Abstract
Background/Objective: Lung ultrasound (LUS) is a valuable tool for detecting pulmonary conditions, but its accuracy depends on user expertise. This study evaluated whether an artificial intelligence (AI) tool could improve clinician performance in detecting pleural effusion and consolidation/atelectasis on LUS scans. Methods: In this multi-reader, multi-case study, 14 clinicians of varying experience reviewed 374 retrospectively selected LUS scans (cine clips from the PLAPS point, obtained using three different probes) from 359 patients across six centers in the U.S. and Canada. In phase one, readers scored the likelihood (0–100) of pleural effusion and consolidation/atelectasis without AI. After a 4-week washout, they re-evaluated all scans with AI-generated bounding boxes. Performance metrics included area under the curve (AUC), sensitivity, specificity, and Fleiss’ Kappa. Subgroup analyses examined effects by reader experience. Results: For pleural effusion, AUC improved from 0.917 to 0.960, sensitivity from 77.3% to 89.1%, and specificity from 91.7% to 92.9%. Fleiss’ Kappa increased from 0.612 to 0.774. For consolidation/atelectasis, AUC rose from 0.870 to 0.941, sensitivity from 70.7% to 89.2%, and specificity from 85.8% to 89.5%. Kappa improved from 0.427 to 0.756. Conclusions: AI assistance enhanced clinician detection of pleural effusion and consolidation/atelectasis in LUS scans, particularly benefiting less experienced users.
1. Introduction
Over the past decade, lung ultrasound (LUS) has become a valuable bedside tool, particularly in emergency departments (EDs) and intensive care units (ICUs) [1,2]. It offers a radiation-free, low-cost, rapid, and portable method for real-time assessment of pulmonary structures [3,4,5]. Compared to chest radiography, LUS demonstrates higher sensitivity and similar specificity for detecting conditions such as pleural effusion, pneumonia, and pulmonary edema [6,7,8,9,10]. The Bedside Lung Ultrasound in Emergency (BLUE) protocol [11] provides a standardized LUS approach in critical care, delivering over 90% diagnostic accuracy for pneumonia, pneumothorax, pulmonary edema, and pulmonary embolism, all in under three minutes [12].
Ultrasound is particularly effective in visualizing pleural fluid. Simple effusions appear anechoic, while complex effusions (e.g., chronic, malignant, hemithorax, empyema) often present with heterogeneous features [13,14]. LUS is superior to chest radiography in detecting and evaluating the type of pleural effusions [7], aiding in interventions like drainage or more aggressive treatments [4,7].
Lung consolidation and sometimes gross atelectasis can cause the lung to appear as solid, non-aerated tissue on ultrasound, resembling the liver, a phenomenon known as hepatization [4]. In these conditions, the normally air-filled lung becomes visible as it becomes a dense and hypoechoic structure [4]. Lung consolidation can result from diseases such as pneumonia, infarction, or tumor infiltration. Atelectasis, which involves the collapse or loss of air in parts or all of the lungs, can be caused by compressive forces (e.g., a large pleural effusion) or internal airway blockages [15].
As LUS use expands, many clinicians advocate for its routine application in ICUs to reduce reliance on chest radiographs, which carry increased costs and cumulative radiation exposure, particularly in pediatric populations [5,16]. However, LUS image quality and diagnostic accuracy remain highly dependent on user expertise. AI has emerged as a potential solution, with tools capable of guiding scan acquisition and detecting conditions such as pleural effusion and consolidation in real time [17,18,19,20,21]. Real-time AI assistance in interpreting LUS scans for conditions like pleural effusion and consolidation/atelectasis could improve accuracy, reliability, and efficiency, regardless of the user’s expertise.
Previous studies have demonstrated the potential of deep learning (DL) models in diagnosing conditions specific to lung ultrasound (LUS). Arntfield et al. developed a DL model that could distinguish between A-line and B-line artifacts [22]. Ebadi et al. proposed a multiclass DL model capable of classifying A-line, B-line, and pleural effusion or consolidation [23]. Shang et al. showed that such models could improve radiologists’ accuracy in identifying COVID-19 pneumonia on ultrasound images [24]. Chaudhary et al. introduced Pleff-Net, a DL model specifically designed to detect pleural effusions of varying sizes [25]. Hong et al. developed a model that improved radiologists’ accuracy for the detection of pleural effusion and consolidation [17]; however, their evaluation was limited to only two radiologists, raising questions about generalizability. More comprehensive reviews of state-of-the-art AI applications in LUS are available in recent survey papers [26,27].
Despite promising results, a key limitation across the existing literature is that most models are evaluated as stand-alone systems, without integration into clinical workflows or real-time interaction with human users. No prior work has employed a multi-reader, multi-case (MRMC) design to systematically assess how AI tools influence diagnostic performance across clinicians with different levels of ultrasound expertise.
This represents an important gap that remains underexplored, especially since LUS is routinely performed in real-world settings by users with varying levels of expertise. To address this gap, the present study investigates whether an AI-based computer-aided detection (CADe) system can enhance diagnostic accuracy for pleural effusion, consolidation, or atelectasis in LUS, specifically through an MRMC study involving 14 readers with varying levels of training in LUS from novice to experts.
2. Materials and Methods
2.1. Study Design
We retrospectively collected 465 LUS scans from 359 patients, aged 18 to 75, across six United States and Canadian centers between January 2019 and July 2024. Lung scans from these external centers were entirely segregated from the data used for internal training/tuning of the AI algorithm. Cases were selected consecutively in the order they were received. Following the BLUE protocol, only PLAPS (Posterolateral Alveolar and/or Pleural Syndrome) point images, known to be sensitive to detecting pleural effusion and consolidation, were included. The dataset was then enriched to include at least 30% abnormal cases per center to ensure sufficient representation of clinically relevant conditions. Scans were acquired using curvilinear, phased-array, or portable transducers (1.5–7 MHz) with Exo Iris (86 scans), Sonosite X-Porte (253 scans), and Philips Lumify (126 scans). Scans that did not meet the quality standards of the American College of Emergency Physicians (ACEP) or the American Institute of Ultrasound in Medicine (AIUM) were excluded. For the MRMC study, a random subset of 374 cases was selected, consisting of 185 positive and 189 negative cases for pleural effusion, and 185 positive and 189 negative cases for consolidation or atelectasis.
The 14 readers selected for this MRMC study represented a range of expertise, from specialists (trained emergency physicians with fellowship training in LUS) to less experienced users (medical students who received targeted training to identify the relevant pathologies for this study). This diversity was intentionally chosen to reflect the spectrum of potential users and to assess the impact of AI assistance more effectively.
2.2. Selection of Participants
The 14 readers participated in two separate reading sessions: one without AI assistance and one with AI assistance, separated by a four-week washout period. In each session, readers assigned a score from 0 to 100 to indicate the likelihood of the condition (either pleural effusion or consolidation/atelectasis) being present in the scan. The first session was conducted without assistance, while the second session incorporated AI-generated bounding boxes provided by the Exo Lung AI (iris 2.5).
For subgroup analysis, readers were divided into three categories based on expertise: novices, experienced, and experts. Novices were medical students with some LUS exposure (R13, R14). Experienced users included residents and physicians who completed extensive didactic and hands-on LUS training during residency and actively use LUS in practice (R2, R5, R6, R7, R8, R11, R12), exceeding ACEP educational standards. Experts were physicians with LUS fellowship training or board certification and years of experience (R1, R3, R4, R9, R10).
2.3. Inclusion/Exclusion Criteria
Inclusion: Adult patients aged above 18 years underwent lung ultrasound at one of six participating centers. Scans were included if they captured the PLAPS point in accordance with the BLUE protocol. Cases were collected consecutively as they became available and were later enriched to ensure at least 30% abnormal cases per site, improving representation of diagnostic conditions relevant to the study aims.
Exclusion criteria were limited to scan quality: images were excluded if they failed to meet quality benchmarks defined by ACEP or AIUM; typically, this would be images with large rib shadows or inappropriate depth (too shallow or too deep) that result in poor visibility of the lung or diaphragm.
The final study dataset used for the MRMC analysis was a randomized selection of 374 cases from the eligible pool, with balanced numbers of positive and negative findings for each target condition (pleural effusion, consolidation/atelectasis).
2.4. Exo Lung AI Software
The system uses a U-Net style architecture [28] for multi-class segmentation to identify pleural effusion and consolidation/atelectasis across individual frames. In parallel, a landmark detection model identifies key anatomical structures of the lung, such as the diaphragm, liver/spleen, and kidney. Per-frame outputs are then stored and processed collectively at the clip level. During this post-processing phase, a contour validation algorithm assesses the validity of the predicted effusion and consolidation masks based on their relative position to the anatomical structures. A clip is classified as positive for a condition if at least one frame contains a validated contour for the corresponding pathology. The algorithm selects the frame with the largest valid pathological contour for the region of interest placement. The box of the region of interest is then overlaid on the corresponding image and shown to readers during interpretation. An example of these bounding boxes is shown in Figure 1.
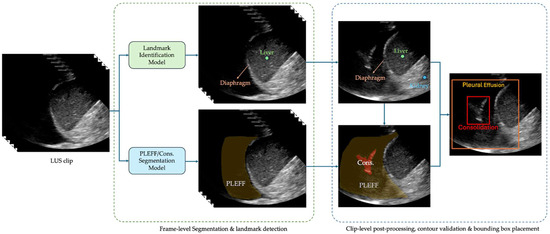
Figure 1.
An example of the produced bounding boxes for pleural effusion and consolidation/atelectasis.
2.5. Ground Truthing
Three board-certified experts, either radiologists or emergency physicians with a minimum of 10 years of experience and more than 5000 prior LUS reviews, established the ground truth labels for each ultrasound image based solely on the LUS data, without access to any additional information such as CT scans or X-rays. Two experts independently analyzed the scans, assigning one binary label for pleural effusion and another for consolidation or atelectasis (1 indicating presence, 0 indicating absence). In cases of disagreement, a third expert served as a tiebreaker to determine the final label.
2.6. Outcomes
The primary objective is to assess Exo Lung AI’s ability to assist clinicians and healthcare professionals in interpreting LUS scans for detecting pleural effusion and consolidation/atelectasis. Subgroup analysis by expertise level was a secondary outcome. Agreement among clinicians and healthcare professionals was also measured before and after AI assistance.
2.7. Analysis
Statistical analysis was performed using iMRMC software [29] to estimate mean AUC differences, p-values, and confidence intervals within an MRMC framework. A two-sided p-value < 0.02 was used to reject the null hypothesis of no AUC difference. The same criteria applied to the experience-based subgroup analysis. A mean AUC difference > 0.02 indicated successful performance. Sensitivity and specificity differences were analyzed using one-sided permutation tests in Python’s SciPy library [30], testing the null hypothesis that the mean difference (aided minus unaided) was less than or equal to zero, based on 100,000 permutations. p-values < 0.02 were considered significant. Confidence intervals were computed in R using the DescTools package [31] with 100,000 BCa bootstrap resamples. Fleiss’ Kappa, calculated using Python’s Statsmodels library [32], assessed inter-rater reliability; improvement and values > 0.6 indicated successful agreement.
3. Results
3.1. Characteristics of Study Subjects
465 LUS scans were collected from 359 patients aged 18 to 75 years (mean age 38.8 ± 14.5 years; 15 percent female, 17 percent male, remainder unspecified) across six United States and Canadian centers between January 2019 and July 2024. All scans were from the PLAPS point, selected consecutively, and following the BLUE protocol [11].
3.2. Main Results
The Exo Lung AI algorithm showed high accuracy compared to the ground truth from three experts. For pleural effusion detection, sensitivity was 0.967 (95% CI: 0.94–0.99) and specificity was 0.912 (95% CI: 0.87–0.95). For consolidation/atelectasis detection, sensitivity was 0.968 (95% CI: 0.94–0.99) and specificity was 0.945 (95% CI: 0.91–0.98). The analysis was conducted on a per-image basis. Subgroup analyses confirmed consistent performance across centers, devices, and patient sex, with sensitivity and specificity remaining above 0.94 and 0.83 in all subgroups (Table 1).

Table 1.
Subgroup analyses of the AI results compared to the ground truth.
The study compared human readers’ performance in detecting pleural effusion with and without AI assistance. The AUC-ROC improved from 0.917 to 0.960 (ΔAUC = 0.044; p < 0.01; 95% CI: 0.0142–0.0729), indicating a statistically significant gain (Figure 2A). Sensitivity increased from 77.3% to 89.1% (ΔSe = 11.8%; p < 0.01; 95% CI: [5.9%, 18.6%]), and specificity slightly improved from 91.7% to 92.9% (ΔSp = 1.1%; p < 0.31; 95% CI: [−1.9%, 4.5%]). AUC-ROC improved in 13 of 14 readers, with 9 showing >2% improvement (Figure 3A). Fleiss Kappa rose from 0.612 to 0.774, reflecting better inter-reader agreement with AI aid. It should be noted that Fleiss Kappa was calculated by converting the readers’ scores into binary labels (positive/negative) using a threshold of 50.
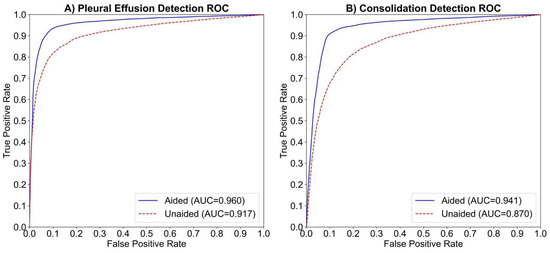
Figure 2.
The average ROC curves and AUCs for the aided and unaided readings of 14 readers in detecting (A) pleural effusion and (B) consolidation/atelectasis, respectively. The dashed red line represents the average ROC curve for unaided readings, while the solid blue line represents the average ROC curve for aided readings.
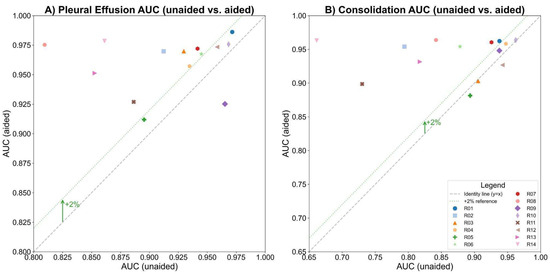
Figure 3.
The AUCs before and after AI assistance for each reader in detecting (A) pleural effusion and (B) consolidation/atelectasis, respectively, with the gray dashed line indicating no change and the green dotted line marking a 2% AUC improvement threshold.
The study compared human readers’ performance in detecting consolidation/atelectasis with and without AI assistance. The AUC-ROC improved from 0.87 to 0.941 (ΔAUC = 0.071; p < 0.01; 95% CI: 0.0221–0.1200), showing a statistically significant gain (Figure 2B). Sensitivity increased from 70.7% to 89.2% (ΔSe = 18.4%; p < 0.001; 95% CI: [10.6%, 27.5%]), and specificity improved from 85.8% to 89.5% (ΔSp = 3.7%; p < 0.11; 95% CI: [−0.005%, 7.5%]). AUC-ROC improved in 11 of 14 readers, with 8 showing >2% improvement (Figure 3B). Inter-reader agreement, measured using Fleiss Kappa, rose from 0.427 to 0.756 after AI assistance.
In MRMC subgroup analyses at a 95% significance level (ɑ = 0.05), AUC improved significantly in pleural effusion detection by 10.8% for novices (p < 0.001) and 5% for experienced readers (p < 0.02), with all subgroups reaching about 0.96 after AI-assisted reading (Figure 4A). Similarly, in consolidation/atelectasis detection, AUC improved significantly by 20.8% for novices and 7.6% for experienced readers (both p < 0.01), with all subgroups reaching about 0.94 post-AI assistance (Figure 4B).
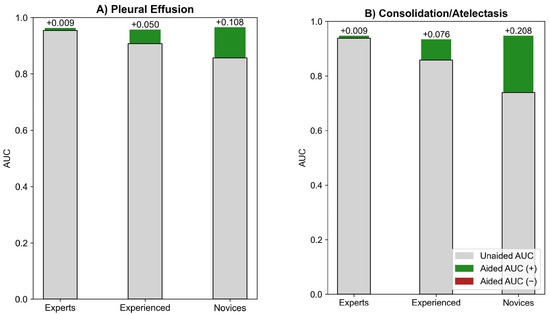
Figure 4.
MRMC experience-based subgroup analysis for (A) pleural effusion and (B) consolidation/atelectasis detection.
The radar chart comparing unaided and AI-aided sensitivity and specificity for detecting pleural effusion and consolidation/atelectasis per reader is presented in Figure 5.
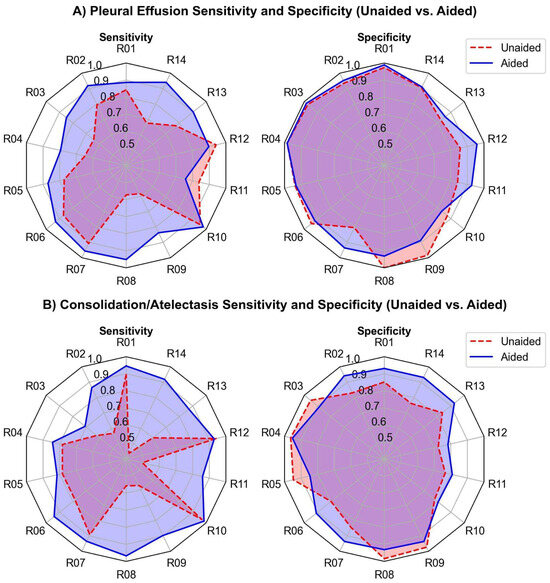
Figure 5.
Sensitivity and specificity (unaided vs. aided) for all 14 readers in detecting (A) pleural effusion and (B) consolidation/atelectasis. The dashed red and solid blue lines represent the unaided and aided reading results, respectively.
4. Discussion
In this study, human readers of varying experience showed substantially improved performance in detecting pleural effusion and consolidation/atelectasis on previously obtained LUS images when they were provided with AI assistance. In our MRMC analysis, the AI assistance significantly improves sensitivity, particularly for less experienced readers, while slightly improving specificity.
AUC-ROC analysis showed significant improvements in detecting pleural effusion (4.4%) and consolidation/atelectasis (7.1%). Both novice and experienced users benefited, with performance approaching expert level. Exo Lung AI significantly increased average sensitivity, with gains of over 11.8% for pleural effusion and 18.4% for consolidation/atelectasis. Specificity improved modestly, as baseline levels were already high (over 90% for pleural effusion and 85% for consolidation). With AI, both sensitivity and specificity reached approximately 90%. MRMC analysis highlighted greater gains in sensitivity, which is crucial for accurately identifying true cases in clinical care.
There is a body of work on AI systems for interpreting lung images, including LUS or chest radiography [33,34,35,36,37,38]. To the best of our knowledge, our study stands as the first of its kind in terms of the data modality and study design. Several AI systems have been developed to classify ultrasound images by detecting conditions such as pleural effusion or consolidation [17,39,40]. For example, one validated deep learning (DL) model distinguished normal (A-line) from abnormal (B-line) lung patterns on LUS with 93% sensitivity and 96% specificity across 400 clips [41]. Another model diagnosed absent lung sliding on 241 LUS scans, achieving 92.1% sensitivity, 80.2% specificity, and 88.5% accuracy [42]. In a study with a similar design, the impact of an AI assistant on users with diverse backgrounds in identifying different abnormal findings from chest radiographs was examined [36]. In the study described [36], 12 readers reviewed half of the radiographs with AI assistance and half without. AI raised sensitivity by 8.5% and 14%, and specificity by 3.9% and 2.9%, for pleural effusion and consolidation, respectively. In another study [38], an experience-based subgroup analysis showed unaided accuracy ranged from 68.7% (beginners) to 95% (experts) for key LUS lung findings, including pleural effusion and consolidation. They also demonstrated that the accuracy of 57 non-expert users in identifying similar lung findings in LUS clips increased from 68.9% to 82.9% after using their AI-based software [38]. The body of work presented just prior, including both the design elements and general findings, provides a foundation and context that guide the study design outlined in this paper. These findings are also broadly in line with the existing literature, further supporting the relevance of the methodological choices made in this work.
These results indicate that the integration of Exo Lung AI assistance not only enhances the detection capabilities of clinicians but also contributes to more reliable and accurate identification of lung conditions, thereby improving overall diagnostic performance.
Strengths and Limitations
The study included 14 clinical users with varying training and experience, reflecting the typical range in point-of-care settings. A strength is the use of data from multiple ultrasound device manufacturers.
This study also has limitations. One limitation is that the analysis was performed per clip rather than per patient, as the tool is designed to flag individual clips needing closer attention. Including data from multiple lung regions could enhance detection accuracy and comprehensiveness.
While multi-center data was used, it is important to note that data is limited to a North American population. Including populations from other continents would be of value in the future. Thirdly, no external reference standard (such as X-ray or CT scan) was used. For future studies, we will incorporate cross-validation using gold-standard modalities such as chest X-ray or CT to enhance diagnostic certainty. AI can also support other aspects of the workflow, such as flagging poor-quality images [20] (done manually in this study), providing segmentation masks for conditions and organs, guiding image acquisition, or enhancing information during diagnosis [43]. While the proposed AI algorithm improved diagnostic performance, its outputs should be interpreted as decision support. AI is not there to replace clinical judgment, so we can avoid potential over-reliance or misinterpretation.
Finally, the algorithm was evaluated solely on clips obtained from the PLAPS point. While clinically relevant, this can limit generalization for full lung assessments. For future iterations of the study, we aim to conduct multi-view validation.
5. Conclusions
This study found that AI algorithms for the detection of pleural effusion and consolidation/atelectasis in LUS scans enhanced the performance of both novice users and highly trained healthcare providers, particularly by improving sensitivity for these pathologies. Since most clinicians have minimal training in interpreting LUS, our model can be instrumental in upskilling clinicians at the point-of-care and facilitating easier integration of this solution into clinical care. The deployment of such systems alongside existing Point-of-Care Ultrasound (POCUS) solutions can improve patients’ clinical care in current healthcare settings, reduce the need for ionizing radiation, and eventually lower healthcare costs.
Author Contributions
Conceptualization, S.E.S.B. and M.D.; methodology, S.E.S.B. and M.D.; software, S.E.S.B.; validation, S.E.S.B. and M.D.; formal analysis, S.E.S.B.; investigation, All authors; resources, B.B. and J.L.J.; writing—original draft preparation, All authors; writing—review and editing, All authors; visualization, M.D.; supervision, D.Z.; project administration, D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Exo supported the study by providing the AI platform used and covering the cost of publication.
Institutional Review Board Statement
Our retrospective study received approval from the health research ethics boards at the participating center (University of Alberta Health Research Ethics Board-Health Panel Pro00100068 and Pro00147192) with an approval date of 5 November 2024. All patient data were anonymized and kept secure throughout the research.
Informed Consent Statement
This study was approved by the Ethics Board at the University of Alberta. A waiver of informed consent was granted as the study involved retrospective analysis of anonymized lung ultrasound scans obtained during routine clinical care.
Data Availability Statement
The dataset presented in this paper is not publicly available due to privacy concerns and the restrictions outlined in the data sharing agreement the authors have with the medical institution that obtained the data.
Acknowledgments
We would like to thank Amir Safarpoor for his valuable comments on the draft of this article.
Conflicts of Interest
Authors S.E.S.B., M.D., M.N., D.Z., and A.N. are employees of Exo Imaging. Authors J.L.J., and J.K. hold equity in Exo Imaging but receive no salary or other compensation. No other disclosures were reported.
References
- Mojoli, F.; Bouhemad, B.; Mongodi, S.; Lichtenstein, D. Lung Ultrasound for Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2019, 199, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Hayward, S.A.; Innes, S.M.; Miller, A.S.C. Point-of-care lung ultrasound in patients with COVID-19—A narrative review. Anaesthesia 2020, 75, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Zieleskiewicz, L.; Cornesse, A.; Hammad, E.; Haddam, M.; Brun, C.; Vigne, C.; Meyssignac, B.; Remacle, A.; Chaumoitre, K.; Antonini, F.; et al. Implementation of lung ultrasound in polyvalent intensive care unit: Impact on irradiation and medical cost. Anaesth. Crit. Care Pain. Med. 2015, 34, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Marini, T.J.; Rubens, D.J.; Zhao, Y.T.; Weis, J.; O’Connor, T.P.; Novak, W.H.; Kaproth-Joslin, K.A. Lung Ultrasound: The Essentials. Radiol. Cardiothorac. Imaging 2021, 3, e200564. [Google Scholar] [CrossRef]
- Bouhemad, B.; Zhang, M.; Lu, Q.; Rouby, J.J. Clinical review: Bedside lung ultrasound in critical care practice. Crit. Care 2007, 11, 205. [Google Scholar] [CrossRef]
- Zaki, H.A.; Albaroudi, B.; Shaban, E.E.; Shaban, A.; Elgassim, M.; Almarri, N.D.; Basharat, K.; Azad, A.M. Advancement in pleura effusion diagnosis: A systematic review and meta-analysis of point-of-care ultrasound versus radiographic thoracic imaging. Ultrasound J. 2024, 16, 3. [Google Scholar] [CrossRef]
- Alzahrani, S.A.; Al-Salamah, M.A.; Al-Madani, W.H.; Elbarbary, M.A. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit. Ultrasound J. 2017, 9, 6. [Google Scholar] [CrossRef]
- Maw, A.M.; Hassanin, A.; Ho, P.M.; McInnes, M.D.F.; Moss, A.; Juarez-Colunga, E.; Soni, N.J.; Miglioranza, M.H.; Platz, E.; DeSanto, K.; et al. Diagnostic Accuracy of Point-of-Care Lung Ultrasonography and Chest Radiography in Adults with Symptoms Suggestive of Acute Decompensated Heart Failure: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e190703. [Google Scholar] [CrossRef] [PubMed]
- Balk, D.S.; Lee, C.; Schafer, J.; Welwarth, J.; Hardin, J.; Novack, V.; Yarza, S.; Hoffmann, B. Lung ultrasound compared to chest X-ray for diagnosis of pediatric pneumonia: A meta-analysis. Pediatr. Pulmonol. 2018, 53, 1130–1139. [Google Scholar] [CrossRef]
- Chavez, M.A.; Shams, N.; Ellington, L.E.; Naithani, N.; Gilman, R.H.; Steinhoff, M.C.; Santosham, M.; Black, R.E.; Price, C.; Gross, M.; et al. Lung ultrasound for the diagnosis of pneumonia in adults: A systematic review and meta-analysis. Respir. Res. 2014, 15, 50. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A. BLUE-protocol and FALLS-protocol: Two applications of lung ultrasound in the critically ill. Chest 2015, 147, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Brogi, E.; Gargani, L.; Bignami, E.; Barbariol, F.; Marra, A.; Forfori, F.; Vetrugno, L. Thoracic ultrasound for pleural effusion in the intensive care unit: A narrative review from diagnosis to treatment. Crit. Care 2017, 21, 325. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Goldstein, I.; Mourgeon, E.; Cluzel, P.; Grenier, P.; Rouby, J.J. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004, 100, 9–15. [Google Scholar] [CrossRef]
- Benson, R.E.C. Lung Cancer: Atelectasis and Consolidation In Chest Imaging; Oxford University Press: Oxford, UK, 2019; pp. 269–273. [Google Scholar]
- Gargani, L.; Picano, E. The risk of cumulative radiation exposure in chest imaging and the advantage of bedside ultrasound. Crit. Ultrasound J. 2015, 7, 4. [Google Scholar] [CrossRef]
- Hong, D.; Choi, H.; Hong, W.; Kim, Y.; Kim, T.J.; Choi, J.; Ko, S.B.; Park, C.M. Deep-learning model accurately classifies multi-label lung ultrasound findings, enhancing diagnostic accuracy and inter-reader agreement. Sci. Rep. 2024, 14, 22228. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lin, Y.; Cao, P.; Zou, X.; Qin, Q.; Lin, Z.; Liang, F.; Li, Z. Automated detection and segmentation of pleural effusion on ultrasound images using an Attention U-net. J. Appl. Clin. Med. Phys. 2024, 25, e14231. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, D.; Wang, A.; Antico, M.; Steffens, M.; Ruvinov, I.; van Sloun, R.J.; van Sloun, R.J.; Canty, D.; Royse, A.; Royse, C. Automatic deep learning-based pleural effusion segmentation in lung ultrasound images. BMC Med. Inf. Decis. Mak. 2023, 23, 274. [Google Scholar] [CrossRef]
- Baloescu, C.; Bailitz, J.; Cheema, B.; Agarwala, R.; Jankowski, M.; Eke, O.; Liu, R.; Nomura, J.; Stolz, L.; Gargani, L.; et al. Artificial Intelligence-Guided Lung Ultrasound by Nonexperts. JAMA Cardiol. 2025, 10, 245–253. [Google Scholar] [CrossRef]
- Marchi, G.; Mercier, M.; Cefalo, J.; Salerni, C.; Ferioli, M.; Candoli, P.; Gori, L.; Cucchiara, F.; Cenerini, G.; Guglielmi, G.; et al. Advanced imaging techniques and artificial intelligence in pleural diseases: A narrative review. Eur. Respir. Rev. 2025, 34, 240263. [Google Scholar] [CrossRef]
- Arntfield, R.; Wu, D.; Tschirhart, J.; VanBerlo, B.; Ford, A.; Ho, J.; McCauley, J.; Wu, B.; Deglint, J.; Chaudhary, R.; et al. Automation of lung ultrasound interpretation via deep learning for the classification of normal versus abnormal lung parenchyma: A multicenter study. Diagnostics 2021, 11, 2049. [Google Scholar] [CrossRef] [PubMed]
- Erfanian Ebadi, S.; Krishnaswamy, D.; Bolouri, S.E.S.; Zonoobi, D.; Greiner, R.; Meuser-Herr, N.; Jaremko, J.L.; Kapur, J.; Noga, M.; Punithakumar, K. Automated detection of pneumonia in lung ultrasound using deep video classification for COVID-19. Inf. Med. Unlocked 2021, 25, 100687. [Google Scholar] [CrossRef]
- Shang, S.; Huang, C.; Yan, W.; Chen, R.; Cao, J.; Zhang, Y.; Guo, Y.; Du, G. Performance of a computer aided diagnosis system for SARS-CoV-2 pneumonia based on ultrasound images. Eur. J. Radiol. 2022, 146, 110066. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Ho, J.; Smith, D.; Hossain, S.; Hargun, J.; VanBerlo, B.; Murphy, N.; Prager, R.; Rikhraj, K.; Tschirhart, J.; et al. Diagnostic accuracy of an automated classifier for the detection of pleural effusions in patients undergoing lung ultrasound. Am. J. Emerg. Med. 2025, 90, 142–150. [Google Scholar] [CrossRef]
- Shoeibi, A.; Khodatars, M.; Jafari, M.; Ghassemi, N.; Sadeghi, D.; Moridian, P.; Khadem, A.; Alizadehsani, R.; Hussain, S.; Zare, A.; et al. Automated detection and forecasting of COVID-19 using deep learning techniques: A review. Neurocomputing 2024, 577, 127317. [Google Scholar] [CrossRef]
- Mento, F.; Khan, U.; Faita, F.; Smargiassi, A.; Inchingolo, R.; Perrone, T.; Demi, L. State of the art in lung ultrasound, shifting from qualitative to quantitative analyses. Ultrasound Med. Biol. 2022, 48, 2398–2416. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In International Conference on Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015; Springer: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Gallas, B.D.; Hillis, S.L. Generalized Roe and Metz receiver operating characteristic model: Analytic link between simulated decision scores and empirical AUC variances and covariances. J. Med. Imaging 2014, 1, 031006. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 10: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Signorell, A. DescTools: Tools for Descriptive Statistics and Exploratory Data Analysis. Github. Available online: https://github.com/AndriSignorell/DescTools (accessed on 3 June 2025).
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. SciPy 2010, 7, 92–96. [Google Scholar] [CrossRef]
- Çallı, E.; Sogancioglu, E.; van Ginneken, B.; van Leeuwen, K.G.; Murphy, K. Deep learning for chest X-ray analysis: A survey. Med. Image Anal. 2021, 72, 102125. [Google Scholar] [CrossRef]
- Baloescu, C.; Toporek, G.; Kim, S.; McNamara, K.; Liu, R.; Shaw, M.M.; McNamara, R.L.; Balasundar, R.; Moore, C.L. Automated Lung Ultrasound B-Line Assessment Using a Deep Learning Algorithm. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 2312–2320. [Google Scholar] [CrossRef]
- Durrani, N.; Vukovic, D.; van der Burgt, J.; Antico, M.; van Sloun, R.J.G.; Canty, D.; Steffens, M.; Wang, A.; Royse, A.; Royse, C.; et al. Automatic deep learning-based consolidation/collapse classification in lung ultrasound images for COVID-19 induced pneumonia. Sci. Rep. 2022, 12, 17581. [Google Scholar] [CrossRef]
- Bennani, S.; Regnard, N.E.; Ventre, J.; Lassalle, L.; Nguyen, T.; Ducarouge, A.; Dargent, L.; Guillo, E.; Gouhier, E.; Zaimi, S.H.; et al. Using AI to Improve Radiologist Performance in Detection of Abnormalities on Chest Radiographs. Radiology 2023, 309, e230860. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Maranna, S.; Watson, C.; Parange, N. A scoping review on the integration of artificial intelligence in point-of-care ultrasound: Current clinical applications. Am. J. Emerg. Med. 2025, 92, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Nhat, P.T.H.; Van Hao, N.; Tho, P.V.; Kerdegari, H.; Pisani, L.; Thu, L.N.M.; Phuong, L.T.; Duong, H.T.H.; Thuy, D.B.; McBride, A.; et al. Clinical benefit of AI-assisted lung ultrasound in a resource-limited intensive care unit. Crit. Care 2023, 27, 257. [Google Scholar] [CrossRef]
- Tsai, C.H.; van der Burgt, J.; Vukovic, D.; Kaur, N.; Demi, L.; Canty, D.; Wang, A.; Royse, A.; Royse, C.; Haji, K.; et al. Automatic deep learning-based pleural effusion classification in lung ultrasound images for respiratory pathology diagnosis. Phys. Med. 2021, 83, 38–45. [Google Scholar] [CrossRef]
- Born, J.; Wiedemann, N.; Cossio, M.; Buhre, C.; Brändle, G.; Leidermann, K.; Goulet, J.; Aujayeb, A.; Moor, M.; Rieck, B.; et al. Accelerating Detection of Lung Pathologies with Explainable Ultrasound Image Analysis. Appl. Sci. 2021, 11, 672. [Google Scholar] [CrossRef]
- Dave, C.; Wu, D.; Tschirhart, J.; Smith, D.; VanBerlo, B.; Deglint, J.; Ali, F.; Chaudhary, R.; VanBerlo, B.B.; Ford, A.B.; et al. Prospective Real-Time Validation of a Lung Ultrasound Deep Learning Model in the ICU. Crit. Care Med. 2023, 51, 301–309. [Google Scholar] [CrossRef]
- Clausdorff Fiedler, H.; Prager, R.; Smith, D.; Wu, D.; Dave, C.; Tschirhart, J.; Wu, B.; Van Berlo, B.; Malthaner, R.; Arntfield, R. Automated Real-Time Detection of Lung Sliding Using Artificial Intelligence: A Prospective Diagnostic Accuracy Study. Chest 2024, 166, 362–370. [Google Scholar] [CrossRef]
- Narang, A.; Bae, R.; Hong, H.; Thomas, Y.; Surette, S.; Cadieu, C.; Chaudhry, A.; Martin, R.P.; McCarthy, P.M.; Rubenson, D.S.; et al. Utility of a Deep-Learning Algorithm to Guide Novices to Acquire Echocardiograms for Limited Diagnostic Use. JAMA Cardiol. 2021, 6, 624–632. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).