Diagnosis and Management of Functional Pancreatic Neuroendocrine Tumors in Children—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Screening Process, Critical Appraisal, and Data Extraction
2.4. Assessment of the Methodological Quality and the Risk of Bias of Studies
2.5. Statistical Analysis
3. Results
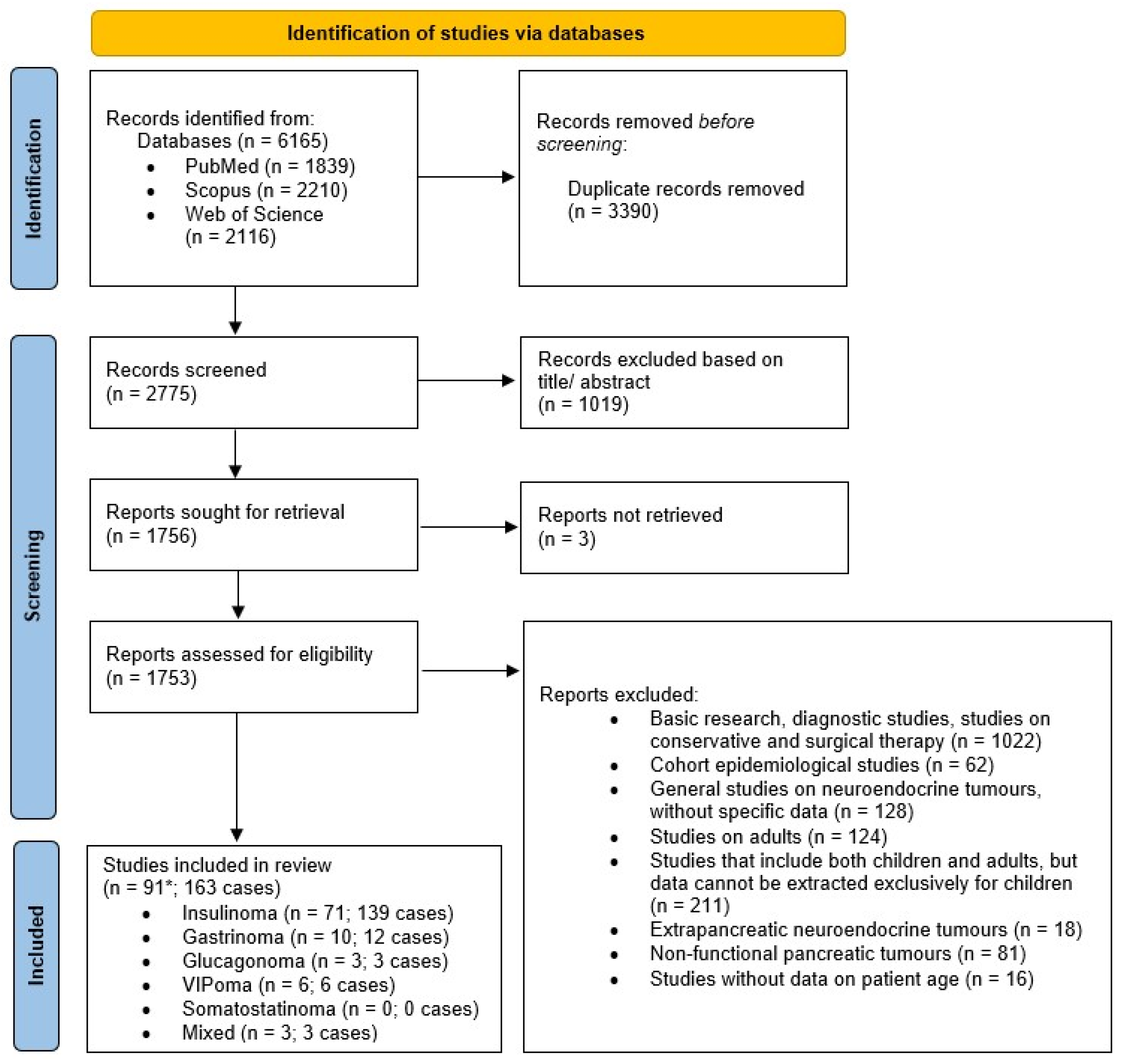
3.1. Study Selection
3.2. Study Characteristics, Risk of Bias, and Summary of Included Studies
| Study | Country | Type of Study | Type of Tumor | Patient’s Gender | Patient’s Age (Years) | Clinical Presentation | Laboratory Findings | Genetic Analysis | Radiological Modality | Tumor Location | Tumor Size (cm) | Type of Treatment | Histopathology | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sales et al., 2025 [15] | Brazil | Case report | I | M | 11 | Generalized tonic–clonic seizures, hypoglycemia | Fasting glucose—33 mg/dL, insulin—12.1 mU/L | MEN1 | MR | - | 1 × 0.8 | Surgical resection | - | Glycemic normalization |
| Murray et al. 2025 [16] | USA | Case report | I | M | 13 | Severe weakness, altered mental status, syncopal episodes | Glucose—98 mg/dL, insulin—44 μIU/mL | MEN1 | CT + MR | Head + body + tail | 0.3, 0,4, 0.7, 2.1 | Surgical enucleation | NET G1/G2 | Glycemic normalization |
| Lemus-Zepeda et al. 2025 [17] | Colombia | Case report | I | M | 8 | Hypoglycemia during an episode of abnormal movements | Glucose—33.5 mg/dL, insulin—13.3 µUI/mL | MEN1 | MR | Uncinate process | 2.7 × 2.2 × 1.6 | Surgical enucleation | - | Glycemic normalization |
| Huang et al., 2024 [18] | China | Case report | I | M | 14 | Recurrent seizure-like episodes | Glucose—2.09 mmol/L, insulin—23.96 μU/mL | MEN1 | MR | Body | 1.7 × 1.2 | Distal partial pancreatectomy | NET G2 | Glycemic normalization |
| Kamińska-Jackowiak et al., 2024 [19] | Poland | Case report | I | F | 15 | Neuroglycopenia | Glucose—47 mg/dL, insulin—43 μUI/mL | MEN1 | US + CT + MR | Head, tail | - | Enucleation | - | Reoperation |
| Moszczyńska et al., 2024 [20] | Poland | Case report | I | F | 13 | Weakness, paleness, profuse sweating, balance impairment, hypoglycemia | Glucose—27 mg/dL, insulin—90.9 μIU/mL | - | CT | Head | +liver mts | Modified Whipple’s operation | NET G2 | Somatostatin analogs + allogeneic liver transplantation + relaparotomy |
| Tian et al., 2024 [21] | China | Original research | I | F | 9 | Dizziness | Glucose—1.8 mmol/L, insulin—129 pmol/L | - | US + MR | Head | 3 | Tumor enucleation | NET G1 | glycemic normalization |
| M | 7 | Dizziness | Glucose—3.9 mmol/L, insulin—1736 pmol/L | US + CT | Tail | 1.5 | Partial resection | NET G2 | Glycemic normalization | |||||
| M | 9 | Dizziness | Glucose—0.5 mmol/L, insulin—69.3 pmol/L | US + MR | Neck | 2 | Tumor enucleation | NET G2 | Glycemic normalization | |||||
| M | 4 | Seizure | Glucose—1.5 mmol/L, insulin—94.2 pmol/L | US + MR | Body | 1.5 | Partial resection | NET G1 | Glycemic normalization | |||||
| M | 4 | Dizziness | Glucose—2.2 mmol/L, insulin—219.2 pmol/L | US + MR | Head | 0.8 | Subtotal resection | NET G1 | Glycemic normalization, postoperative Creon | |||||
| Vinhosa Bastos et al., 2023 [22] | Brazil | Case report | I | M | 16 | Episodes of muscle spasms in thighs and legs | Glucose—71 mg/dL | - | US + MR | Body–tail transition | 2.2 × 1.3 | Pancreatic nodulectomy | NET G2 | Glycemic normalization, mild chronic neurogenic changes |
| Melikyan et al., 2023 [6] | Russia | Original research | I | M:F/9:13 | 11 | - | Glucose—1.9 (0.5–2.2) mmol/L, insulin—20.9 (8.13–149) U/L | MEN1 | US (7), CT and/or MR (18) | Head + tail | 1.2, 0.3 | Partial resection | NET G2 | Pituitary adenoma at 13 years, hPTH at 15 years |
| 8 | MEN1 | Head | 2.5 | Subtotal pancreatectomy | - | hPRL at 21 years (on cabergoline), gastrinoma at 25 years | ||||||||
| 13 | MEN1 | Tail | 3 | Enucleation | - | hPTH, hPRL, adrenal nodular hyperplasia at 19 years | ||||||||
| 11 | MEN1 | Body + tail | 1.5, 1.4 | Enucleation | NET G2 | hPTH at 13 years, hPRL at 16 tears (on cabergoline) | ||||||||
| 9 | MEN1 | Head + body + tail | 2, 0.5, 0.6 | Partial resection | NET G1 | Somatotropinoma at 12 years, pNET at 13 years, hPTH at 14 years | ||||||||
| 14 | MEN1 | Head + tail | 3.7, 0.6 | Enucleation | NET G2 | hPTH at 18 years | ||||||||
| 8 | MEN1 | Tail | 1.1 | Partial resection | NET G2 | Glycemic normalization | ||||||||
| 12 | MEN1 | Tail | 2.3 | Enucleation | NET G2 | Glycemic normalization | ||||||||
| 17 | - | Tail | 3.5 | Enucleation | NET G2 | Glycemic normalization | ||||||||
| 13 | - | Tail | 3 | Enucleation | NET G1 | Glycemic normalization | ||||||||
| 12 | - | Body | 1.5 | Enucleation | NET G2 | Glycemic normalization | ||||||||
| 11 | - | Body | 6 + liver mts | Subtotal pancreatectomy + splenectomy | NET G2 in tumor, G3 in mts | Deceased at 11 years 8 months | ||||||||
| 8 | - | Tail | 0.95 | Enucleation | NET G1 | - | ||||||||
| 15 | - | Head | 1.56 | Pancreatic resection | NET G1 | - | ||||||||
| 14 | - | Head | 2 | Partial resection | NET G1 | Creon | ||||||||
| 13 | - | Head | 1.5 | Partial resection | NET G2 | Glycemic normalization | ||||||||
| 16 | - | Tail | 1.1 | Partial resection | NET G1 | - | ||||||||
| 14 | - | Head | 1.9 | Enucleation | - | Epilepsy at 18 years | ||||||||
| 11 | - | Body | 1 | Enucleation | - | Glycemic normalization | ||||||||
| 11 | - | Head + body | 1.5, 0.5, 1, 3 | Enucleation at 11 years, partial resection at 12 years, pancreato-gastro-duodenal resection at 13 years | NET G1/G2 | Postoperative DM, liver mts at 21 years, nephropathy at 32 years | ||||||||
| 9 | - | Body | 1.9 | Enucleation | NET G1 | - | ||||||||
| 17 | - | Body | 2.5 | Partial resection | - | - | ||||||||
| Shariq et al., 2022 [23] | United Kingdom | Original research | I | F | 15 | - | - | MEN1 | EUS + CT + MR | Tail | 6 | Distal pancreatectomy | - | No recurrence of hypoglycemia |
| F | 10 | MEN1 | EUS + CT + MR | Tail | 1.7 | Distal pancreatectomy | NET G2 | No recurrence of hypoglycemia | ||||||
| M | 10 | MEN1 | EUS + CT + MR | Tail | 1 | Distal pancreatectomy | - | No recurrence of hypoglycemia | ||||||
| M | 6 | MEN1 | EUS + CT + MR | Body | 0.4 | Distal pancreatectomy | NET G1 | No recurrence of hypoglycemia | ||||||
| F | 16 | MEN1 | EUS + CT + MR | Tail | 2.1 | Distal pancreatectomy | NET G2 | No recurrence of hypoglycemia | ||||||
| Sherafati et al., 2022 [24] | Iran | Case report | I | M | 16 | Generalized tonic–clonic seizures | Glucose—60 mg/dL, insulin—30.1 mIU/L | - | PET CT | Tail | 1 | Subtotal pancreatectomy | - | No recurrence |
| Yu et al., 2021 [25] | China | Case report | I | F | 14 | Increased daytime, walked unsteadily, twitching of the limbs | Glucose—1.9 mmol/L, insulin—23.2 μIU/mL | - | EUS + CT + MR | Head | 1 | Surgical enucleation | - | Glycemic normalization |
| Schulte Am Esch et al., 2021 [26] | Germany | Case report | I | M | 10 | Absence-like condition | Glucose—46 mg/dL, insulin—7.2 µU/L | MEN1 | EUS + PET MR | Body | 1.5 | Robotic enucleation | NET G2 | Glycemic normalization |
| Mahdi et al., 2020 [27] | Saudi Arabia | Case report | I | M | 8 | Multiple hypoglycemic attacks | Glucose—1.9 mmol/L, insulin—20 μU/mL | - | CT | Tail | 1.2 | Laparoscopic enucleation | NET G1 | No recurrence |
| Al Azmi et al., 2020 [28] | Saudi Arabia | Case report | I | M | 7 | Hypoglycemia episodes | Glucose—1.9 mmol/L, insulin—20 µU/mL | - | CT + MR | Tail | 2.5 × 1 | Laparoscopic enucleation | - | No recurrence |
| Selberherr et al., 2019 [29] | Austria | Original research | I | M | 15 | Hypoglycemia | - | MEN1 | EUS + CT + MR | Body + tail | 2, 1.5, 0.6, 0.5 | Left pancreatic resection | NET G2 | Glycemic normalization |
| Escartín et al., 2018 [30] | Spain | Case report | I | M | 11 | Episode of fainting | Glucose—42 mg/dL, insulin—10.6 µU/mL | - | EUS + CT | Body–tail transition | 1.1 | Laparoscopic partial pancreatectomy | - | Glycemic normalization |
| Liang et al., 2018 [31] | China | Case report | I | F | 9 | Loss of consciousness, palpitations, convulsions | Glucose—2.2 mmol/L, insulin—15.35 μIU/mL | MEN1 | PET CT + MR | Tail | 1.1 × 1.3 | Robotic enucleation | NET G2 | No recurrence |
| Nakano et al., 2018 [32] | Japan | Case report | I | F | 14 | Convulsions | Glucose—33 mg/dL, insulin—11.2 µIU/mL | MEN1 | MR | - | 1 | Surgical resection | - | - |
| Hu et al., 2017 [33] | China | Case report | I | F | 9 | Episodic fainting attacks with sweatiness | Glucose—1.84 mmol/L, insulin—8.2 mU/L | - | MR | Tail | 2 × 2 × 1.2 | Robot-assisted, distal pancreatectomy | NET G2 | Glycemic normalization |
| Beisang et al., 2017 [34] | USA | Case report | I | M | 16 | Limb shaking | Glucose—41 mg/dL, insulin—21 uU/mL | - | EUS + MR | Uncinate process | 1.6 × 1 | Pancreaticoduodenectomy | - | Glycemic normalization |
| Yao et al., 2017 [35] | China | Original research | I | F | 17 | Hypoglycemia, somnolence | Insulin—25.7 mU/L | - | - | Tail | 0.5 | Enucleation | - | PNET recurrence—reenucleation |
| M | 14 | Hypoglycemia | Insulin—19.7 mU/L | CT + MR | Head | 7 + liver mts | Enucleation | PNET recurrence—pancreaticoduodenectomy | ||||||
| F | 15 | Confusion | Insulin—7.9 mU/L | - | Uncinate process | 2 | Radical surgery | No recurrence | ||||||
| Kwon et al., 2016 [36] | Korea | Case report | I | F | 9 | Sudden loss of balance, tremor, generalized tonic–clonic seizure | Glucose—34 mg/dL, insulin—142.7 uIU/mL | MEN1 | MR | Head | 1.3 × 1.5 | Enucleation | - | Glycemic normalization |
| Miron et al., 2016 [37] | Romania | Case report | I | M | 11 | Diffuse abdominal pain, cold sweats, confusion, tremor, paresthesias | Glucose 14—38 mg/dL, insulin—12.6 μU/mL | - | US + MR | Tail | 1 | Enucleation | - | Glycemic normalization |
| Vasikasin et al., 2016 [38] | Thailand | Case report | I | M | 15 | Lightheadedness, diaphoresis, palpitations | Glucose—1.5 mmol/L, insulin—13.34 μU/mL | - | CT | Tail | 12.5 × 10 × 8.3 | Distal pancreatectomy | . | Glycemic normalization |
| Goudet et al., 2015 [39] | France | Original research | I | F | 5 | Confusion | - | MEN1 | - | - | 1.3 | Left pancreatectomy | - | No recurrence |
| Smith et al., 2015 [40] | USA | Case report | I | F | 14 | Unusual behaviors | Glucose—44 mg/dL, insulin—104 pmol/L | - | US + CT + MR + ASVS | Head/neck | 2.1 × 1.3 × 0.8 | Enucleation | NET G2 | Glycemic normalization |
| Abu-Zaid et al., 2014 [41] | Saudi Arabia | Case report | I | M | 10 | Tremulousness, diaphoresis, increased hunger, confusion, fainting | Glucose—64 mg/dL, insulin—6 μU/mL | - | CT | Body | 2.3 × 1.6 × 1.1 | Enucleation | NET G2 | Glycemic normalization |
| Padidela et al., 2014 [42] | United Kingdom | Original research | I | F | 3 | Neurological symptoms of hypoglycemia | Glucose—1.9 mmol/L, insulin—5.7 mIU/L | - | PET CT | Uncinate process | 1 | Enucleation | - | Glycemic normalization |
| M | 5 | glucose—2.2 mmol/L, insulin—4.5 mIU/L | MEN1 | MR | Head | 1 | Subtotal pancreatectomy | |||||||
| M | 8 | Glucose—1.5 mmol/L, insulin—5.8 mIU/L | MEN1 | PET CT + MR | Uncinate process + tail | 1.5 | Partial pancreatectomy | |||||||
| F | 8 | Glucose—1.1 mmol/L, insulin—66.3 mIU/L | - | MR | Head | 1.5 | Subtotal pancreatectomy | |||||||
| M | 8 | Glucose—0.8 mmol/L, insulin—85 mIU/L | - | PET CT + MR | Tail | 1.2 | Partial pancreatectomy | |||||||
| F | 11 | Glucose—1.6 mmol/L, insulin—9 mIU/L | - | MR | Head | 1.2 | Enucleation | |||||||
| F | 13 | Glucose—2 mmol/L, insulin—7 mIU/L | - | MR | Uncinate process | 0.8 | Enucleation + pancreaticojejunostomy | |||||||
| F | 13 | Glucose—2.1 mmol/L, insulin—44.6 mIU/L | - | PET CT + MR | Uncinate process | 2 | Enucleation + pancreaticojejunostomy | |||||||
| M | 15 | Glucose—1.9 mmol/L, insulin—14.4 mIU/L | - | MR | Head | 1.2 | Subtotal pancreatectomy | |||||||
| Gozzi Graf et al., 2014 [43] | Switzerland | Case report | I | M | 14 | Seizures | Glucose—2.9 mmol/L, insulin—47.9 pmol/L | MEN1 | MR | Tail | 2.5 | Laparoscopic distal pancreatectomy | - | No recurrence |
| M | 11 | Dizziness, disorientation, vomiting | Glucose—2.6 mmol/L, insulin—93.3 pmol/L | - | PET CT + MR | Head | 1.2 | Enucleation | - | No recurrence | ||||
| Peranteau et al., 2013 [44] | USA | Original research | I | F | 4 | - | - | - | CT + ASVS | Head | 0.7 | Enucleation | - | Glycemic normalization |
| M | 8 | - | EUS + PET CT + MR | Tail | 1.5 | Enucleation | ||||||||
| M | 9 | - | EUS + CT + MR | Head | 1.5 | Enucleation | ||||||||
| M | 11 | - | US + CT | Tail | 1.5 | Distal pancreatectomy | ||||||||
| F | 5 | - | PET CT | Tail | 1.2 | Distal pancreatectomy | ||||||||
| M | 14 | - | EUS + CT | Neck | 0.7 | Enucleation | ||||||||
| M | 11 | MEN1 | MR | Head, body, tail | 0.3 | Distal pancreatectomy + enucleation | ||||||||
| Horváth et al., 2013 [45] | Romania | Case report | I | M | 16 | Confusion, generalized tonic–clonic seizures | Glucose—25 mg/dL, insulin—23.8 μU/mL | - | CT | Tail | 1.6 | Enucleation | - | No recurrence |
| Bartsch et al., 2013 [46] | Germany | Original research | I | M | 12 | Hypoglycemia-related symptoms | I/G ratio > 0.3 | MEN1 | EUS or CT or MR | - | 1.7 | Enucleation | NET G1 | No recurrence, dead of unrelated cause |
| M | 9 | MEN1 | - | 1.3 | Subtotal distal pancreatectomy | NET G1 | No recurrence | |||||||
| F | 15 | MEN1 | - | 4 | Enucleation | NET G1 | No recurrence | |||||||
| F | 11 | MEN1 | - | 0.8 | Enucleation | - | No recurrence | |||||||
| Ahmed et al., 2013 [47] | Canada | Original research | I | F | - | Shakiness, seizures, hypoglycemia | - | - | US + MR | Tail | 2.2 | Resection | - | - |
| Toaiari et al., 2013 [48] | Italy | Original research | I | M | 17 | - | - | - | - | - | 1.3 | - | NET G1 | - |
| F | 15 | MEN1 | 2.5 | NET G2 | ||||||||||
| M | 17 | - | 3.3 | NET G1 | ||||||||||
| van den Akker et al., 2012 [49] | Belgium | Original research | I | F | 15 | - | - | - | - | - | 4 | Distal pancreatectomy | - | No recurrence |
| F | 13 | 2 | Distal pancreatectomy | No recurrence | ||||||||||
| M | 8 | 0.9 | Distal pancreatectomy | No recurrence | ||||||||||
| de Paiva et al., 2012 [50] | Brazil | Case report | I | M | 17 | Tonic–clonic generalized seizure | Glucose—17 mg/dL | MEN1 | MR | - | - | Partial pancreatectomy | - | No recurrence |
| Marchegiani et al., 2011 [51] | Italy | Original research | I | F | 15 | Asymptomatic (incidental diagnosis) | - | MEN1 | - | Head, body | 2.5 | Enucleation | NET G1 | No recurrence |
| Fabbri et al., 2010 [52] | Brazil | Case report | I | M | 8 | Sweating, palpitation, tremulousness, hunger, anxiety | Glucose—19 mg/dL, insulin—26.2 IU/mL | MEN1 | US + CT | Body | 0.6, 0.3 | Partial pancreatectomy | - | No recurrence |
| Janem et al., 2010 [53] | Jordan | Case report | I | M | 12 | Abdominal pain, weight loss, generalized weakness | Glucose—25 mg/dL, insulin—55.7 mIU/mL | - | CT | - | Liver, bone, bone marrow mts | Chemotherapy | - | Died |
| Ozen et al., 2009 [54] | Turkey | Case report | I | M | 16 | Generalized tonic–clonic seizure | Glucose—20 mg/dL, insulin—8.8 IU/L | - | EUS + CT | Tail | 0.8 | Resection | - | No recurrence |
| Concolino et al., 2008 [55] | Italy | Case report | I | F | 15 | Episodes of absences, behavioral disturbances, seizures | Glucose—576.6 mmol/L, insulin—4.5 pmol/L | MEN1 | MR | Head, body, tail | 2 × 1.8, 0.8, 0.85 | Enucleation | - | - |
| Bonfig et al., 2007 [56] | Germany | Case report | I | M | 15 | Hypoglycemic seizures | Glucose—41 mg/dL, insulin—15.6 μU/mL | - | EUS + PET CT + MR | Tail | 1 × 1.5 | Laparoscopic enucleation | - | Glycemic normalization |
| Blasetti et al., 2007 [57] | Italy | Case report | I | F | 17 | Refractory seizures | Glucose—1.9 mM/L, insulin—22.1 μΙΙ/mL | - | CT | Tail | 1.5 | Resection | - | No recurrence |
| Nakagawa et al., 2007 [58] | Japan | Case report | I | F | 9 | Generalized convulsion | Glucose—32 mg/dL, insulin—13 μU/mL | - | CT + MR + ASVS | Head | 4 × 3.5 × 3.3 | Enucleation | - | No recurrence |
| de Vogelaere et al., 2006 [59] | Belgium | Case report | I | M | 8 | Episodes of absences, headache, visual and auditive disturbances | Insulin > 10 μU/mL | MEN1 | US + CT + MR | Body | 1 × 1.5 | Laparoscopic enucleation | - | No recurrence |
| Karachaliou et al., 2006 [60] | Greece | Case report | I | F | 10 | Pallor, sweating, weakness, loss of consciousness, convulsions | Glucose—2.05 mmol/L, insulin—12.67 μU/mL | - | CT + MR | Tail | 2 | Distal pancreatectomy | - | Glycemic normalization |
| Zografos et al., 2005 [61] | Greece | Case report | I | F | 17 | Loss of consciousness | Glucose—1.6 to 3 mmol/L, insulin > 6 iu/L | - | EUS + CT + MR | Tail | 0.98 × 0.82 | enucleation | - | no recurrence |
| Kann et al., 2005 [62] | Germany | Case report | I | M | 15 | Severe hypoglycemia, multiple convulsions | - | - | EUS | Tail | 1.5 | Laparoscopic enucleation | - | No recurrence |
| Langer et al., 2005 [63] | Germany | Case report | I | M | 15 | Tonic–clonic convulsions, tremor, hunger | Glucose—28 mg/dL, insulin—15.9 µU/L | - | EUS + CT + MR | Tail | 0.8 × 1.3 | Laparoscopic enucleation | - | No recurrence |
| Ardengh et al., 2004 [64] | Brazil | Original research | I | M | 14 | - | - | - | EUS + CT | Tail | 2.2 | Distal pancreatectomy | - | - |
| Van Nieuwenhove et al., 2003 [65] | Belgium | Original research | I | M | 8 | Hypoglycemia | - | MEN1 | MR | Neck | >1 | Enucleation | - | No recurrence |
| Lo et al., 2003 [66] | Hong Kong | Case report | I | M | 13 | Fainting attacks, dizziness, sweatiness, decreased consciousness | Glucose—2 mmol/L, insulin—20 mIU/L | - | EUS + CT + MR | Tail | 2.3 | Laparoscopic distal pancreatectomy | - | No recurrence |
| Hussain et al., 2002 [67] | United Kingdom | Case report | I | F | 8 | Tonic–clonic seizures | Glucose—1.9 mmol/L, insulin—37.2 pmol/L | - | MR (-) | Head body transition | 1.5 | Subtotal pancreatectomy | - | No recurrence |
| Nollet et al., 2001 [68] | Belgium | Case report | I | M | 16 | Muscle fatigue and moderate paresthesia | Glucose—2.4 mmol/L, insulin—48 mU/L | - | US + CT + MR | Tail | 2.5 × 3 | Distal pancreatectomy | - | No recurrence |
| Chavan et al., 2000 [69] | Germany | Original research | I | M | 11 | - | - | MEN1 | US + CT + MR + ASVS | Tail | 1, 0.8 | - | - | No recurrence |
| Proye et al., 1998 [70] | France | Original research | I | F | 16 | - | - | - | EUS | Head | 1.4 | Enucleation | - | No recurrence |
| M | 12 | EUS | Distal pancreas | 1 | Enucleation | No recurrence | ||||||||
| Beccaria et al., 1997 [71] | Italy | Case report | I | M | 11 | Loss of consciousness, vertigo, amaurosis, ataxic gait | Glucose—1.7 mmol/L, insulin—24 mU/L; | - | US + MR | Body, tail | 2 × 1.4, 0.6, 2 × 1, 0.2 | Enucleation | - | Glycemic normalization |
| Waeber et al., 1997 [72] | Switzerland | Case report | I | M | 17 | Episodes of sudden absence, inappropriate response to verbal stimuli, somnolence | Glucose—1.5 mmol/L, insulin 51 mU/L | MEN1 | EUS + CT | Head | 1.5 | Enucleation | - | No recurrence |
| Maioli et al., 1992 [73] | Italy | Case report | I | M | 14 | Loss of consciousness, sweating | Glucose/insulin ratio < 3 | - | US + CT | Tail | 1.5 | Partial pancreatectomy—reoperation | - | No recurrence |
| F | 6 | Vertigo | - | - | - | Tail | 1 | Enucleation | - | No recurrence | ||||
| Winocour et al., 1992 [74] | United Kingdom | Case report | I | F | 15 | Reversible neurological disturbances | Glucose—1.5 mmol/L, insulin—245 pmol/L | MEN1 | CT | Head, body, tail | 1.5 × 1 × 0.5 | Sub-total pancreatectomy, pancreaticoduodenectomy (reoperation) | - | Residual insulinoma, death |
| Telander et al., 1986 [75] | USA | Original research | I | M | 15 | - | - | MEN1 | US | Head, body | 1.5, 0.5, 0.5, 0.2 | 85% pancreatectomy + enucleation | - | - |
| F | 13 | MEN1 | US | Tail | 0.2–0.9 | 85% pancreatectomy | ||||||||
| M | 14 | - | US + CT | Tail | 2.5 | Distal pancreatectomy | ||||||||
| M | 16 | - | US + CT | Head | 1.6 | 85% pancreatectomy + enucleation | ||||||||
| M | 12 | - | US | Head | 1.5 | 85% pancreatectomy + enucleation | ||||||||
| Rasbach et al., 1985 [76] | USA | Original research | I | - | 10 | Dizziness, weakness, blurred vision, confusion, headaches, seizures | - | MEN1 | Angiography | - | 0.4–3 | Enucleation | - | Recurrent hyperinsulinism—reoperation |
| 10 | MEN1 | Angiography | 2.7 | Resection (80%) | Persistent hyperinsulinism—reoperation | |||||||||
| 17 | MEN1 | Angiography | 0.3–2.3 | Resection (85%) | No recurrence | |||||||||
| 14 | MEN1 | US | 0.2–2.4 | Resection (85%) | No recurrence | |||||||||
| 12 | MEN1 | US | 0.2–0.9 | Resection (85%) | Persistent hyperinsulinism | |||||||||
| Stringel et al., 1985 [77] | USA | Case report | I | M | 12 | Seizures, pallor, clammy skin, confusion, tachycardia | Glucose—2 mmol/L | - | US + CT + angiography | Body | - | Distal pancreatectomy | - | No recurrence |
| Gough, 1984 [78] | United Kingdom | Case report | I | M | 30 h | Jittery, convulsion | - | - | - | Neck | 0.5 | Enucleation | - | No recurrence |
| F | 9 | Fainting attacks with convulsions | Angiography | Tail | 1 | Distal pancreatectomy | No recurrence | |||||||
| MacDonald et al., 1983 [79] | USA | Case report | I | F | 2 months | Listlessness, tremulousness, eye rolling, weak suck | Glucose < 30 mg/dL, insulin—29 µU/mL | - | - | - | - | Pancreatectomy (50%) | - | No recurrence |
| F | 2 months | Generalized seizures | Glucose—30 mg/dL, insulin—49 µU/mL | Head | 1 | Pancreatectomy (65%) | No recurrence | |||||||
| Bordi et al., 1982 [80] | Italy | Case report | I | M | 3 days | Convulsions | Glucose—0.3 mmol/L, insulin >12 µU/mL | - | - | Body | 0.4 | Pancreatectomy (80%) | - | No recurrence |
| Glickman et al., 1980 [81] | USA | Original research | I | M | 9 | Convulsions | Glucose—5 mg/100 mL | - | - | - | 1 | Enucleation | - | Recurrent 10 cm adenoma. died after resection |
| M | 16 | Seizure | Glucose—40 mg/100 mL | Angiography | Head, neck | 2 | Enucleation | No recurrence | ||||||
| M | 7 | Fainting, seizure | Glucose—35 mg/100 mL | - | Body | 1.5 | Enucleation | No recurrence | ||||||
| F | 5 days | Seizure | Glucose—4 mg/100 mL | - | Head | 0.3 | Pancreatectomy (85%) | No recurrence | ||||||
| Ginsberg-Fellner et al., 1980 [82] | USA | Case report | I | M | 8 | Recurring seizures | Glucose—25 mg/dL, insulin—96 µU/mL | - | Angiography | Body | 1.5 | Enucleation | - | No recurrence |
| Cameron et al., 1972 [83] | Canada | Case report | I | M | 6 | Unconsciousness, irritable | Glucose—56 mg/100 mL | - | Angiography | Body tail transition | 2 × 1.5 | Enucleation | - | No recurrence |
| Heitz et al., 1971 [84] | Switzerland | Case report | I | M | 12 | Hypoglycemic attacks | Glucose—27 mg/100 mL, insulin—150 μU/mL | - | Angiography + US | Head | 2 × 1.5 × 1 | Enucleation | - | No recurrence |
| Study | Country | Type of Study | Type of Tumor | Patient’s Gender | Patient’s Age (Years) | Clinical Presentation | Laboratory Findings | Genetic Analysis | Radiological Modality | Tumor Location | Tumor Size (cm) | Type of Treatment | Histopathology | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nath et al., 2017 [8] | India | Case report | G | M | 12 | Epigastric pain, vomiting, occasional loose stools, peptic ulcer | Gastrin—940 pg/mL | - | US + CT | Head | 3.8 × 2.8 | Excised in toto | - | No recurrence |
| Goyal et al., 2016 [85] | India | Case report | G | M | 12 | Abdominal pain, diarrhea, vomiting, weight loss, peptic ulcer | Gastrin > 8000 pg/mL | - | US + CT | Head | 3.3 × 2.3 + liver mts | - | - | Oncology follow-up |
| Murase et al., 2015 [86] | Japan | Case report | G | M | 9 | Vomiting, peptic ulcer | Gastrin—834 pg/mL | - | US + CT + MR | Head | - | Laparoscopic-assisted Pancreaticoduodenectomy | - | No recurrence |
| Goudet et al., 2015 [39] | France | Original research | G | F | 6 | Diarrhea, esophagitis, multiple ulcerations of duodenum and antral stomach | Gastrin—3000 pg/mL | MEN1 | US | - | 0.2 | - | - | Reoperated on at 14, 18, and 19 yr old for local recurrence |
| M | 16 | Abdominal pain, intermittent vomiting episodes, esophagitis, multiple duodenal ulcers | Gastrin—870 pg/mL | MEN1 | - | - | - | - | - | |||||
| F | 17 | Abdominal pain, heart burn, diarrhea, esophagitis, multiple gastrointestinal ulcers | Gastrin—610 pg/mL | MEN1 | - | Head + tail | - | Enucleation + left pancreatectomy- | - | |||||
| Massaro et al., 2014 [87] | USA | Case report | G | F | 11 | Intermittent abdominal pain, vomiting, diarrhea, duodenal peptic ulcer | Gastrin > 100,000 pg/mL | - | US + PET CT + MR | Tail + liver mts | - | Distal pancreatectomy + liver transplant | - | Asymptomatic after transplant and chemotherapy |
| Dall’igna et al., 2010 [88] | Italy | Original research | G | F | 14 | Zollinger–Ellison syndrome | - | - | - | Head + liver mts | 2 | Biopsy + octreotide | - | Alive with stable disease at 16 months |
| Schettini et al., 2009 [89] | Brazil | Case report | G | M | 11 | Epigastric pain, retching, vomiting, diarrhea, gastric ulcer | Gastrin—1000 pg/mL | - | CT | Head + liver mts | 1.5 | Enucleation | - | No recurrence |
| Gurevich et al., 2003 [90] | Russia | Original research | G | M | 15 | Zollinger–Ellison syndrome | Gastrin—260 pg/mL | - | - | Head + liver mts | 4.5 | - | - | - |
| Wamsteker et al., 2003 [91] | USA | Original research | G | F | 15 | Asymptomatic | Gastrin—169 pg/mL | MEN1 | EUS | Head, uncinate process, body, tail | 0.5–0.7 | - | - | EUS surveillance |
| Eire et al., 1996 [92] | Spain | Case report | G | F | 14 | Suffered stomach, diarrhea | Gastrin—1500 pg/mL | - | US | Head | - | Partial gastrectomy and subtotal pancreaticoduodenectomy | - | No recurrence |
| Study | Country | Type of Study | Type of Tumor | Patient’s Gender | Patient’s Age (Years) | Clinical Presentation | Laboratory Findings | Genetic Analysis | Radiological Modality | Tumor Location | Tumor Size (cm) | Type of Treatment | Histopathology | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Luber et al., 2016 [9] | USA | Case report | GL | F | 15 | Persistent, pruritic, and painful rash; hair loss; brittle nails; diarrhea; poor weight gain | Glucagon—3076 pg/mL | - | CT | Uncinate process | 3.4 × 4.3 × 2.9 | Whipple procedure | NET G1 | No recurrence |
| van den Akker et al., 2012 [49] | Belgium | Original research | GL | M | 12 | - | - | - | - | - | 4 | Distal pancreatectomy | - | No recurrence |
| van Beek et al., 2004 [93] | Netherlands | Case report | GL | M | 16 | No complaints | Glucagon—145 pmol/L | MEN1 | Angiography + MR | Body | 2 | Subtotal pancreatectomy | - | No recurrence |
| Study | Country | Type of Study | Type of Tumor | Patient’s Gender | Patient’s Age (Years) | Clinical Presentation | Laboratory Findings | Genetic Analysis | Radiological Modality | Tumor Location | Tumor Size (cm) | Type of Treatment | Histopathology | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bonilla Gonzalez et al., 2021 [94] | Colombia | Case report | V | F | 14 | Diarrhea, abdominal pain, vomiting, hypoxia, arthralgia, myalgia | VIP—91.2 pmol/L | - | Scintigraphy + MR | Body | 2 × 1.6, 1 × 0.9 | Distal pancreatectomy | NET G2 | No recurrence |
| Yeh et al., 2020 [11] | Taiwan | Case report | V | F | 7 | Blood-tinged and mucoid diarrhea, poor appetite, general weakness | VIP—743.82 pg/mL | - | CT + MRCP | Body | - | Partial pancreatectomy | - | Therapy: everolimus + octreotide |
| Acosta-Gualandri et al., 2019 [95] | Canada | Case report | V | F | 13 | Profuse watery diarrhea, nausea, vomiting, dehydration, abdominal pain | VIP—1105 pg/mL | MEN1 | CT + MR | Tail | 4.5 | Distal pancreatectomy | NET G1 | Withdrawal of symptoms |
| Bourcier et al., 2013 [96] | USA | Case report | V | M | 12 | Dehydration, diarrhea, fainting, flushing | VIP—134.5 pg/mL | - | CT + MR | Tail | 1.5 × 1.0 × 0.8 + liver mts | Distal pancreatectomy | - | Therapy |
| Masulovic et al., 2012 [97] | Serbia | Case report | V | M | 15 | Chronic diarrhea, abdominal pain, nausea, vomiting | - | MEN1 | CT + MR | Head, body | 3.8, 1.2 | Whipple procedure + enucleation | - | No recurrence |
| Brenner et al., 1986 [98] | USA | Case report | V | F | 15 | Diarrhea, vomiting | VIP—2150 pg/mL | - | ERCP | Body, tail | 6 × 5 | Distal pancreatectomy (85%) | - | No recurrence |
| Study | Country | Type of Study | Type of Tumor | Patient’s Gender | Patient’s Age (Years) | Clinical Presentation | Laboratory Findings | Genetic Analysis | Radiological Modality | Tumor Location | Tumor Size (cm) | Type of Treatment | Histopathology | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Petriczko et al., 2022 [99] | Poland | Case report | I/GL | M | 17 | Hypoglycemia, epileptic seizures | Glucose—1.94 mmol/L, insulin—5.13 uIU/mL | MEN1 | US + EUS + CT | Head + body + tail | 0.7, 1.1, 1.2 | Surgical resection + reoperation | NET G1/G2 | Glycemic normalization |
| Erichsen et al., 2020 [100] | Denmark | Case report | I/GL | F | 14 | Unrecognized hypoglycemia symptoms | Glucose—2.5 mmol/L, p-insulin 158 pmol/L, glucagon—1000 pmol/L | MEN1 | EUS + PET CT + MR | Head + tail + uncinate process | 1 | Surgical resection x4 | - | - |
| Winston et al., 2014 [101] | Canada | Case report | I/GL | M | 14 | Abnormal behavior | Glucose—2.1 mmol/L, insulin—82.9 pmol/L | MEN1 | US + MR | Body + tail | 2.9 × 3.2 × 2.4, 2.5 × 1.5 × 2 | Sub-total pancreatectomy | - | Glycemic normalization |
3.2.1. Insulinomas
3.2.2. Gastrinomas
3.2.3. Glucagonomas
3.2.4. VIPomas
3.2.5. Mixed Functional pNETs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| pNETs | Pancreatic neuroendocrine tumors |
| MEN1 | Multiple endocrine neoplasia type 1 |
| VHL | Von Hippel–Lindau disease |
| NF1 | Neurofibromatosis type 1 |
| TSC | Tuberous sclerosis complex |
| ZES | Zollinger–Ellison syndrome |
| NME | Necrolytic migratory erythema |
| VIP | Vasoactive intestinal peptide |
| US | Ultrasound |
| EUS | Endoscopic ultrasound |
| MR | Magnetic resonance |
| CT | Computed tomography |
| ASVS | Arterial calcium stimulation with venous sampling |
| PET | Positron emission tomography |
| NET | Neuroendocrine tumor |
| G | Gradus |
| hPTH | Hyperparathyroidism |
| hPRL | Hyperprolactinemia |
| DM | Diabetes mellitus |
| Mts | Metastases |
| MRCP | Magnetic resonance cholangiopancreatography |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| PPIs | Proton pump inhibitors |
References
- Young, K.; Iyer, R.; Morganstein, D.; Chau, I.; Cunningham, D.; Starling, N. Pancreatic neuroendocrine tumors: A review. Future Oncol. 2015, 11, 853–864. [Google Scholar] [CrossRef]
- Karges, K.; Kunstreich, M.; Pape, U.F.; Fuchs, J.; Vokuhl, C.; Abele, M.; Schneider, D.T.; Brecht, I.B.; Lapa, C.; Frühwald, M.C.; et al. Pancreatic neuroendocrine tumors in children and adolescents-Data from the German MET studies (1997–2023). J. Neuroendocrinol. 2025, 37, e70039. [Google Scholar] [CrossRef]
- Alshareefy, Y.; Cummins, S.; Mazzoleni, A.; Sharma, V.; Guggilapu, S.; Leong, A.W.Y.; Wireko, A.A. A review of functional pancreatic neuroendocrine tumors: Exploring the molecular pathogenesis, diagnosis and treatment. Medicine 2023, 102, e36094. [Google Scholar] [CrossRef]
- Geurts, J.L. Inherited syndromes involving pancreatic neuroendocrine tumors. J. Gastrointest. Oncol. 2020, 11, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Nasher, O.; Hall, N.J.; Sebire, N.J.; de Coppi, P.; Pierro, A. Pancreatic tumours in children: Diagnosis, treatment and outcome. Pediatr. Surg. Int. 2015, 31, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Melikyan, M.; Gubaeva, D.; Shadrina, A.; Bolmasova, A.; Kareva, M.; Tiulpakov, A.; Efremenkov, A.; Sokolov, Y.; Brusgaard, K.; Christesen, H.T.; et al. Insulinoma in childhood: A retrospective review of 22 patients from one referral centre. Front. Endocrinol. 2023, 14, 1127173. [Google Scholar] [CrossRef] [PubMed]
- Giannis, D.; Moris, D.; Karachaliou, G.S.; Tsilimigras, D.I.; Karaolanis, G.; Papalampros, A.; Felekouras, E. Insulinomas: From diagnosis to treatment. A review of the literature. J. Balk. Union Oncol. 2020, 25, 1302–1314. [Google Scholar]
- Nath, A.L.; Saxena, N.A.; Kulkarni, B.K.; Borwankar, S.S.; Lahoti, H.N.; Oak, S.N. Zollinger-Ellison Syndrome in a 12-year-old Child. J. Indian Assoc. Pediatr. Surg. 2017, 22, 168–169. [Google Scholar]
- Luber, A.J.; Ackerman, L.S.; Culpepper, K.S.; Buschmann, C.M.; Koep, L.J. Paediatric necrolytic migratory erythema as a presenting sign of glucagonoma syndrome. Br. J. Dermatol. 2016, 174, 1092–1095. [Google Scholar] [CrossRef]
- Karele, E.N. All you need to know about VIPoma: Review on the latest studies. Presse Med. 2024, 53, 104222. [Google Scholar] [CrossRef]
- Yeh, P.J.; Chen, S.H.; Lai, J.Y.; Lai, M.W.; Chiu, C.H.; Chao, H.C.; Chen, S.H.; Wu, R.C.; Wang, C.J.; Chen, C.C. Rare Cases of Pediatric Vasoactive Intestinal Peptide Secreting Tumor With Literature Review: A Challenging Etiology of Chronic Diarrhea. Front. Pediatr. 2020, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Nesi, G.; Marcucci, T.; Rubio, C.A.; Brandi, M.L.; Tonelli, F. Somatostatinoma: Clinico-pathological features of three cases and literature reviewed. J. Gastroenterol. Hepatol. 2008, 23, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Sales, M.T.A.; Branco, R.C.C.; Granjeiro, C.H.P.; Sousa, M.S.; Aragão, L.F.F.; Carvalho, A.B.; Montenegro, A.P.D.R.; Quidute, A.R.P. Multiple endocrine neoplasia type 1 in childhood and description of a novel variant. Rev. Paul. Pediatr. 2025, 43, e2024175. [Google Scholar] [CrossRef]
- Murray, A.; Rodas Marquez, S.P.; Krishnamurthy, M.; Lopez-Nunez, O.; Gurria, J.P.; Trout, A.T.; Almazan, S.; Mutyala, K.; Grisotti, G.; Shah, A.; et al. Multifocal Insulinoma as the Unique Presenting Feature of Multiple Endocrine Neoplasia Type 1 in an Adolescent. Horm. Res. Paediatr. 2025, 98, 75–83. [Google Scholar] [CrossRef]
- Lemus-Zepeda, R.; Salazar-Solarte, A.M.; Vasquez-Forero, D.M.; Angulo-Mosquera, M., Jr.; Mejía-Zapata, L. Insulinoma Associated with MEN1 Syndrome: A Case of Persistent Hypoglycemia in a School-aged Child. J. Clin. Res. Pediatr. Endocrinol. 2025, 17, 226–230. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Zhang, K.; Tang, Y.; Zhang, M.; Fan, Z.; Wang, T.; Liu, Y. Case report: Novel germline c.587delA pathogenic variant in familial multiple endocrine neoplasia type 1. Front. Endocrinol. 2024, 15, 1467882. [Google Scholar] [CrossRef]
- Kamińska-Jackowiak, O.; Kordek, J.; Stefanowicz, M.; Chobot, A. Neuroglycopaenia as the first manifestation of MEN1 syndrome. Pediatr. Med. Rodz. 2024, 20, 238–241. [Google Scholar] [CrossRef]
- Moszczyńska, E.; Wydra, A.; Zasada, K.; Baszyńska-Wilk, M.; Majak, D.; Śliwińska, A.; Grajkowska, W. Long-term Survival in a Child with Malignant Insulinoma After Liver Transplantation. J. Clin. Res. Pediatr. Endocrinol. 2024, 16, 106–110. [Google Scholar] [CrossRef]
- Tian, F.; Zhao, J.; Ding, D.; Feng, J.; Ma, Y. Clinicopathological Features of Pediatric Insulinoma: A Single-Centre Study. Br. J. Hosp. Med. 2024, 85, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vinhosa Bastos, M.A., Jr.; da Silva Caires, I.; Boschi Portella, R.; Nascimento Martins, R.; Reverdito, R.; Reverdito, S.; Moro, N., Jr. Insulinoma with peripheral neuropathy: A case report. J. Med. Case Rep. 2023, 17, 241. [Google Scholar] [CrossRef] [PubMed]
- Shariq, O.A.; Lines, K.E.; English, K.A.; Jafar-Mohammadi, B.; Prentice, P.; Casey, R.; Challis, B.G.; Selberherr, A.; Boon, H.; Cranston, T.; et al. Multiple endocrine neoplasia type 1 in children and adolescents: Clinical features and treatment outcomes. Surgery 2022, 171, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Sherafati, H.; Joodi, M.; Fathi, M.; Aref Emami, M.; Ameri, L.; Bahrami Taqanaki, P.; Mehdi Zarif Soltani, M.; Ghodsi, A.; Parvizi Mashhadi, M. Insulinoma in a teenager with seizures. J. Pediatr. Surg. Case Rep. 2022, 79, 102231. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Sun, Y.; Wang, Y.; Tian, Y.; Ma, Q.; Fu, Y. Case Report: Insulinoma Presenting as Excessive Daytime Somnolence. Front. Endocrinol. 2021, 12, 712392. [Google Scholar] [CrossRef]
- Schulte Am Esch, J.; Krüger, M.; Barthlen, W.; Förster, C.; Mohnike, K.; Empting, S.; Benhidjeb, T.; Vossschulte, H. Technical aspects of paediatric robotic pancreatic enucleation based on a case of an insulinoma. Int. J. Med. Robot. 2021, 17, e2317. [Google Scholar] [CrossRef]
- Mahdi, M.; Almehman, B.; Nassan, S.; Binyahib, S. Pancreatic insulinoma causing hypoglycemic episodes. J. Pediatr. Surg. Case Rep. 2020, 57, 101466. [Google Scholar] [CrossRef]
- Al Azmi, F.; Al Shaikh, A. Persistent Hypoglycemia in Seven-year-old Saudi Child: A Case Report. Oman Med. J. 2020, 35, e154. [Google Scholar] [CrossRef]
- Selberherr, A.; Koperek, O.; Riss, P.; Scheuba, C.; Niederle, M.B.; Kaderli, R.; Perren, A.; Niederle, B. Intertumor heterogeneity in 60 pancreatic neuroendocrine tumors associated with multiple endocrine neoplasia type 1. Orphanet J. Rare Dis. 2019, 14, 54. [Google Scholar] [CrossRef]
- Escartín, R.; Brun, N.; García Monforte, M.N.; Ferreres, J.C.; Corripio, R. Insulinoma: A Rare Cause of Hypoglycemia in Childhood. Am. J. Case Rep. 2018, 19, 1121–1125. [Google Scholar] [CrossRef]
- Liang, M.; Jiang, J.; Dai, H.; Hong, X.; Han, X.; Cong, L.; Tong, A.; Li, F.; Luo, Y.; Liu, W.; et al. Robotic enucleation for pediatric insulinoma with MEN1 syndrome: A case report and literature review. BMC Surg. 2018, 18, 44. [Google Scholar] [CrossRef]
- Nakano, S.; Sato, T.; Hosokawa, M.; Takagi, C.; Yoshida, F.; Ishii, T.; Sato, S.; Hasegawa, T. A pediatric case of insulinoma and a novel MEN1 mutation: The efficacy of the combination therapy of diazoxide and cornstarch. Clin. Pediatr. Endocrinol. 2018, 27, 197–199. [Google Scholar] [CrossRef]
- Hu, M.G.; Xiao, Y.H.; Song, D.D.; Zhao, G.D.; Liu, Y.Z.; Wang, Z.; Li, H.Y.; Liu, R. First experience of robotic spleen-preserving distal pancreatectomy in a child with insulinoma. World J. Surg. Oncol. 2017, 15, 199. [Google Scholar] [CrossRef]
- Beisang, D.; Forlenza, G.P.; Luquette, M.; Sarafoglou, K. Sporadic Insulinoma Presenting as Early Morning Night Terrors. Pediatrics 2017, 139, e20162007. [Google Scholar] [CrossRef]
- Yao, L.; Xie, Z.B.; Jin, C.; Jiang, Y.J.; Li, J.; Yang, F.; Lin, Q.J.; Fu, D.L. Radical resection and enucleation in Chinese adolescents with pancreatic tumors: A 15-year case series. Medicine 2017, 96, e6438. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.B.; Jeong, H.R.; Shim, Y.S.; Lee, H.S.; Hwang, J.S. Multiple Endocrine Neoplasia Type 1 Presenting as Hypoglycemia due to Insulinoma. J. Korean Med. Sci. 2016, 31, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Miron, I.; Diaconescu, S.; Aprodu, G.; Ioniuc, I.; Diaconescu, M.R.; Miron, L. Diagnostic Difficulties in a Pediatric Insulinoma: A Case Report. Medicine 2016, 95, e3045. [Google Scholar] [CrossRef] [PubMed]
- Vasikasin, V.; Watthanatham, J.; Napatharatip, P.; Termmathurapoj, S. Giant insulinoma in a 15-year-old man: A case report. Int. J. Surg. Case Rep. 2016, 24, 135–138. [Google Scholar] [CrossRef][Green Version]
- Goudet, P.; Dalac, A.; Le Bras, M.; Cardot-Bauters, C.; Niccoli, P.; Lévy-Bohbot, N.; du Boullay, H.; Bertagna, X.; Ruszniewski, P.; Borson-Chazot, F.; et al. MEN1 disease occurring before 21 years old: A 160-patient cohort study from the Groupe d’étude des Tumeurs Endocrines. J. Clin. Endocrinol. Metab. 2015, 100, 1568–1577. [Google Scholar] [CrossRef]
- Smith, A.; Thornton, P.S.; Galliani, C.A.; Miller, J.P.; Dykes, J.; Leung-Pineda, V. A 14-year old girl with reversible hypoglycemic episodes: The role of ASVS. Pediatr. Dev. Pathol. 2015, 18, 80–83. [Google Scholar] [CrossRef]
- Abu-Zaid, A.; Alghuneim, L.A.; Metawee, M.T.; Elkabbani, R.O.; Almana, H.; Amin, T.; Azzam, A. Sporadic insulinoma in a 10-year-old boy: A case report and literature review. JOP 2014, 15, 53–57. [Google Scholar] [PubMed]
- Padidela, R.; Fiest, M.; Arya, V.; Smith, V.V.; Ashworth, M.; Rampling, D.; Newbould, M.; Batra, G.; James, J.; Wright, N.B.; et al. Insulinoma in childhood: Clinical, radiological, molecular and histological aspects of nine patients. Eur. J. Endocrinol. 2014, 170, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Gozzi Graf, T.; Brändle, M.; Clerici, T.; l’Allemand, D. Insulinoma: Only in adults?-case reports and literature review. Eur. J. Pediatr. 2014, 173, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Peranteau, W.H.; Palladino, A.A.; Bhatti, T.R.; Becker, S.A.; States, L.J.; Stanley, C.A.; Adzick, N.S. The surgical management of insulinomas in children. J. Pediatr. Surg. 2013, 48, 2517–2524. [Google Scholar] [CrossRef]
- Horváth, E.; Gozar, H.; Chira, L.; Dunca, I.; Kiss, E.; Pávai, Z. Insulinoma diagnosed as drug-refractory epilepsy in an adolescent boy: A case report. Rom. J. Morphol. Embryol. 2013, 54, 1147–1151. [Google Scholar]
- Bartsch, D.K.; Albers, M.; Knoop, R.; Kann, P.H.; Fendrich, V.; Waldmann, J. Enucleation and limited pancreatic resection provide long-term cure for insulinoma in multiple endocrine neoplasia type 1. Neuroendocrinology 2013, 98, 290–298. [Google Scholar] [CrossRef]
- Ahmed, T.S.; Chavhan, G.B.; Navarro, O.M.; Traubici, J. Imaging features of pancreatic tumors in children: 13-year experience at a pediatric tertiary hospital. Pediatr. Radiol. 2013, 43, 1435–1443. [Google Scholar] [CrossRef]
- Toaiari, M.; Davì, M.V.; Dalle Carbonare, L.; Boninsegna, L.; Castellani, C.; Falconi, M.; Francia, G. Presentation, diagnostic features and glucose handling in a monocentric series of insulinomas. J. Endocrinol. Investig. 2013, 36, 753–758. [Google Scholar]
- van den Akker, M.; Angelini, P.; Taylor, G.; Chami, R.; Gerstle, J.T.; Gupta, A. Malignant pancreatic tumors in children: A single-institution series. J. Pediatr. Surg. 2012, 47, 681–687. [Google Scholar] [CrossRef]
- de Paiva, A.R.; Castro, L.H.; Rodrigues, W., Jr.; Passarelli, V.; Jorge, C.L.; Brotto, M.W.; Hirata, M.T.; Marchiori, P.E. Multiple endocrine neoplasia type 1 presenting as refractory epilepsy and polyneuropathy—A case report. J. Neurol. Sci. 2012, 315, 172–175. [Google Scholar] [CrossRef]
- Marchegiani, G.; Crippa, S.; Malleo, G.; Partelli, S.; Capelli, P.; Pederzoli, P.; Falconi, M. Surgical treatment of pancreatic tumors in childhood and adolescence: Uncommon neoplasms with favorable outcome. Pancreatology 2011, 11, 383–389. [Google Scholar] [CrossRef]
- Fabbri, H.C.; Mello, M.P.; Soardi, F.C.; Esquiaveto-Aun, A.M.; Oliveira, D.M.; Denardi, F.C.; Moura-Neto, A.; Garmes, H.M.; Baptista, M.T.; Matos, P.S.; et al. Long-term follow-up of an 8-year-old boy with insulinoma as the first manifestation of a familial form of multiple endocrine neoplasia type 1. Arq. Bras. Endocrinol. Metabol. 2010, 54, 754–760. [Google Scholar] [CrossRef]
- Janem, W.; Sultan, I.; Ajlouni, F.; Deebajeh, R.; Haddad, H.; Sughayer, M.A.; Goussous, R.Y. Malignant insulinoma in a child. Pediatr. Blood Cancer 2010, 55, 1423–1426. [Google Scholar] [CrossRef]
- Ozen, S.; Saz, E.U.; Celik, A.; Aydin, A.; Simsek, D.G.; Darcan, S. Many admissions to the emergency departments with recurrent syncope attacks and seizures in an adolescent boy. Eur. J. Pediatr. 2009, 168, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Concolino, P.; Rossodivita, A.; Carrozza, C.; Raffaelli, M.; Lombardi, C.P.; Rigante, D.; Pitocco, D.; Stabile, A.; Bellantone, R.; Zuppi, C.; et al. A novel MEN1 frameshift germline mutation in two Italian monozygotic twins. Clin. Chem. Lab. Med. 2008, 46, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Bonfig, W.; Kann, P.; Rothmund, M.; Schwarz, H.P. Recurrent hypoglycemic seizures and obesity: Delayed diagnosis of an insulinoma in a 15 year-old boy—Final diagnostic localization with endosonography. J. Pediatr. Endocrinol. Metab. 2007, 20, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Blasetti, A.; Di Pietro, L.; di Corcia, G.; Franzoni, E.; Chiarelli, F.; Verrotti, A. Can insulinoma cause generalised epilepsy? J. Pediatr. Endocrinol. Metab. 2007, 20, 837–840. [Google Scholar] [CrossRef]
- Nakagawa, A.; Ueno, K.; Ito, M.; Okamoto, S.; Uehara, K.; Ito, H.; Mishina, S.; Kinoshita, E.; Nojima, T.; Takahashi, H.; et al. Insulin responses to selective arterial calcium infusion under hyperinsulinemic euglycemic glucose clamps: Case studies in adult nesidioblastosis and childhood insulinoma. Endocr. J. 2007, 54, 27–33. [Google Scholar] [CrossRef][Green Version]
- de Vogelaere, K.; De Schepper, J.; Vanhoeij, M.; De Mey, J.; Goossens, A.; Vanbesien, J.; De Backer, A.; Delvaux, G. Laparoscopic management of insulinoma in a child with multiple endocrine neoplasia type 1. J. Laparoendosc. Adv. Surg. Tech. A 2006, 16, 335–338. [Google Scholar] [CrossRef]
- Karachaliou, F.; Vlachopapadopoulou, E.; Kaldrymidis, P.; Simatos, G.; Zacharea, M.; Spanidou-Karvouni, E.; Michalacos, S.; Voros, D. Malignant insulinoma in childhood. J. Pediatr. Endocrinol. Metab. 2006, 19, 757–760. [Google Scholar] [CrossRef]
- Zografos, G.N.; Stathopoulou, A.; Mitropapas, G.; Karoubalis, J.; Kontogeorgos, G.; Kaltsas, G.; Piaditis, G.; Papastratis, G.I. Preoperative imaging and localization of small sized insulinoma with EUS-guided fine needle tattoing: A case report. Hormones 2005, 4, 111–116. [Google Scholar]
- Kann, P.H.; Rothmund, M.; Zielke, A. Endoscopic ultrasound imaging of insulinomas: Limitations and clinical relevance. Exp. Clin. Endocrinol. Diabetes 2005, 113, 471–474. [Google Scholar] [CrossRef]
- Langer, P.; Bartsch, D.K.; Fendrich, V.; Kann, P.H.; Rothmund, M.; Zielke, A. Minimal-invasive operative treatment of organic hyperinsulinism. Dtsch. Med. Wochenschr. 2005, 130, 514–518. [Google Scholar] [CrossRef]
- Ardengh, J.C.; de Paulo, G.A.; Ferrari, A.P. EUS-guided FNA in the diagnosis of pancreatic neuroendocrine tumors before surgery. Gastrointest. Endosc. 2004, 60, 378–384. [Google Scholar] [CrossRef]
- Van Nieuwenhove, Y.; Vandaele, S.; Op de Beeck, B.; Delvaux, G. Neuroendocrine tumors of the pancreas. Surg. Endosc. 2003, 17, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.Y.; Tam, P.K. Laparoscopic pancreatic resection of an insulinoma in a child. Asian J. Surg. 2003, 26, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Cosgrove, K.E.; Shepherd, R.M.; Chapman, J.C.; Swift, S.M.; Smith, V.V.; Kassem, S.A.; Glaser, B.; Lindley, K.J.; Aynsley-Green, A.; et al. Uncontrolled insulin secretion from a childhood pancreatic beta-cell adenoma is not due to the functional loss of ATP-sensitive potassium channels. Endocr. Relat. Cancer 2002, 9, 221–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nollet, A.; Bruyninckx, F.; Van Den Bruel, A.; Muls, E.; Bouillon, R.; Mathieu, C. Muscle fibre membrane instability in a young boy with an insulinoma. Clin. Endocrinol. 2001, 55, 559–561. [Google Scholar] [CrossRef]
- Chavan, A.; Kirchhoff, T.D.; Brabant, G.; Scheumann, G.F.; Wagner, S.; Galanski, M. Role of the intra-arterial calcium stimulation test in the preoperative localization of insulinomas. Eur. Radiol. 2000, 10, 1582–1586. [Google Scholar] [CrossRef]
- Proye, C.; Malvaux, P.; Pattou, F.; Filoche, B.; Godchaux, J.M.; Maunoury, V.; Palazzo, L.; Huglo, D.; Lefebvre, J.; Paris, J.C. Noninvasive imaging of insulinomas and gastrinomas with endoscopic ultrasonography and somatostatin receptor scintigraphy. Surgery 1998, 124, 1134–1143; discussion 1143–1144. [Google Scholar] [CrossRef]
- Beccaria, L.; Bosio, L.; Burgio, G.; Paesano, P.L.; Del Maschio, A.; Chiumello, G. Multiple insulinomas of the pancreas: A patient report. J. Pediatr. Endocrinol. Metab. 1997, 10, 309–314. [Google Scholar] [CrossRef]
- Waeber, G.; Gomez, F.; Bishof-Delaloye, A.; Chaubert, P.; Francke, M.L.; Haefliger, J.A.; Scherrer, U.; Centeno, G.; Temler, E.; Nicod, P. Insulinoma associated with a case of multiple endocrine neoplasia type I: Functional somatostatin receptors and abnormal glucose-induced insulin secretion. Horm. Res. 1997, 48, 76–82. [Google Scholar] [CrossRef]
- Maioli, M.; Ciccarese, M.; Pacifico, A.; Tonolo, G.; Ganau, A.; Cossu, S.; Tanda, F.; Realdi, G. Familial insulinoma: Description of two cases. Acta Diabetol. 1992, 29, 38–40. [Google Scholar] [CrossRef]
- Winocour, P.H.; Moriarty, K.J.; Hales, C.N.; Adams, J.; Reeve, R.; Wynick, D.; Allison, D.; Bloom, S.R.; Anderson, D.C. Difficulties in localization and treatment of insulinomas in type 1 multiple endocrine adenomatosis (MEA). Postgrad. Med. J. 1992, 68, 196–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Telander, R.L.; Charboneau, J.W.; Haymond, M.W. Intraoperative ultrasonography of the pancreas in children. J. Pediatr. Surg. 1986, 21, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Rasbach, D.A.; van Heerden, J.A.; Telander, R.L.; Grant, C.S.; Carney, J.A. Surgical management of hyperinsulinism in the multiple endocrine neoplasia, type 1 syndrome. Arch. Surg. 1985, 120, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Stringel, G.; Dalpé-Scott, M.; Perelman, A.H.; Heick, H.M. The occult insulinoma operative localization by quick insulin radioimmunoassay. J. Pediatr. Surg. 1985, 20, 734–736. [Google Scholar] [CrossRef]
- Gough, M.H. The surgical treatment of hyperinsulinism in infancy and childhood. Br. J. Surg. 1984, 71, 75–78. [Google Scholar] [CrossRef]
- MacDonald, M.J.; Warner, T.F.; Pellett, J.R. Increased mitochondrial glycerol phosphate dehydrogenase activity in insulinomas of two hypoglycemic infants. J. Clin. Endocrinol. Metab. 1983, 57, 662–664. [Google Scholar] [CrossRef]
- Bordi, C.; Ravazzola, M.; Pollak, A.; Lubec, G.; Orci, L. Neonatal islet cell adenoma: A distinct type of islet cell tumor? Diabetes Care 1982, 5, 122–125. [Google Scholar] [CrossRef]
- Glickman, M.H.; Hart, M.J.; White, T.T. Insulinoma in Seattle: 39 cases in 30 years. Am. J. Surg. 1980, 140, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg-Fellner, F.; Rayfield, E.J. Metabolic studies in a child with a pancreatic insulinoma. Am. J. Dis. Child. 1980, 134, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Cameron, G.S.; Wright, A.D.; Tuttle, R.J.; Cole, F.M.; Birchard, E. Insulinoma diagnosed by insulin immunoassay and angiography. J. Pediatr. Surg. 1972, 7, 717–718. [Google Scholar] [CrossRef]
- Heitz, P.; Steiner, H.; Halter, F.; Egli, F.; Kapp, J.P. Multihormonal, amyloid-producing tumour of the islets of Langerhans in a twelve-year-old boy. Clinical, morphological and biochemical data and review of the literature. Virchows Arch. A Pathol. Anat. 1971, 353, 312–324. [Google Scholar] [CrossRef]
- Goyal, R.; Debi, U.; Dey, P.; Prasad, K.K.; Thapa, B.R. Zollinger-Ellison syndrome: An unusual case of chronic diarrhoea in a child. Malays. J. Pathol. 2016, 38, 321–325. [Google Scholar]
- Murase, N.; Uchida, H.; Tainaka, T.; Kawashima, H.; Tanaka, Y.; Amano, H.; Kishimoto, H. Laparoscopic-assisted pancreaticoduodenectomy in a child with gastrinoma. Pediatr. Int. 2015, 57, 1196–1198. [Google Scholar] [CrossRef]
- Massaro, S.A.; Emre, S.H. Metastatic gastrinoma in a pediatric patient with Zollinger-Ellison syndrome. J. Pediatr. Hematol. Oncol. 2014, 36, e13–e15. [Google Scholar] [CrossRef]
- Dall’igna, P.; Cecchetto, G.; Bisogno, G.; Conte, M.; Chiesa, P.L.; D’Angelo, P.; De Leonardis, F.; De Salvo, G.; Favini, F.; Ferrari, A. TREP Group Pancreatic tumors in children and adolescents: The Italian TREP project experience. Pediatr. Blood Cancer 2010, 54, 675–680. [Google Scholar] [CrossRef]
- Schettini, S.T.; Ribeiro, R.C.; Facchin, C.G.; Abib, S.C. Gastrinoma in childhood: Case report and update on diagnosis and treatment. Eur. J. Pediatr. Surg. 2009, 19, 38–40. [Google Scholar] [CrossRef]
- Gurevich, L.; Kazantseva, I.; Isakov, V.A.; Korsakova, N.; Egorov, A.; Kubishkin, V.; Bulgakov, G. The analysis of immunophenotype of gastrin-producing tumors of the pancreas and gastrointestinal tract. Cancer 2003, 98, 1967–1976. [Google Scholar] [CrossRef]
- Wamsteker, E.J.; Gauger, P.G.; Thompson, N.W.; Scheiman, J.M. EUS detection of pancreatic endocrine tumors in asymptomatic patients with type 1 multiple endocrine neoplasia. Gastrointest. Endosc. 2003, 58, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Eire, P.F.; Rodriguez Pereira, C.; Barca Rodriguez, P.; Varela Cives, R. Uncommon case of gastrinoma in a child. Eur. J. Pediatr. Surg. 1996, 6, 173–174. [Google Scholar] [CrossRef]
- van Beek, A.P.; de Haas, E.R.; van Vloten, W.A.; Lips, C.J.; Roijers, J.F.; Canninga-van Dijk, M.R. The glucagonoma syndrome and necrolytic migratory erythema: A clinical review. Eur. J. Endocrinol. 2004, 151, 531–537. [Google Scholar] [CrossRef]
- Bonilla Gonzalez, C.; Rusinque, J.; Uribe, C.; Carias, A.; Contreras, M.L. Pancreatic VIPoma as a Differential Diagnosis in Chronic Pediatric Diarrhea: A Case Report and Review of the Literature. J. Med. Cases 2021, 12, 195–201. [Google Scholar] [CrossRef]
- Acosta-Gualandri, A.; Kao, K.T.; Wong, T.; Webber, E.; Armstrong, L.; Panagiotopoulos, C. Perioperative Hypotensive Crisis in an Adolescent with a Pancreatic VIPoma and MEN1-Gene Variant. Horm. Res. Paediatr. 2019, 91, 285–289. [Google Scholar] [CrossRef]
- Bourcier, M.E.; Vinik, A.I. Sunitinib for the treatment of metastatic paraganglioma and vasoactive intestinal polypeptide-producing tumor (VIPoma). Pancreas 2013, 42, 348–352. [Google Scholar] [CrossRef]
- Masulovic, D.; Stevic, R.; Knezevic, S.; Micev, M.; Saranovic, D.; Filipovic, A.; Knezevic, D.; Ivanovic, A.; Djuric-Stefanovic, A. Education and imaging. Hepatobiliary and pancreatic: Pancreatic VIPomas associated with multiple endocrine neoplasia type I. J. Gastroenterol. Hepatol. 2012, 27, 619. [Google Scholar] [CrossRef]
- Brenner, R.W.; Sank, L.I.; Kerner, M.B.; Schrager, G.O.; Elguezabal, A.; Roth, J. Resection of a vipoma of the pancreas in a 15-year-old girl. J. Pediatr. Surg. 1986, 21, 983–985. [Google Scholar] [CrossRef]
- Petriczko, E.; Marcinkiewicz, K.; Prokurat, A.; Sagan, L.; Malkowski, B.; Biczysko-Mokosa, A.; Horodnicka-Jozwa, A.; Andrysiak-Mamos, E.; Syrenicz, A.; Kostrzeba, E.; et al. Rare clinical manifestation of multiple endocrine neoplasia type 1. Neuro Endocrinol. Lett. 2022, 43, 199–207. [Google Scholar] [PubMed]
- Erichsen, T.D.; Detlefsen, S.; Andersen, K.Ø.; Pedersen, H.; Rasmussen, L.; Gotthardt, M.; Pörksen, S.; Christesen, H.T. Occult insulinoma, glucagonoma and pancreatic endocrine pseudotumour in a patient with multiple endocrine neoplasia type 1. Pancreatology 2020, 20, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Winston, K.Y.; Dawrant, J. A rare case of hypoglycaemia due to insulinoma in an adolescent with acutely altered mental status. J. Pediatr. Endocrinol. Metab. 2014, 27, 773–776. [Google Scholar] [CrossRef]
- Svensson, E.; Muth, A.; Hedenström, P.; Ragnarsson, O. The incidence of insulinoma in Western Sweden between 2002 and 2019. Ann. Gastroenterol. 2022, 35, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Kurakawa, K.I.; Okada, A.; Manaka, K.; Konishi, T.; Jo, T.; Ono, S.; Uda, K.; Michihata, N.; Matsui, H.; Fushimi, K.; et al. Clinical Characteristics and Incidences of Benign and Malignant Insulinoma Using a National Inpatient Database in Japan. J. Clin. Endocrinol. Metab. 2021, 106, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Placzkowski, K.A.; Vella, A.; Thompson, G.B.; Grant, C.S.; Reading, C.C.; Charboneau, J.W.; Andrews, J.C.; Lloyd, R.V.; Service, F.J. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987–2007. J. Clin. Endocrinol. Metab. 2009, 94, 1069–1073. [Google Scholar] [CrossRef]
- Sözen, M.; Cantürk, Z.; Selek, A.; Çetinarslan, B.; Eryılmaz, B.H.; Gezer, E.; Köksalan, D. Clinicopathological Features of Insulinoma: A Single Tertiary Center Experience. Indian. J. Surg. Oncol. 2023, 14, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Sada, A.; Habermann, E.B.; Szabo Yamashita, T.; Thompson, G.B.; Lyden, M.L.; Foster, T.R.; Dy, B.M.; Halfdanarson, T.R.; Vella, A.; McKenzie, T.J. Comparison Between Sporadic and Multiple Endocrine Neoplasia Type 1-Associated Insulinoma. J. Am. Coll. Surg. 2022, 235, 756–763. [Google Scholar] [CrossRef]
- Dizon, A.M.; Kowalyk, S.; Hoogwerf, B.J. Neuroglycopenic and other symptoms in patients with insulinomas. Am. J. Med. 1999, 106, 307–310. [Google Scholar] [CrossRef]
- Peltola, E.; Hannula, P.; Huhtala, H.; Metso, S.; Kiviniemi, U.; Vornanen, M.; Sand, J.; Laukkarinen, J.; Tiikkainen, M.; Schalin-Jäntti, C.; et al. Characteristics and Outcomes of 79 Patients with an Insulinoma: A Nationwide Retrospective Study in Finland. Int. J. Endocrinol. 2018, 2018, 2059481. [Google Scholar] [CrossRef]
- Finlayson, E.; Clark, O.H. Surgical treatment of insulinomas. Surg. Clin. N. Am. 2004, 84, 775–785. [Google Scholar] [CrossRef]
- Demeure, M.J.; Klonoff, D.C.; Karam, J.H.; Duh, Q.Y.; Clark, O.H. Insulinomas associated with multiple endocrine neoplasia type I: The need for a different surgical approach. Surgery 1991, 110, 998–1004. [Google Scholar]
- Service, F.J.; McMahon, M.M.; O’Brien, P.C.; Ballard, D.J. Functioning insulinoma—Incidence, recurrence, and long-term survival of patients: A 60-year study. Mayo Clin. Proc. 1991, 66, 711–719. [Google Scholar] [CrossRef]
- Calabrò, D.; Argalia, G.; Ambrosini, V. Role of PET/CT and Therapy Management of Pancreatic Neuroendocrine Tumors. Diagnostics 2020, 10, 1059. [Google Scholar] [CrossRef]
- Lee, L.; Ito, T.; Jensen, R.T. Imaging of pancreatic neuroendocrine tumors: Recent advances, current status, and controversies. Expert. Rev. Anticancer Ther. 2018, 18, 837–860. [Google Scholar] [CrossRef] [PubMed]
- Thoeni, R.F.; Mueller-Lisse, U.G.; Chan, R.; Do, N.K.; Shyn, P.B. Detection of small, functional islet cell tumors in the pancreas: Selection of MR imaging sequences for optimal sensitivity. Radiology 2000, 214, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, M.; Warshaw, A.L.; Axelrod, L.; Deshpande, V.; Thayer, S.P.; Ferrone, C.R.; Fernández-del Castillo, C. Improved contemporary surgical management of insulinomas: A 25-year experience at the Massachusetts General Hospital. Ann. Surg. 2008, 247, 165–172. [Google Scholar] [CrossRef]
- James, P.D.; Tsolakis, A.V.; Zhang, M.; Belletrutti, P.J.; Mohamed, R.; Roberts, D.J.; Heitman, S.J. Incremental benefit of preoperative EUS for the detection of pancreatic neuroendocrine tumors: A meta-analysis. Gastrointest. Endosc. 2015, 81, 848–856. [Google Scholar] [CrossRef]
- Lo, C.Y.; Chan, F.L.; Tam, S.C.; Cheng, P.W.; Fan, S.T.; Lam, K.S. Value of intra-arterial calcium-stimulated venous sampling for regionalization of pancreatic insulinomas. Surgery 2000, 128, 903–909. [Google Scholar] [CrossRef]
- Kulke, M.H.; Anthony, L.B.; Bushnell, D.L.; de Herder, W.W.; Goldsmith, S.J.; Klimstra, D.S.; Marx, S.J.; Pasieka, J.L.; Pommier, R.F.; Yao, J.C.; et al. North American Neuroendocrine Tumor Society (NANETS). NANETS treatment guidelines: Well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010, 39, 735–752. [Google Scholar] [CrossRef]
- Mansour, J.C.; Chen, H. Pancreatic endocrine tumors. J. Surg. Res. 2004, 120, 139–161. [Google Scholar] [CrossRef]
- Sulciner, M.L.; Clancy, T.E. Surgical Management of Pancreatic Neuroendocrine Tumors. Cancers 2023, 15, 2006. [Google Scholar] [CrossRef]
- Bidani, K.; Marinovic, A.G.; Moond, V.; Harne, P.; Broder, A.; Thosani, N. Treatment of Pancreatic Neuroendocrine Tumors: Beyond Traditional Surgery and Targeted Therapy. J. Clin. Med. 2025, 14, 3389. [Google Scholar] [CrossRef]
- Crippa, S.; Zerbi, A.; Boninsegna, L.; Capitanio, V.; Partelli, S.; Balzano, G.; Pederzoli, P.; Di Carlo, V.; Falconi, M. Surgical management of insulinomas: Short- and long-term outcomes after enucleations and pancreatic resections. Arch. Surg. 2012, 147, 261–266. [Google Scholar] [CrossRef]
- Thompson, G.B.; Service, F.J.; van Heerden, J.A.; Carney, J.A.; Charboneau, J.W.; O’Brien, P.C.; Grant, C.S. Reoperative insulinomas, 1927 to 1992: An institutional experience. Surgery 1993, 114, 1196–1204. [Google Scholar]
- Sharma, A.; Varshney, P.; Kasliwal, R.; Nagar, A.; Venkatatelikicherla, K.; Sarin, S.; Choubey, R.P.; Kapoor, V.K. Insulinoma-Accurate Preoperative Localization Is the Key to Management: An Initial Experience. Indian J. Surg. Oncol. 2022, 13, 403–411. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Wiseman, J.T.; Pawlik, T.M. Surgical management of pancreatic neuroendocrine liver metastases. J. Gastrointest. Oncol. 2020, 11, 590–600. [Google Scholar] [CrossRef]
- Oberg, K. Pancreatic endocrine tumors. Semin. Oncol. 2010, 37, 594–618. [Google Scholar] [CrossRef]
- Berna, M.J.; Hoffmann, K.M.; Serrano, J.; Gibril, F.; Jensen, R.T. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine 2006, 85, 295–330. [Google Scholar] [CrossRef]
- Norton, J.A. Neuroendocrine tumors of the pancreas and duodenum. Curr. Probl. Surg. 1994, 31, 77–156. [Google Scholar] [CrossRef] [PubMed]
- Metz, D.C.; Cadiot, G.; Poitras, P.; Ito, T.; Jensen, R.T. Diagnosis of Zollinger-Ellison syndrome in the era of PPIs, faulty gastrin assays, sensitive imaging and limited access to acid secretory testing. Int. J. Endocr. Oncol. 2017, 4, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.A.; Foster, D.S.; Ito, T.; Jensen, R.T. Gastrinomas: Medical or Surgical Treatment. Endocrinol. Metab. Clin. N. Am. 2018, 47, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Venzon, D.J.; Shojamanesh, H.; Abou-Saif, A.; Peghini, P.; Doppman, J.L.; Gibril, F.; Jensen, R.T. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine 2000, 79, 379–411. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T.; Shelat, V.G. Perforated peptic ulcer-an update. World J. Gastrointest. Surg. 2017, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, A.H.; Donowitz, M. Catching the Zebra: Clinical Pearls and Pitfalls for the Successful Diagnosis of Zollinger-Ellison Syndrome. Dig. Dis. Sci. 2017, 62, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Elvevi, A.; Citterio, D.; Coppa, J.; Invernizzi, P.; Mazzaferro, V.; Massironi, S. Gastrinoma and Zollinger Ellison syndrome: A roadmap for the management between new and old therapies. World J. Gastroenterol. 2021, 27, 5890–5907. [Google Scholar] [CrossRef]
- Yang, R.H.; Chu, Y.K. Zollinger-Ellison syndrome: Revelation of the gastrinoma triangle. Radiol. Case. Rep. 2015, 10, 827. [Google Scholar] [CrossRef][Green Version]
- Metz, D.C.; Jensen, R.T. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology 2008, 135, 1469–1492. [Google Scholar] [CrossRef]
- Jensen, R.T.; Berna, M.J.; Bingham, D.B.; Norton, J.A. Inherited pancreatic endocrine tumor syndromes: Advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer 2008, 113, 1807–1843. [Google Scholar] [CrossRef]
- Fishbeyn, V.A.; Norton, J.A.; Benya, R.V.; Pisegna, J.R.; Venzon, D.J.; Metz, D.C.; Jensen, R.T. Assessment and prediction of long-term cure in patients with the Zollinger-Ellison syndrome: The best approach. Ann. Intern. Med. 1993, 119, 199–206. [Google Scholar] [CrossRef]
- Howe, J.R.; Merchant, N.B.; Conrad, C.; Keutgen, X.M.; Hallet, J.; Drebin, J.A.; Minter, R.M.; Lairmore, T.C.; Tseng, J.F.; Zeh, H.J.; et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 1–33. [Google Scholar] [CrossRef]
- Hofland, J.; Falconi, M.; Christ, E.; Castaño, J.P.; Faggiano, A.; Lamarca, A.; Perren, A.; Petrucci, S.; Prasad, V.; Ruszniewski, P.; et al. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J. Neuroendocrinol. 2023, 35, e13318. [Google Scholar] [CrossRef]
- Jadhav, A.; Ayyat, M.; Serafini, F.M. Presentation, Diagnosis, and Treatment of a Sporadic Gastrinoma With Liver Metastases. Cureus 2024, 16, e61426. [Google Scholar] [CrossRef] [PubMed]
- van Beek, D.J.; Nell, S.; Pieterman, C.R.C.; de Herder, W.W.; van de Ven, A.C.; Dekkers, O.M.; van der Horst-Schrivers, A.N.; Drent, M.L.; Bisschop, P.H.; Havekes, B.; et al. Prognostic factors and survival in MEN1 patients with gastrinomas: Results from the DutchMEN study group (DMSG). J. Surg. Oncol. 2019, 120, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Wermers, R.A.; Fatourechi, V.; Wynne, A.G.; Kvols, L.K.; Lloyd, R.V. The glucagonoma syndrome. Clinical and pathologic features in 21 patients. Medicine 1996, 75, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Lévy-Bohbot, N.; Merle, C.; Goudet, P.; Delemer, B.; Calender, A.; Jolly, D.; Thiéfin, G.; Cadiot, G.; Groupe des Tumeurs Endocrines. Prevalence, characteristics and prognosis of MEN 1-associated glucagonomas, VIPomas, and somatostatinomas: Study from the GTE (Groupe des Tumeurs Endocrines) registry. Gastroenterol. Clin. Biol. 2004, 28, 1075–1081. [Google Scholar] [CrossRef]
- Anelli, S.; Mazzilli, R.; Zamponi, V.; Giorgini, B.; Golisano, B.; Mancini, C.; Russo, F.; Panzuto, F.; Faggiano, A. Glucagonoma and Glucagonoma Syndrome: An Updated Review. Clin. Endocrinol. 2025. Online ahead of print. [Google Scholar]
- Song, X.; Zheng, S.; Yang, G.; Xiong, G.; Cao, Z.; Feng, M.; Zhang, T.; Zhao, Y. Glucagonoma and the glucagonoma syndrome. Oncol. Lett. 2018, 15, 2749–2755. [Google Scholar]
- Kitamura, Y.; Sato, M.; Hatamochi, A.; Yamazaki, S. Necrolytic migratory erythema without glucagonoma associated with hepatitis B. Eur. J. Dermatol. 2005, 15, 49–51. [Google Scholar]
- Lv, W.F.; Han, J.K.; Liu, X.; Wang, S.C.; Pan, B.O.; Xu, A.O. Imaging features of glucagonoma syndrome: A case report and review of the literature. Oncol. Lett. 2015, 9, 1579–1582. [Google Scholar] [CrossRef][Green Version]
- King, C.M.; Reznek, R.H.; Dacie, J.E.; Wass, J.A. Imaging islet cell tumours. Clin. Radiol. 1994, 49, 295–303. [Google Scholar] [CrossRef]
- Yacine, O.; Ksontini, F.L.; Ben Mahmoud, A.; Magherbi, H.; Fterich, S.F.; Kacem, M. Case report of a recurrent resected glucagonoma. Ann. Med. Surg. 2022, 77, 103604. [Google Scholar] [CrossRef]
- Ferrarese, A.; Borello, A.; Gentile, V.; Bindi, M.; Ferrara, Y.; Solej, M.; Martino, V.; Nano, M. Meso-pancreatectomy for pancreatic neuroendocrine tumor. Int. J. Surg. 2014, 12, 123–125. [Google Scholar] [CrossRef][Green Version]
- Dimitriadis, G.K.; Weickert, M.O.; Randeva, H.S.; Kaltsas, G.; Grossman, A. Medical management of secretory syndromes related to gastroenteropancreatic neuroendocrine tumours. Endocr. Relat. Cancer 2016, 23, 423–436. [Google Scholar] [CrossRef]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef]
- Perry, R.R.; Vinik, A.I. Clinical review 72: Diagnosis and management of functioning islet cell tumors. J. Clin. Endocrinol. Metab. 1995, 80, 2273–2278. [Google Scholar] [PubMed]
- Smith, S.L.; Branton, S.A.; Avino, A.J.; Martin, J.K.; Klingler, P.J.; Thompson, G.B.; Grant, C.S.; van Heerden, J.A. Vasoactive intestinal polypeptide secreting islet cell tumors: A 15-year experience and review of the literature. Surgery 1998, 124, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Una Cidon, E. Vasoactive intestinal peptide secreting tumour: An overview. World J. Gastrointest. Oncol. 2022, 14, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Schizas, D.; Mastoraki, A.; Bagias, G.; Patras, R.; Moris, D.; Lazaridis, I.I.; Arkadopoulos, N.; Felekouras, E. Clinicopathological data and treatment modalities for pancreatic vipomas: A systematic review. J. Balk. Union Oncol. 2019, 24, 415–423. [Google Scholar]
- Ammori, B.J.; El-Dhuwaib, Y.; Ballester, P.; Augustine, T. Laparoscopic distal pancreatectomy for neuroendocrine tumors of the pancreas. Hepatogastroenterology 2005, 52, 620–624. [Google Scholar]
- Azizian, A.; König, A.; Ghadimi, M. Treatment options of metastatic and nonmetastatic VIPoma: A review. Langenbecks Arch. Surg. 2022, 407, 2629–2636. [Google Scholar] [CrossRef]
- Damaskos, C.; Dimitroulis, D.; Garmpi, A.; Antoniou, E.A.; Kouraklis, G.; Psilopatis, I.; Mavri, M.; Diamantis, E.; Marinos, G.; Kyriakos, G.; et al. Synchronous Insulinoma and Glucagonoma: A Review of the Literature. In Vivo 2023, 37, 2402–2408. [Google Scholar] [CrossRef]
- Ozbas, B.; Karaca, Z.; Okcesiz, I.; Karahan, I.; Topaloglu, N.; Abdulrezzak, U.; Ozturk, F.; Unluhizarci, K. Co-existence of two different types of pancreatic neuroendocrine tumors in a patient with multiple endocrine neoplasia type-1. J. Clin. Transl. Endocrinol. 2021, 21, 100088. [Google Scholar] [CrossRef]
- Nishiuchi, T.; Imachi, H.; Murao, K.; Fujiwara, M.; Muraoka, T.; Kikuchi, F.; Nishiuchi, Y.; Kushida, Y.; Haba, R.; Ishida, T. Co-existence of glucagonoma with recurrent insulinoma in a patient with multiple endocrine neoplasia-type 1 (MEN-1). Endocrine 2009, 36, 20–24. [Google Scholar] [CrossRef]
- Yagihashi, S.; Yagihashi, N.; Nagai, K. Cystic pancreatic glucagonoma in contact with insulinoma found in a hypoglycemic patient. Pathol. Res. Pract. 1992, 188, 751–756. [Google Scholar] [CrossRef] [PubMed]
- de Herder, W.W.; Hofland, J. Somatostatinoma. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Sandru, F.; Carsote, M.; Valea, A.; Albu, S.E.; Petca, R.C.; Dumitrascu, M.C. Somatostatinoma: Beyond neurofibromatosis type 1 (Review). Exp. Ther. Med. 2020, 20, 3383–3388. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Pieterman, C.R.C.; English, K.A.; Lines, K.E.; Shariq, O.A.; Marini, F.; Cuny, T.; Lewis, M.A.; Stratakis, C.A.; Perrier, N.D.; et al. Delphi Expert Panel. Multiple endocrine neoplasia type 1 (MEN1): Recommendations and guidelines for best practice. Lancet Diabetes Endocrinol. 2025, 13, 699–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, S.; Zhang, B.; Ni, Q.; Yu, X.; Xu, J. Circulating biomarkers for early diagnosis of pancreatic cancer: Facts and hopes. Am. J. Cancer Res. 2018, 8, 332–353. [Google Scholar]
- Ma, Z.Y.; Gong, Y.F.; Zhuang, H.K.; Zhou, Z.X.; Huang, S.Z.; Zou, Y.P.; Huang, B.W.; Sun, Z.H.; Zhang, C.Z.; Tang, Y.Q.; et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J. Gastroenterol. 2020, 26, 2305–2322. [Google Scholar] [CrossRef]
- Modica, R.; Benevento, E.; Liccardi, A.; Cannavale, G.; Minotta, R.; DI Iasi, G.; Colao, A. Recent advances and future challenges in the diagnosis of neuroendocrine neoplasms. Minerva Endocrinol. 2024, 49, 158–174. [Google Scholar] [CrossRef]
- Wu, H.; Ou, S.; Zhang, H.; Huang, R.; Yu, S.; Zhao, M.; Tai, S. Advances in biomarkers and techniques for pancreatic cancer diagnosis. Cancer Cell Int. 2022, 22, 220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keretić, D.; Bašković, M. Diagnosis and Management of Functional Pancreatic Neuroendocrine Tumors in Children—A Systematic Review. Diagnostics 2025, 15, 2176. https://doi.org/10.3390/diagnostics15172176
Keretić D, Bašković M. Diagnosis and Management of Functional Pancreatic Neuroendocrine Tumors in Children—A Systematic Review. Diagnostics. 2025; 15(17):2176. https://doi.org/10.3390/diagnostics15172176
Chicago/Turabian StyleKeretić, Dorotea, and Marko Bašković. 2025. "Diagnosis and Management of Functional Pancreatic Neuroendocrine Tumors in Children—A Systematic Review" Diagnostics 15, no. 17: 2176. https://doi.org/10.3390/diagnostics15172176
APA StyleKeretić, D., & Bašković, M. (2025). Diagnosis and Management of Functional Pancreatic Neuroendocrine Tumors in Children—A Systematic Review. Diagnostics, 15(17), 2176. https://doi.org/10.3390/diagnostics15172176






