Testicular Stiffness and Volume in Varicocele Patients: A Prospective Comparative Shear Wave Elastography Study
Abstract
1. Introduction
2. Materials and Methods
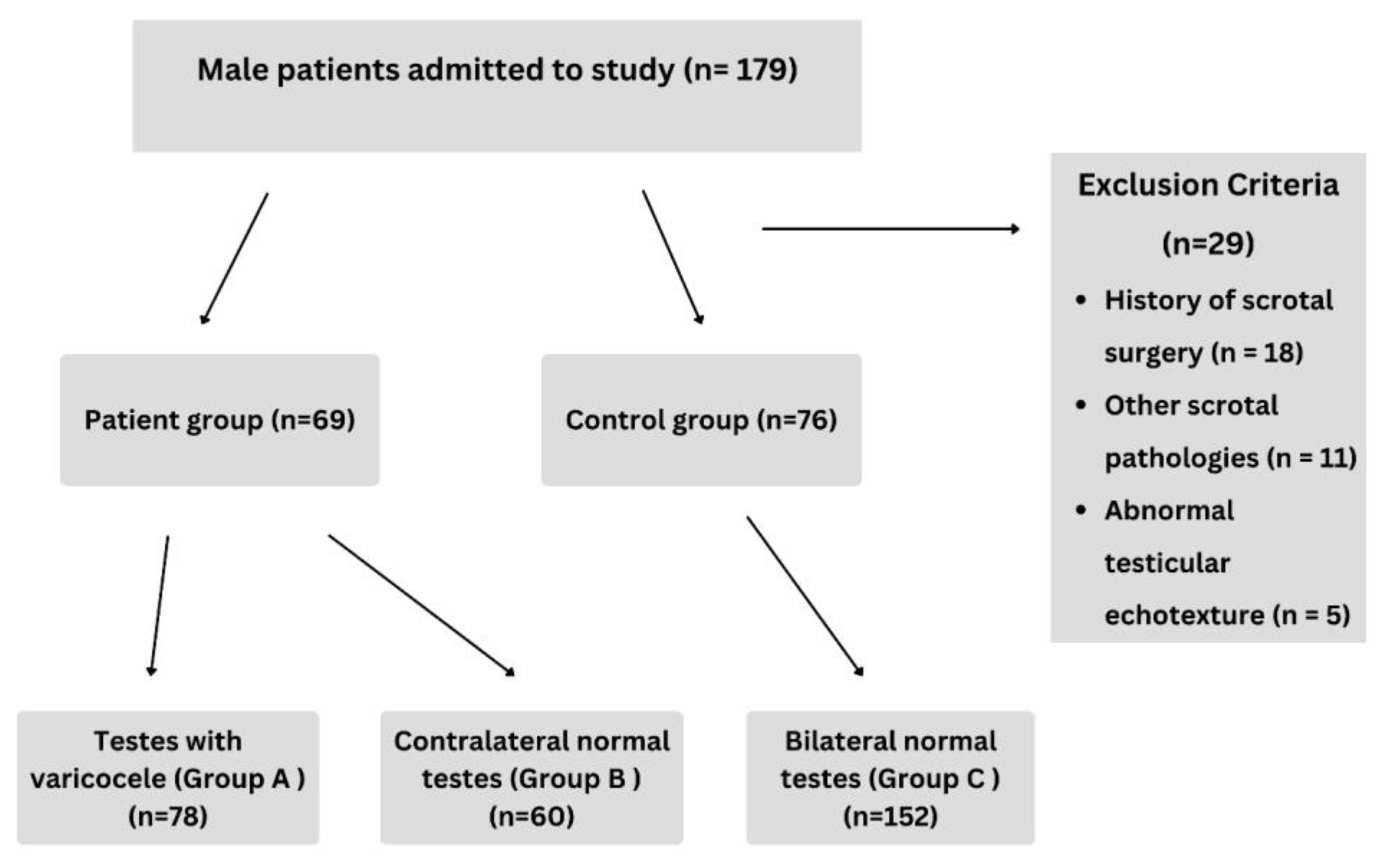
2.1. Study Design and Participants
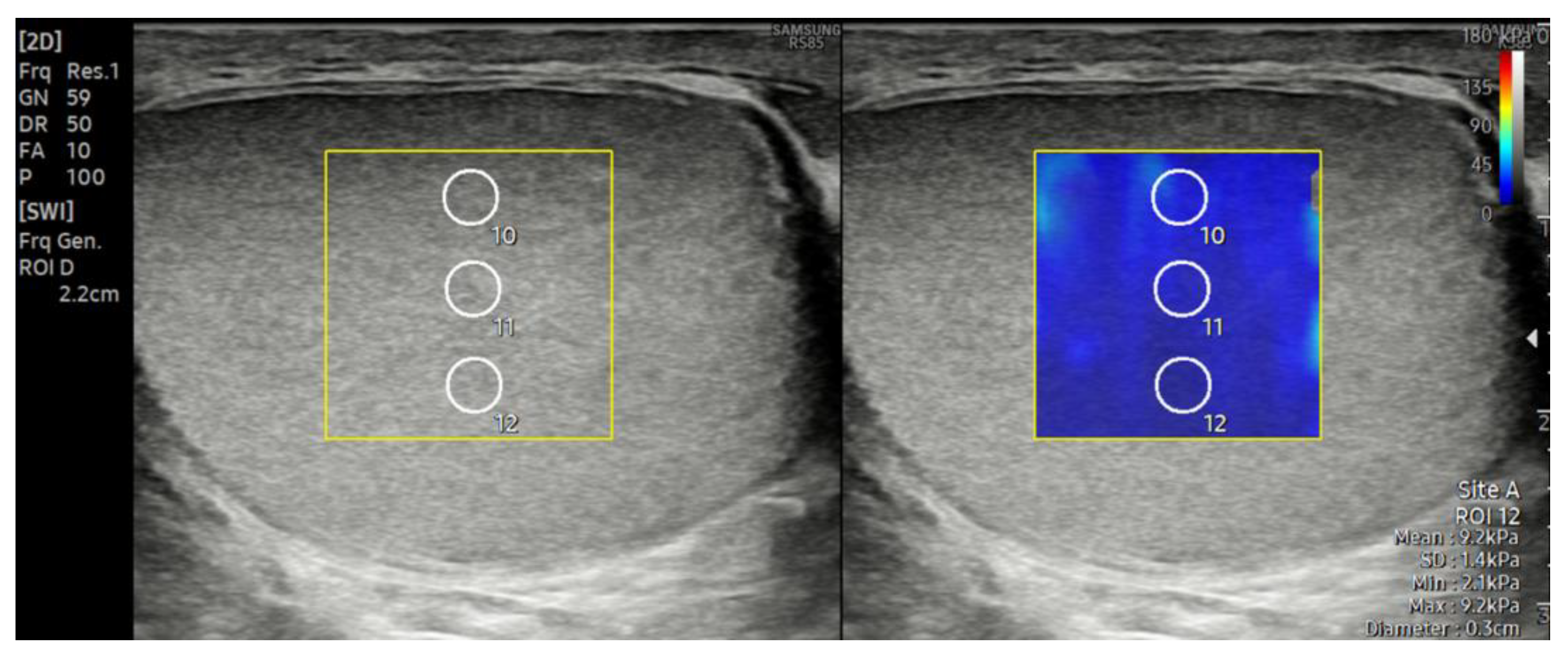
2.2. Ultrasonography and Shear Wave Elastography Examination
2.3. Statistical Analysis
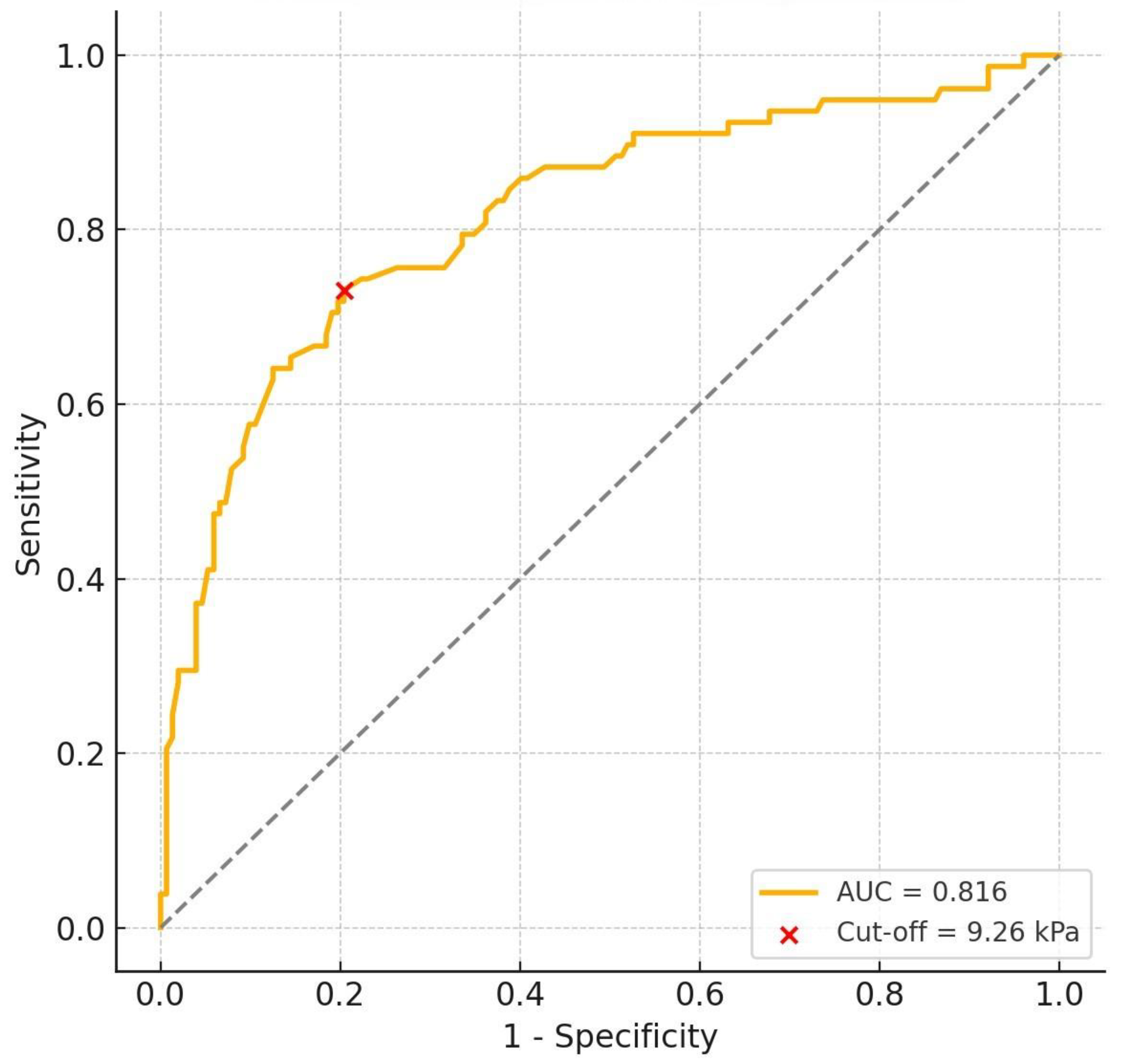
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pauroso, S.; Di Leo, N.; Fulle, I.; Di Segni, M.; Alessi, S.; Maggini, E. Varicocele: Ultrasonographic Assessment in Daily Clinical Practice. J. Ultrasound 2011, 14, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.; Bertolotto, M.; Richenberg, J.; Belfield, J.; Dogra, V.; Huang, D.Y.; Lotti, F.; Markiet, K.; Nikolic, O.; Ramanathan, S.; et al. Ultrasound Evaluation of Varicoceles: Guidelines and Recommendations of the European Society of Urogenital Radiology Scrotal and Penile Imaging Working Group (ESUR-SPIWG) for Detection, Classification, and Grading. Eur. Radiol. 2020, 30, 11–25. [Google Scholar] [CrossRef]
- Zini, A.; Boman, J. Varicocele: Red Flag or Red Herring? Semin. Reprod. Med. 2009, 27, 171–178. [Google Scholar] [CrossRef]
- Marmar, J.L. Varicocele and Male Infertility: Part II: The Pathophysiology of Varicoceles in the Light of Current Molecular and Genetic Information. Hum. Reprod. Update 2001, 7, 461–472. [Google Scholar] [CrossRef]
- Jung, A.; Schuppe, H.-C. Influence of Genital Heat Stress on Semen Quality in Humans. Andrologia 2007, 39, 203–215. [Google Scholar] [CrossRef]
- Dubin, L.; Amelar, R.D. Varicocele Size And Results of Varicocelectomy in Selected Subfertile Men with Varicocele. Fertil. Steril. 1970, 21, 606–609. [Google Scholar] [CrossRef]
- Sagir, L.; Kaba, E.; Huner Yigit, M.; Tasci, F.; Uzun, H. Predicting Semen Analysis Parameters from Testicular Ultrasonography Images Using Deep Learning Algorithms: An Innovative Approach to Male Infertility Diagnosis. J. Clin. Med. 2025, 14, 516. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.H.; Kim, S.H.; Cho, J.Y.; Seo, J.T.; Chun, Y.K. Scrotal US for Evaluation of Infertile Men with Azoospermia. Radiology 2006, 239, 168–173. [Google Scholar] [CrossRef]
- Charoenchue, P.; Khorana, J.; Chitapanarux, T.; Inmutto, N.; Na Chiangmai, W.; Amantakul, A.; Pojchamarnwiputh, S.; Tantraworasin, A. Two-Dimensional Shear-Wave Elastography: Accuracy in Liver Fibrosis Staging Using Magnetic Resonance Elastography as the Reference Standard. Diagnostics 2024, 15, 62. [Google Scholar] [CrossRef]
- Deeg, J.; Swoboda, M.; Egle, D.; Wieser, V.; Soleiman, A.; Ladenhauf, V.; Galijasevic, M.; Amort, B.; Gruber, L. Shear-Wave Elastography Gradient Analysis of Newly Diagnosed Breast Tumours: A Critical Analysis. Diagnostics 2024, 14, 1657. [Google Scholar] [CrossRef] [PubMed]
- Kavvadas, D.; Rafailidis, V.; Partovi, S.; Tegos, T.; Kallia, Z.; Savvoulidis, P.; Papamitsou, T.; Prassopoulos, P. Shear Wave Elastography for Carotid Artery Stiffness: Ready for Prime Time? Diagnostics 2025, 15, 303. [Google Scholar] [CrossRef]
- Agarwal, D.; Bhatia, A.; Saxena, A.K.; Dayal, D.; Sodhi, K.S. Role of Shear Wave Elastography of Thyroid Gland in Children With Newly Diagnosed Hashimoto’s Thyroiditis: Preliminary Study. J. Ultrasound Med. 2022, 41, 2217–2225. [Google Scholar] [CrossRef]
- Balaban, M.; Cilengir, A.H.; Idilman, I.S. Evaluation of Tendon Disorders With Ultrasonography and Elastography. J. Ultrasound Med. 2021, 40, 1267–1286. [Google Scholar] [CrossRef] [PubMed]
- Alperen, K.; Ayca, S.; Unal, T.; Han, G.K.; Sadik, G. Testes Parenchymal Shear Wave Elastography Findings in Varicocele. J. Coll. Physicians Surg. Pak. 2022, 32, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Yüzkan, S.; Çilengir, A.H. Shear Wave Elastography for Assessment of Testicular Stiffness in Patients with Varicocele. J. Med. Ultrasound 2022, 30, 277–281. [Google Scholar] [CrossRef]
- Erdogan, H.; Durmaz, M.S.; Arslan, S.; Gokgoz Durmaz, F.; Cebeci, H.; Ergun, O.; Sogukpinar Karaagac, S. Shear Wave Elastography Evaluation of Testes in Patients With Varicocele. Ultrasound Q. 2020, 36, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Turna, O.; Aybar, M.D. Testicular Stiffness in Varicocele: Evaluation with Shear Wave Elastography. Ultrasonography 2020, 39, 350–355. [Google Scholar] [CrossRef]
- Joo, I.; Kim, S.Y.; Park, H.S.; Lee, E.S.; Kang, H.J.; Lee, J.M. Validation of a New Point Shear-Wave Elastography Method for Noninvasive Assessment of Liver Fibrosis: A Prospective Multicenter Study. Korean J. Radiol. 2019, 20, 1527. [Google Scholar] [CrossRef]
- Jeong, W.K. Liver Stiffness Measurement Using S-Shearwave: Initial Experience. Case Study, Article #CS201505-S-Shearwave; Samsung Medison Co., Ltd.: Seoul, Republic of Korea, 29 May 2015. Available online: https://www.samsungmedison.com (accessed on 14 July 2025).
- Dag, N.; Sinanoglu, M.S. Evaluation of Meniscal Elasticity Using Shear Wave Elastography in Obese Children and Adolescents: A Preliminary Cross-Sectional Study. Pediatr. Radiol. 2023, 54, 293–298. [Google Scholar] [CrossRef]
- Sinanoğlu, M.S.; Dağ, N.; Sinanoğlu, B.; Özdemir, F. Evaluation of the Core Muscles of Children and Adolescents with Nocturnal Enuresis Using Shear Wave Elastography: A Preliminary Study. Sci. Rep. 2025, 15, 2734. [Google Scholar] [CrossRef]
- Salama, N.; Samir, M.; Blgozah, S. Evaluation of Normal and Varicocele-Bearing Testes Using Real-time Strain Elastography. J. Ultrasound Med. 2019, 38, 621–627. [Google Scholar] [CrossRef]
- Kervancıoğlu, S.; Sarıca, A.; Mete, A.; Özkur, A.; Bayram, M. Effect of Varicocele on Testicular Volume. Eur. J. Ther. 2008, 14, 11–14. [Google Scholar] [CrossRef]
- Ku, J.A.H.; Son, H.; Kwak, C.; Lee, S.E.; Lee, N.K.; Park, Y.H.O. Impact Of Varicocele On Testicular Volume In Young Men: Significance Of Compensatory Hypertorphy Of Contralateral Testis. J. Urol. 2002, 168, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Kass, E.J.; Stork, B.R.; Steinert, B.W. Varicocele in Adolescence Induces Left and Right Testicular Volume Loss. BJU Int. 2001, 87, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Mbaeri, T.U.; Orakwe, J.C.; Nwofor, A.M.E.; Oranusi, C.K.; Mbonu, O.O. Ultrasound Measurements of Testicular Volume: Comparing the Three Common Formulas with the True Testicular Volume Determined by Water Displacement. Afr. J. Urol. 2013, 19, 69–73. [Google Scholar] [CrossRef]
- Sakamoto, H.; Saito, K.; Oohta, M.; Inoue, K.; Ogawa, Y.; Yoshida, H. Testicular Volume Measurement: Comparison of Ultrasonography, Orchidometry, and Water Displacement. Urology 2007, 69, 152–157. [Google Scholar] [CrossRef]
- Camoglio, F.S.; Bruno, C.; Peretti, M.; Bianchi, F.; Bucci, A.; Scirè, G.; Patanè, S.; Zampieri, N. The Role of Sonoelastography in the Evaluation of Testes With Varicocele. Urology 2017, 100, 203–206. [Google Scholar] [CrossRef]
- Ahmed, A.T. Utility of Shear Wave Ultrasound Elastography in Evaluation of Testicular Stiffness in Patients with Varicocele. Egypt. J. Radiol. Nucl. Med. 2021, 52, 273. [Google Scholar] [CrossRef]
- Bitkin, A.; Başak Ozbalci, A.; Aydin, M.; Keles, M.; Akgunes, E.; Atilla, M.K.; Irkilata, L. Effects of Varicocele on Testicles: Value of Strain Elastography: A Prospective Controlled Study. Andrologia 2019, 51, e13161. [Google Scholar] [CrossRef]
- Dede, O.; Teke, M.; Daggulli, M.; Utangaç, M.; BAŞ, O.; Penbegül, N. Elastography to Assess the Effect of Varicoceles on Testes: A Prospective Controlled Study. Andrologia 2016, 48, 257–261. [Google Scholar] [CrossRef]
- Jedrzejewski, G.; Osemlak, P.; Wieczorek, A.P.; Nachulewicz, P. Prognostic Values of Shear Wave Elastography in Adolescent Boys with Varicocele. J. Pediatr. Urol. 2019, 15, 223.e1–223.e5. [Google Scholar] [CrossRef] [PubMed]
- Kucukdurmaz, F.; Sarica, M.A.; Emre, O.; Baykara, M.; Kizildag, B.; Resim, S. Evaluation of the Diagnostic Efficacy of Strain Elastography in Infertile Population with Normal and Abnormal Semen Parameters. Türk Urol. Derg./Turk. J. Urol. 2017, 43, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, H.; Durmaz, M.S.; Özbakır, B.; Cebeci, H.; Özkan, D.; Gökmen, İ.E. Experience of Using Shear Wave Elastography in Evaluation of Testicular Stiffness in Cases of Male Infertility. J. Ultrasound 2020, 23, 529–534. [Google Scholar] [CrossRef] [PubMed]
| Group | Age (Year) | Volume (mL) | SWE Value (kPa) |
|---|---|---|---|
| A | 36.27 ± 14.51 | 21.07 ± 3.69 | 10.42 ± 2.50 |
| B | 38.52 ± 15.60 | 23.37 ± 4.62 | 7.73 ± 2.13 |
| C | 39.09 ± 14.00 | 23.94 ± 4.17 | 8.09 ± 1.59 |
| A + B + C | 38.21 ± 14.48 | 23.05 ± 4.31 | 8.64 ± 2.26 |
| Semen Analysis | Testicular Volume (mL) | SWE (kPa) |
|---|---|---|
| Oligospermic (n = 16) | 19.45 ± 2.89 | 10.99 ± 1.45 |
| Normospermic (n = 27) | 21.02 ± 4.08 | 10.10 ± 2.18 |
| p | 0.184 | 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salbas, A.; Büyüktoka, R.E. Testicular Stiffness and Volume in Varicocele Patients: A Prospective Comparative Shear Wave Elastography Study. Diagnostics 2025, 15, 2150. https://doi.org/10.3390/diagnostics15172150
Salbas A, Büyüktoka RE. Testicular Stiffness and Volume in Varicocele Patients: A Prospective Comparative Shear Wave Elastography Study. Diagnostics. 2025; 15(17):2150. https://doi.org/10.3390/diagnostics15172150
Chicago/Turabian StyleSalbas, Ali, and Raşit Eren Büyüktoka. 2025. "Testicular Stiffness and Volume in Varicocele Patients: A Prospective Comparative Shear Wave Elastography Study" Diagnostics 15, no. 17: 2150. https://doi.org/10.3390/diagnostics15172150
APA StyleSalbas, A., & Büyüktoka, R. E. (2025). Testicular Stiffness and Volume in Varicocele Patients: A Prospective Comparative Shear Wave Elastography Study. Diagnostics, 15(17), 2150. https://doi.org/10.3390/diagnostics15172150






