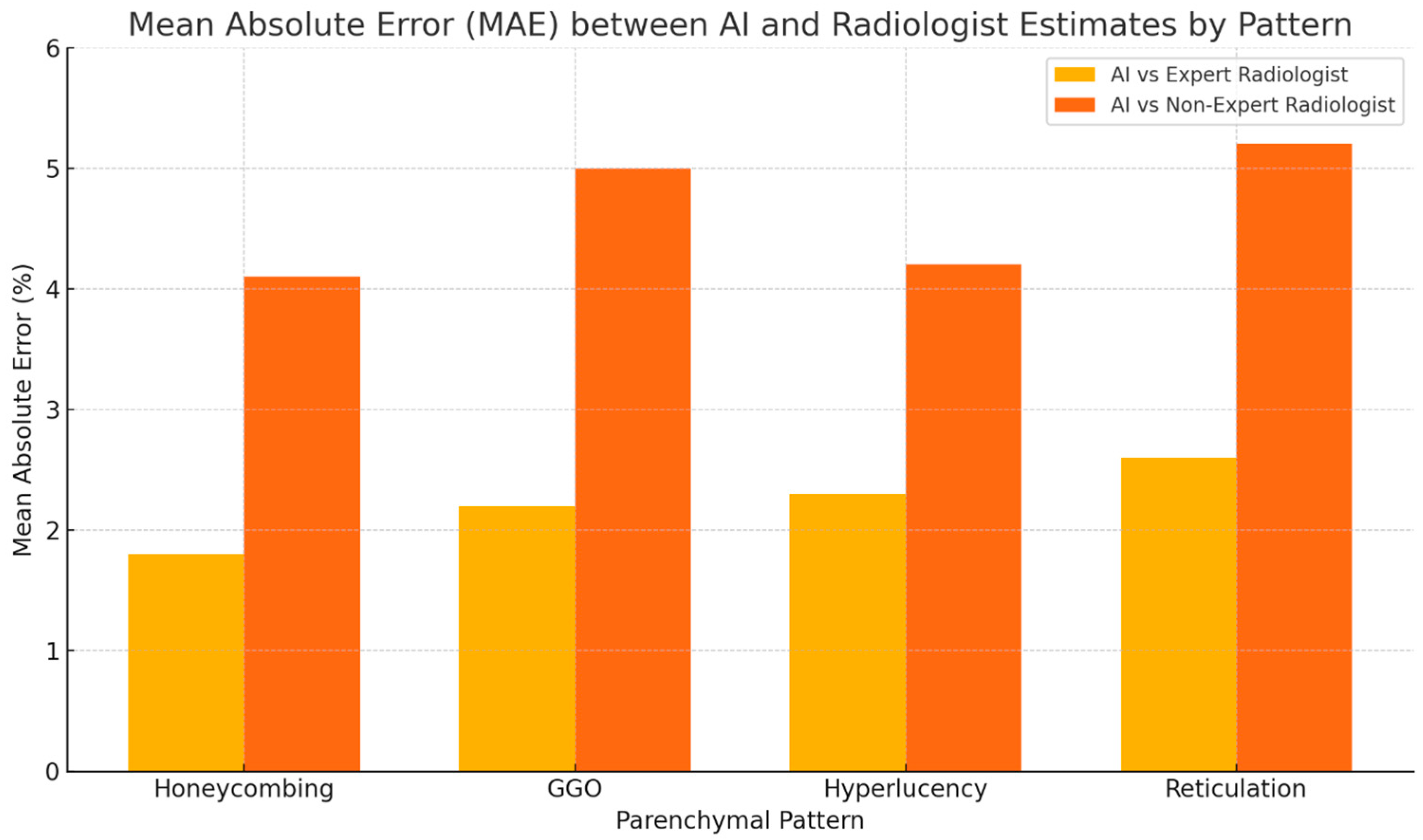
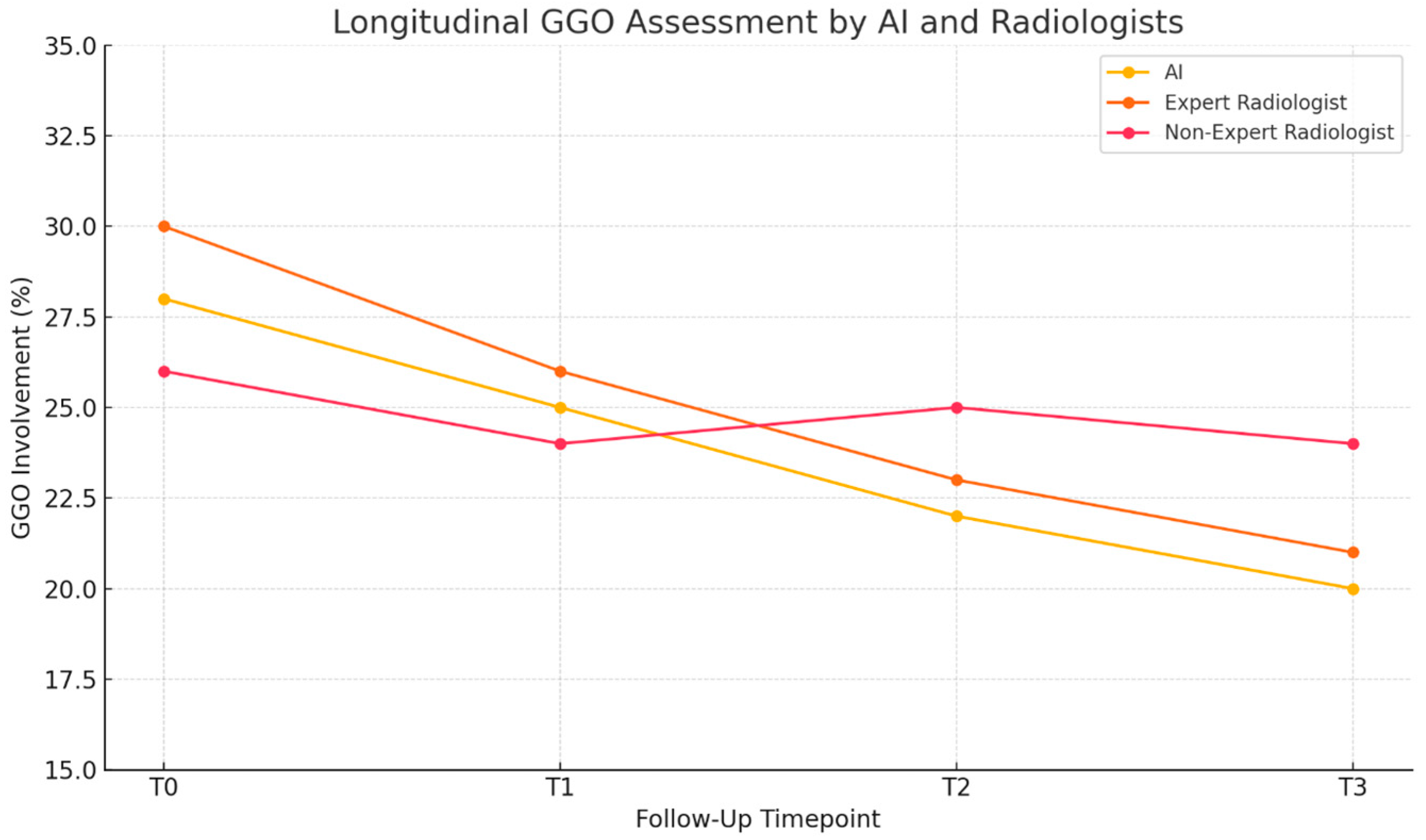
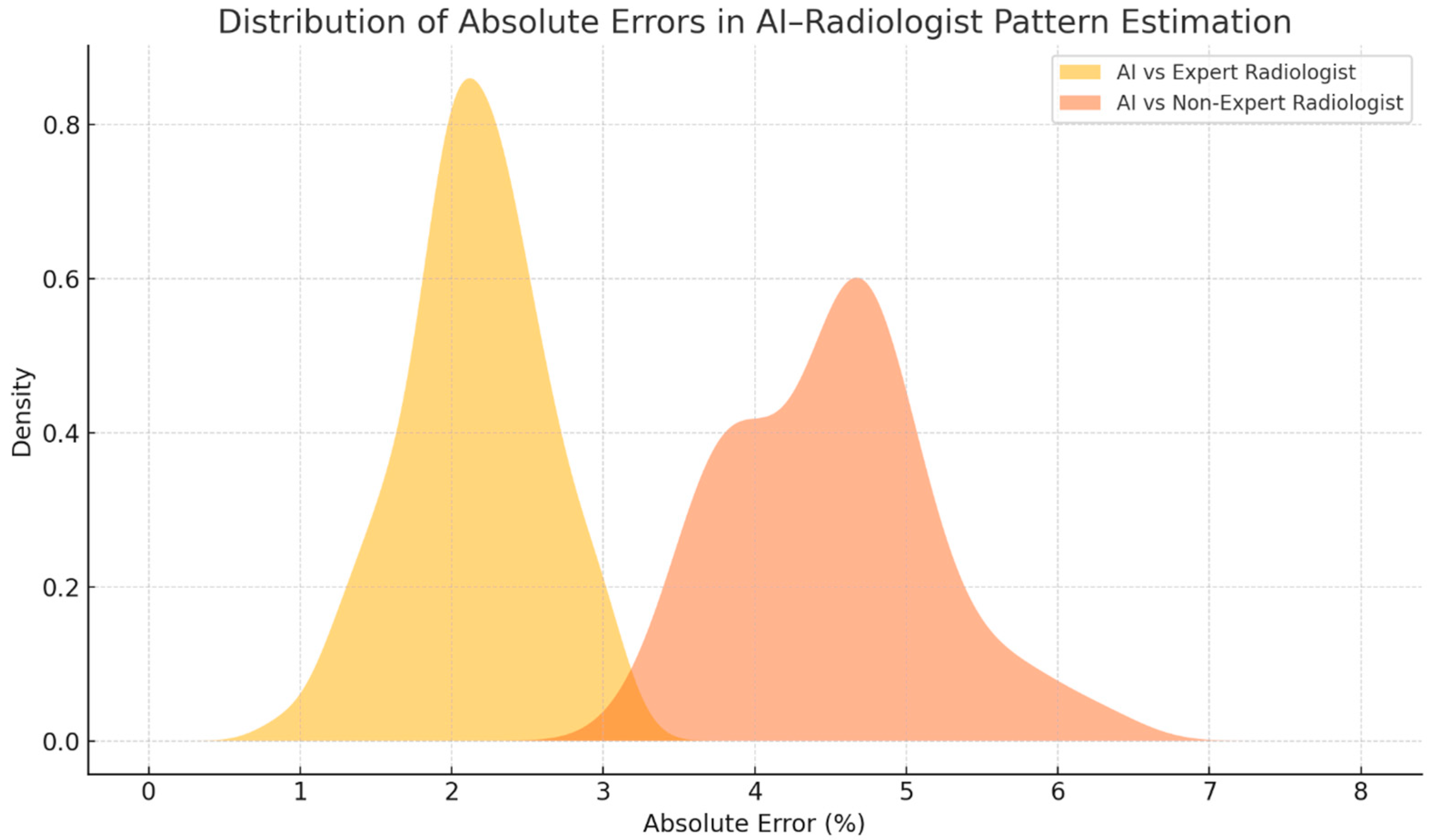
| Underlying CTD Subtype, n (%) | Value |
|---|---|
| Systemic Sclerosis | 20 (41.7%) |
| Rheumatoid Arthritis | 11 (22.9%) |
| Idiopathic Inflammatory Myopathies (dermatomyositis/polymiotis) | 7 (14.6%) |
| Primary Sjogren’s Syndrome | 4 (8.3%) |
| Mixed Connective Tissue Disease | 4 (8.3%) |
| Undifferentiated Connective Tissue Disease | 2 (4.2%) |
| Clinical Scenario | Common Limitation in Practice | How AI Can Help | Clinical Benefit |
|---|---|---|---|
| Routine HRCT follow-up | Visual comparison of serial scans is subjective and inconsistent | Provides continuous, objective quantification of parenchymal patterns across timepoints | More reliable assessment of disease progression or stability |
| Limited radiologist experience | Under- or overestimation of fibrotic involvement | Standardized measurements independent of reader expertise | Reduces variability, improves reporting consistency |
| Multidisciplinary team discussions (MDT) | Lack of reproducible metrics to support imaging interpretation | Offers numerical data on pattern extent that can be shared across MDT members | Improves communication and alignment of clinical decisions |
| Treatment response evaluation | Subtle changes not easily detected visually | Captures minor improvements or worsening, even when not clearly visible on HRCT | Enables timely therapy escalation or continuation |
| Suspected PPF (Progressive Fibrosing Phenotype) | Difficult to meet radiologic progression criteria based on visual read alone | AI provides quantifiable evidence of parenchymal increase supportive of PPF criteria | Aids in identifying patients who may benefit from antifibrotic therapy |
| Patients with limited PFT interpretability | Restrictive lung defects from musculoskeletal or chest wall disease | Imaging-based estimates unaffected by extrapulmonary factors | Complements PFTs, prevents misclassification of disease severity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, A.; Patanè, V.; Oliva, A.; Viglione, V.; Franzese, L.; Forte, G.; Liakouli, V.; Perrotta, F.; Reginelli, A. AI-Based HRCT Quantification in Connective Tissue Disease-Associated Interstitial Lung Disease. Diagnostics 2025, 15, 2179. https://doi.org/10.3390/diagnostics15172179
Russo A, Patanè V, Oliva A, Viglione V, Franzese L, Forte G, Liakouli V, Perrotta F, Reginelli A. AI-Based HRCT Quantification in Connective Tissue Disease-Associated Interstitial Lung Disease. Diagnostics. 2025; 15(17):2179. https://doi.org/10.3390/diagnostics15172179
Chicago/Turabian StyleRusso, Anna, Vittorio Patanè, Alessandra Oliva, Vittorio Viglione, Linda Franzese, Giulio Forte, Vasiliki Liakouli, Fabio Perrotta, and Alfonso Reginelli. 2025. "AI-Based HRCT Quantification in Connective Tissue Disease-Associated Interstitial Lung Disease" Diagnostics 15, no. 17: 2179. https://doi.org/10.3390/diagnostics15172179
APA StyleRusso, A., Patanè, V., Oliva, A., Viglione, V., Franzese, L., Forte, G., Liakouli, V., Perrotta, F., & Reginelli, A. (2025). AI-Based HRCT Quantification in Connective Tissue Disease-Associated Interstitial Lung Disease. Diagnostics, 15(17), 2179. https://doi.org/10.3390/diagnostics15172179






