Evaluating NT-proBNP-to-Albumin (NTAR) and RDW-to-eGFR (RGR) Ratios as Biomarkers for Predicting Hospitalization Duration and Mortality in Pulmonary Arterial Hypertension (PAH) and Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. Treatment, Management, and Disease Severity Parameters
2.4. Laboratory Assessments
2.5. Comorbidities
2.6. LOS, ELOS, and Mortality Assessment
2.7. Proposed New Biomarkers
2.7.1. RDW-SD-to-eGFR Ratio (RGR)
2.7.2. NT-proBNP-to-Albumin Ratio (NTAR)
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Study Cohort
3.2. Comorbidities
3.3. Length of Hospital Stay and Extended Length of Hospital Stay
3.4. Laboratory Data
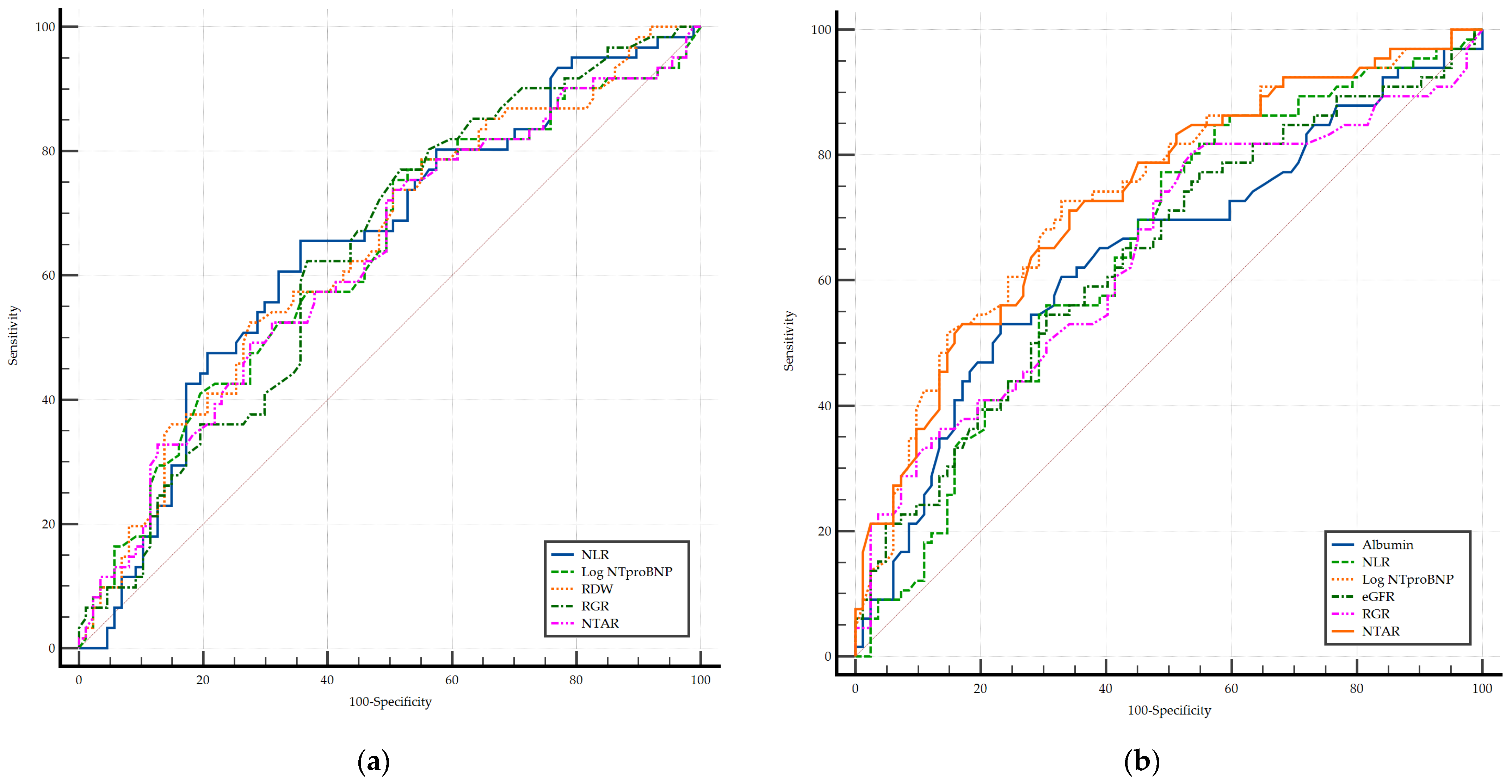
3.5. Biomarkers and LOS
3.6. Biomarkers and ELOS
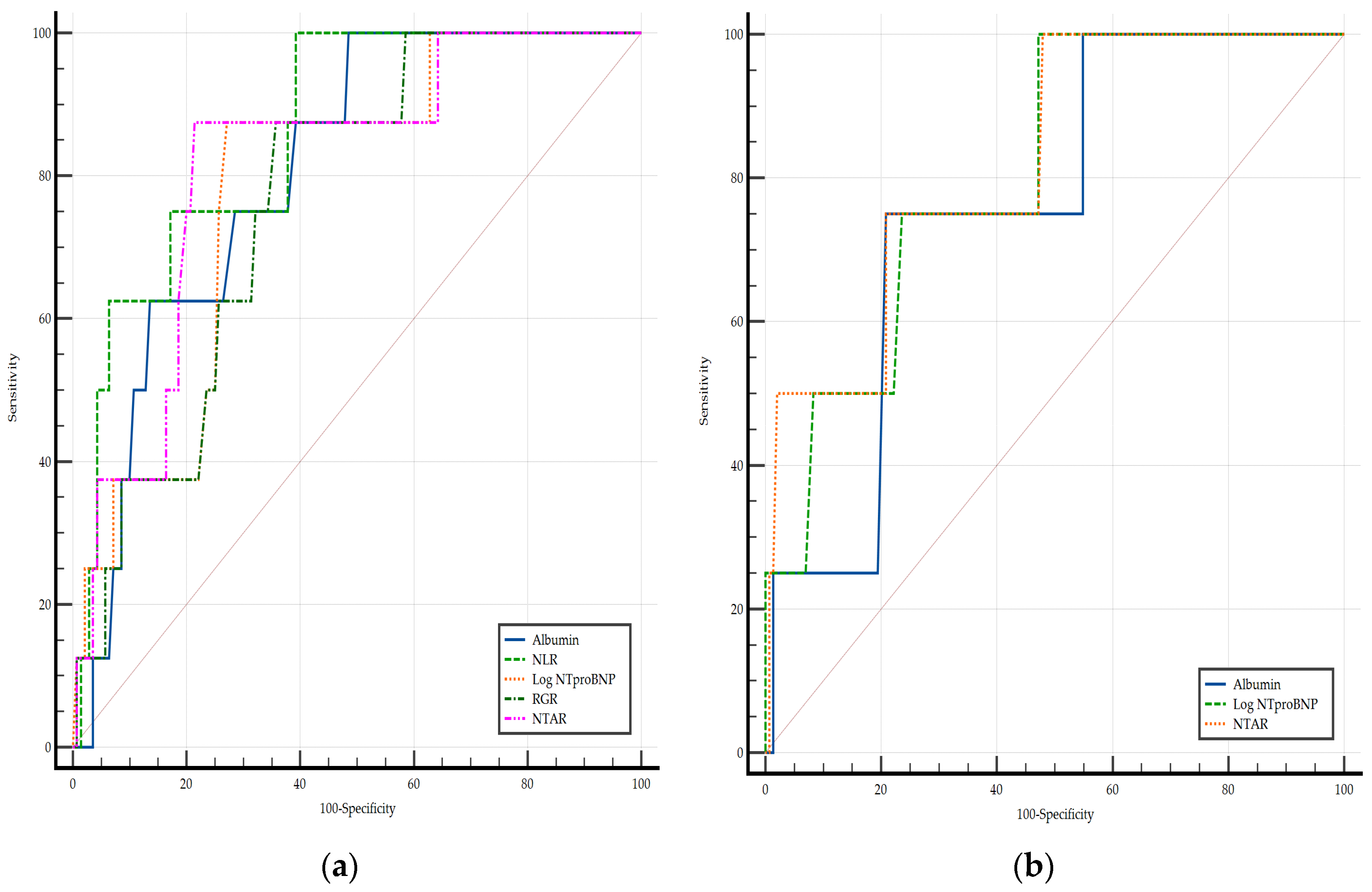
3.7. Biomarkers and In-Hospital Mortality
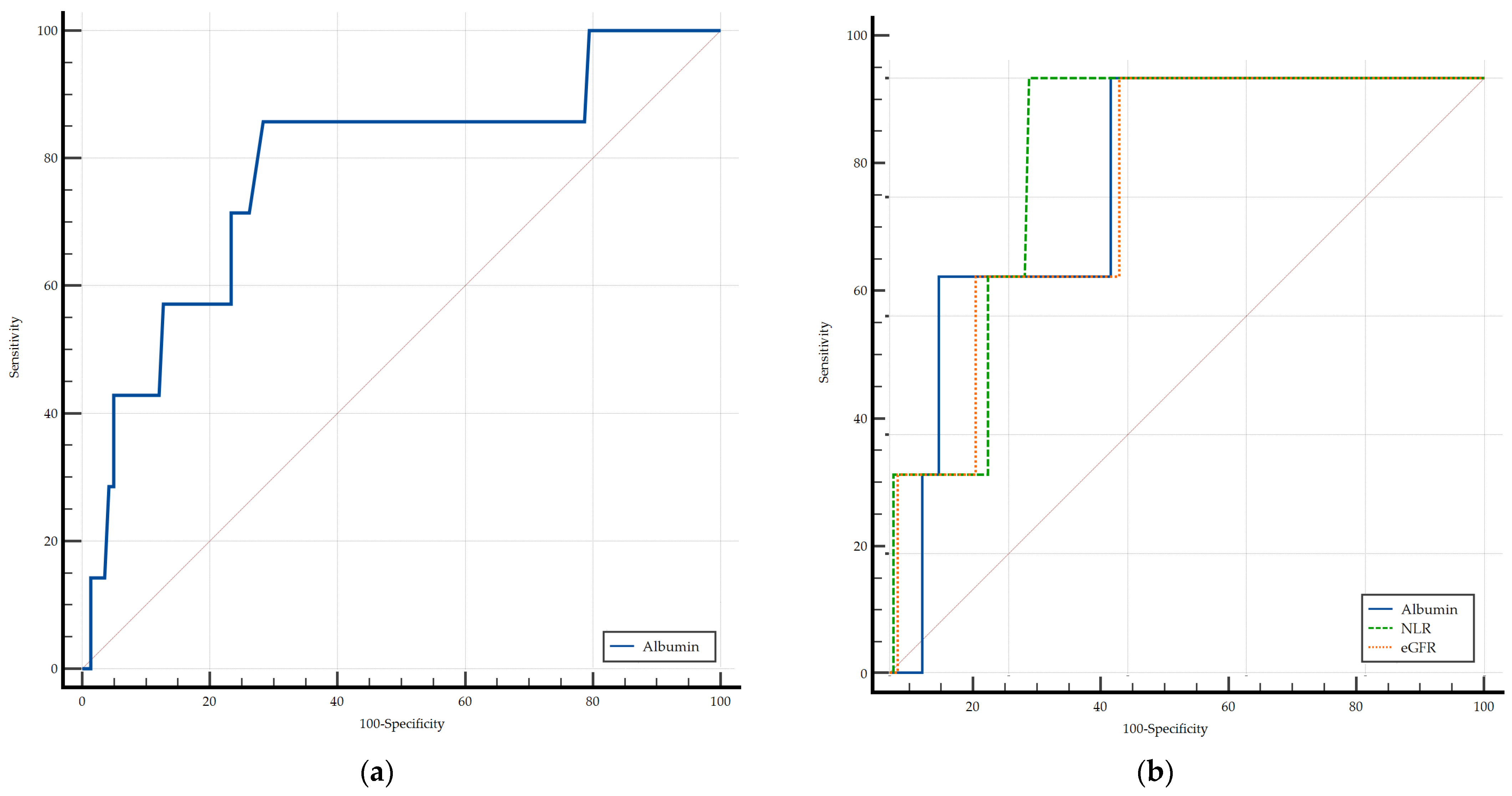
3.8. Biomarkers and 3-Month All-Cause Mortality
4. Discussion
4.1. Prognostic Utility of Conventional Biomarkers and Cut-Off Determination
4.2. RGR and NTAR in Focus: New Predictive Tools for Risk Stratification
4.3. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BMI | Body mass index |
| CHD-PAH | Pulmonary arterial hypertension associated with congenital heart disease |
| CI | Confidence interval |
| CKD | Chronic kidney disease |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| COPD | Chronic obstructive pulmonary disease |
| CTD-PAH | Pulmonary arterial hypertension associated with connective tissue disease |
| CTEPH | Chronic thromboembolic pulmonary hypertension |
| DVT | Deep vein thrombosis |
| eGFR | Estimated glomerular filtration rate |
| ELOS | Extended length of hospital stay |
| ESC/ERS | European Society of Cardiology/European Respiratory Society |
| GDPR | General Data Protection Regulation |
| IQR | Interquartile range |
| IPAH | Idiopathic pulmonary arterial hypertension |
| LOS | Length of hospital stay |
| NLR | Neutrophil-to-lymphocyte ratio |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| NTAR | NT-proBNP-to-albumin ratio |
| OR | Odds ratio |
| PAH | Pulmonary arterial hypertension |
| PH | Pulmonary hypertension |
| PoPH | Pulmonary arterial hypertension associated with portal hypertension |
| RDW | Red cell distribution width |
| REVEAL | Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management |
| RGR | Red cell distribution width-to-estimated glomerular filtration rate ratio |
| ROC | Receiver operating characteristic |
| SD | Standard deviation |
| T2DM | Type 2 diabetes mellitus |
| WHO-FC | World Health Organization functional classification |
| WBC | White blood cell |
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- GBD 2021 Pulmonary Arterial Hypertension Collaborators. Global, Regional, and National Burden of Pulmonary Arterial Hypertension, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Respir. Med. 2025, 13, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Galiè, N.; Ghofrani, H.A.; Gomez Sanchez, M.A.; Kumar, R.K.; Langleben, D.; Lincoln, D.; et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Keogh, A.; Lam, C.S.P.; Mayosi, B.M.; Provencher, S.; Reinaque, J.M.; et al. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef]
- Studer, S.; Hull, M.; Pruett, J.; Koep, E.; Tsang, Y.; Drake, W., III. Treatment Patterns, Healthcare Resource Utilization, and Healthcare Costs Among Patients with Pulmonary Arterial Hypertension in a Real-World US Database. Pulm. Circ. 2019, 9, 2045894018816294. [Google Scholar] [CrossRef]
- Dufour, R.; Pruett, J.; Hu, N.; Lickert, C.; Stemkowski, S.; Tsang, Y.; Lane, D.; Drake, W., III. Healthcare Resource Utilization and Costs for Patients with Pulmonary Arterial Hypertension: Real-World Documentation of Functional Class. J. Med. Econ. 2017, 20, 1178–1186. [Google Scholar] [CrossRef]
- Burger, C.D.; Long, P.K.; Shah, M.R.; McGoon, M.D.; Miller, D.P.; Romero, A.J.; Benton, W.W.; Safford, R.E. Characterization of First-Time Hospitalizations in Patients with Newly Diagnosed Pulmonary Arterial Hypertension in the REVEAL Registry. Chest 2014, 146, 1263–1273. [Google Scholar] [CrossRef]
- Bowman, J.K.; Dutta, S.; Zheng, H.; Kraus, C.; Wilcox, S.R. Patients with Pulmonary Hypertension Presenting to the Emergency Department. Am. J. Emerg. Med. 2020, 38, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.B.; Jackson, K.E.; Wang, C.; Siew, E.D.; Vincz, A.J.; Shaver, C.M.; Bastarache, J.A.; Ware, L.B. Linear Association Between Hypoalbuminemia and Increased Risk of Acute Respiratory Distress Syndrome in Critically III Adults. Crit. Care Explor. 2021, 3, e0527. [Google Scholar] [CrossRef] [PubMed]
- Arques, S. Serum Albumin and Heart Failure: Recent Advances on a New Paradigm. Ann. Cardiol. Angeiol. 2011, 60, 272–278. [Google Scholar] [CrossRef]
- Ebert, E.C.; Hagspiel, K.D. Gastrointestinal and Hepatic Manifestations of Systemic Lupus Erythematosus. J. Clin. Gastroenterol. 2011, 45, 436–441. [Google Scholar] [CrossRef]
- Arques, S. Human Serum Albumin in Cardiovascular Diseases. Eur. J. Intern. Med. 2018, 52, 8–12. [Google Scholar] [CrossRef]
- Snipelisky, D.; Jentzer, J.; Batal, O.; Dardari, Z.; Mathier, M. Serum Albumin Concentration as an Independent Prognostic Indicator in Patients with Pulmonary Arterial Hypertension. Clin. Cardiol. 2018, 41, 782–787. [Google Scholar] [CrossRef]
- Jutras-Beaudoin, N.; Toro, V.; Lajoie, A.C.; Breuils-Bonnet, S.; Paulin, R.; Potus, F. Neutrophil-Lymphocyte Ratio as an Independent Predictor of Survival in Pulmonary Arterial Hypertension: An Exploratory Study. CJC Open 2021, 4, 357–363. [Google Scholar] [CrossRef]
- Efros, O.; Beit Halevi, T.; Meisel, E.; Soffer, S.; Barda, N.; Cohen, O.; Kenet, G.; Lubetsky, A. The Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Patients Hospitalized with Acute Pulmonary Embolism. J. Clin. Med. 2021, 10, 4058. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Zhang, L.; Zhang, W.; Yuan, Q.; Xiao, X. Neutrophil-to-Lymphocyte Ratio Predicts Poor Prognosis in Patients with Chronic Kidney Disease-Related Pulmonary Hypertension: A Retrospective Study. Medicine 2024, 103, e40161. [Google Scholar] [CrossRef] [PubMed]
- Yogeswaran, A.; Tello, K.; Lund, J.; Klose, H.; Harbaum, L.; Sommer, N.; Oqueka, T.; Hennigs, J.K.; Grimminger, F.; Seeger, W.; et al. Risk Assessment in Pulmonary Hypertension Based on Routinely Measured Laboratory Parameters. J. Heart Lung Transplant. 2022, 41, 400–410. [Google Scholar] [CrossRef]
- Mueller, T.; Gegenhuber, A.; Dieplinger, B.; Poelz, W.; Haltmayer, M. Long-Term Stability of Endogenous B-Type Natriuretic Peptide (BNP) and Amino Terminal proBNP (NT-proBNP) in Frozen Plasma Samples. Clin. Chem. Lab. Med. 2004, 42, 942–944. [Google Scholar] [CrossRef]
- Fradley, M.G.; Larson, M.G.; Cheng, S.; McCabe, E.; Coglianese, E.; Shah, R.V.; Levy, D.; Vasan, R.S.; Wang, T.J. Reference Limits for N-Terminal-Pro-B-Type Natriuretic Peptide in Healthy Individuals (from the Framingham Heart Study). Am. J. Cardiol. 2011, 108, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ping, P.; Wang, F.; Luo, L. Synthesis, Secretion, Function, Metabolism and Application of Natriuretic Peptides in Heart Failure. J. Biol. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Fava, C.; Cattazzo, F.; Hu, Z.D.; Lippi, G.; Montagnana, M. The Role of Red Blood Cell Distribution Width (RDW) in Cardiovascular Risk Assessment: Useful or Hype? Ann. Transl. Med. 2019, 7, 581. [Google Scholar] [CrossRef]
- Petrauskas, L.A.; Saketkoo, L.A.; Kazecki, T.; Saito, S.; Jaligam, V.; deBoisblanc, B.P.; Lammi, M.R. Use of Red Cell Distribution Width in a Population at High Risk for Pulmonary Hypertension. Respir. Med. 2019, 150, 131–135. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Li, L.; Tu, X.; Lu, Z. Red Blood Cell Distribution Width Predicts Pulmonary Hypertension Secondary to Chronic Obstructive Pulmonary Disease. Can. Respir. J. 2019, 2019, 3853454. [Google Scholar] [CrossRef]
- Hampole, C.V.; Mehrotra, A.K.; Thenappan, T.; Gomberg-Maitland, M.; Shah, S.J. Usefulness of Red Cell Distribution Width as a Prognostic Marker in Pulmonary Hypertension. Am. J. Cardiol. 2009, 104, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Iancu, D.G.; Varga, A.; Cristescu, L.; Dumbrava, R.A.; Stoica, F.; Moldovan, D.A.; Suteu, R.A.; Tilea, I. Kidney Dysfunction, Hepatic Impairment, and Lipid Metabolism Abnormalities in Patients with Precapillary Pulmonary Hypertension. Diagnostics 2024, 14, 1824. [Google Scholar] [CrossRef]
- Chakinala, M.M.; Coyne, D.W.; Benza, R.L.; Frost, A.E.; McGoon, M.D.; Hartline, B.K.; Frantz, R.P.; Selej, M.; Zhao, C.; Mink, D.R.; et al. Impact of Declining Renal Function on Outcomes in Pulmonary Arterial Hypertension: A REVEAL Registry Analysis. J. Heart Lung Transplant. 2018, 37, 696–705. [Google Scholar] [CrossRef]
- Shah, S.J.; Thenappan, T.; Rich, S.; Tian, L.; Archer, S.L.; Gomberg-Maitland, M. Association of Serum Creatinine with Abnormal Hemodynamics and Mortality in Pulmonary Arterial Hypertension. Circulation 2008, 117, 2475–2483. [Google Scholar] [CrossRef]
- Lenth, R.V. Some Practical Guidelines for Effective Sample Size Determination. Am. Stat. 2001, 55, 187–193. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Çorbacıoğlu, Ş.K.; Aksel, G. Receiver operating characteristic curve analysis in diagnostic accuracy studies: A guide to interpreting the area under the curve value. Turk. J. Emerg. Med. 2023, 23, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Chen, H. Relationship between Serum Albumin Level and Hospitalization Duration following Percutaneous Coronary Intervention for Acute Coronary Syndrome. Sci. Rep. 2024, 14, 23883. [Google Scholar] [CrossRef]
- Pokhrel Bhattarai, S.; Dzikowicz, D.J.; Carey, M.G. Association Between Serum Albumin and the Length of Hospital Stay Among Patients With Acute Heart Failure. Biol. Res. Nurs. 2025, 27, 37–46. [Google Scholar] [CrossRef]
- Ling, M.; Huiyin, L.; Shanglin, C.; Haiming, L.; Zhanyi, D.; Shuchun, W.; Meng, B.; Murong, L. Relationship between Human Serum Albumin and In-Hospital Mortality in Critical Care Patients with Chronic Obstructive Pulmonary Disease. Front. Med. 2023, 10, 1109910. [Google Scholar] [CrossRef]
- Chao, P.; Cui, X.; Wang, S.; Zhang, L.; Ma, Q.; Zhang, X. Serum Albumin and the Short-Term Mortality in Individuals with Congestive Heart Failure in Intensive Care Unit: An Analysis of MIMIC. Sci. Rep. 2022, 12, 16251. [Google Scholar] [CrossRef]
- Özpelit, E.; Akdeniz, B.; Özpelit, M.E.; Tas, S.; Bozkurt, S.; Tertemiz, K.C.; Sevinç, C.; Badak, Ö. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Pulmonary Arterial Hypertension. J. Int. Med. Res. 2015, 43, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Yanartas, M.; Kalkan, M.E.; Arslan, A.; Tas, S.G.; Koksal, C.; Bekiroglu, N.; Yildizeli, B. Neutrophil/Lymphocyte Ratio Can Predict Postoperative Mortality in Patients with Chronic Thromboembolic Pulmonary Hypertension. Ann. Thorac. Cardiovasc. Surg. 2015, 21, 229–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maurer, S.J.; Habdank, V.; Hörer, J.; Ewert, P.; Tutarel, O. NT-proBNP Is a Predictor of Mortality in Adults with Pulmonary Arterial Hypertension Associated with Congenital Heart Disease. J. Clin. Med. 2023, 12, 3101. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Sakhuja, R.; O’Donoghue, M.; Baggish, A.L.; Anwaruddin, S.; Chae, C.U.; Cameron, R.; Krauser, D.G.; Tung, R.; Camargo, C.A., Jr.; et al. Utility of Amino-Terminal Pro-Brain Natriuretic Peptide Testing for Prediction of 1-Year Mortality in Patients with Dyspnea Treated in the Emergency Department. Arch. Intern. Med. 2006, 166, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Nerlekar, R.; Tranah, G.J.; Browner, W.S.; Cummings, S.R. Higher Red Cell Distribution Width and Poorer Hospitalization-Related Outcomes in Elderly Patients. J. Am. Geriatr. Soc. 2022, 70, 2354–2362. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, Q.; Guo, N.; Zhang, Z.; Luo, L.; Luo, Y.; Qin, Z.; Ge, L. Association between Red Blood Cell Distribution Width and In-Hospital Mortality in Acute Myocardial Infarction. Medicine 2021, 100, e25404. [Google Scholar] [CrossRef]
- Tonelli, M.; Sacks, F.; Arnold, M.; Moye, L.; Davis, B.; Pfeffer, M.; for the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People with Coronary Disease. Circulation 2008, 117, 163–168. [Google Scholar] [CrossRef]
- Haas, L.; Eckart, A.; Haubitz, S.; Mueller, B.; Schuetz, P.; Segerer, S. Estimated Glomerular Filtration Rate Predicts 30-Day Mortality in Medical Emergency Departments: Results of a Prospective Multi-National Observational Study. PLoS ONE 2020, 15, e0230998. [Google Scholar] [CrossRef]
- Verstreken, S.; Beles, M.; Oeste, C.L.; Moya, A.; Masuy, I.; Dierckx, R.; Heggermont, W.; Dauw, J.; Hens, D.; Bartunek, J.; et al. eGFR Slope as Predictor of Mortality in Heart Failure Patients. ESC Heart Fail. 2024, 12, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Suk, C.W.; Hsu, S.C.; Chen, C.Y.; Hsieh, H.L.; Kuo, H.T.; Hsu, Y.P.; Sue, Y.M.; Chen, T.H.; Lin, F.Y.; Shih, C.M.; et al. Point of Care eGFR and the Prediction of Outcomes in Pneumonia. Sci. Rep. 2019, 9, 8478. [Google Scholar] [CrossRef]
- Deng, X.; Gao, B.; Wang, F.; Zhao, M.H.; Wang, J.; Zhang, L. Red Blood Cell Distribution Width Is Associated With Adverse Kidney Outcomes in Patients With Chronic Kidney Disease. Front. Med. 2022, 9, 877220. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, A.; Wharton, J.; Thayer, T.E.; Swietlik, E.M.; Assad, T.R.; Desai, A.A.; Gräf, S.; Harbaum, L.; Humbert, M.; Morrell, N.W.; et al. Mendelian Randomisation Analysis of Red Cell Distribution Width in Pulmonary Arterial Hypertension. Eur. Respir. J. 2020, 55, 1901486. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Xu, S.; Zhu, Y.; Xu, S.; Wei, L.; Qian, P.; Lv, Y.; Zhang, C.; Xing, X.; et al. Prognostic Impact of Red Blood Cell Distribution Width in Pulmonary Hypertension Patients: A Systematic Review and Meta-Analysis. Medicine 2020, 99, e19089. [Google Scholar] [CrossRef]
- Smukowska-Gorynia, A.; Tomaszewska, I.; Malaczynska-Rajpold, K.; Marcinkowska, J.; Komosa, A.; Janus, M.; Olasinska-Wisniewska, A.; Slawek, S.; Araszkiewicz, A.; Jankiewicz, S.; et al. Red Blood Cells Distribution Width as a Potential Prognostic Biomarker in Patients With Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension. Heart Lung Circ. 2018, 27, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Chewcharat, A.; Mao, M.A.; Thirunavukkarasu, S.; Kashani, K.B. Impacts of Admission Serum Albumin Levels on Short-Term and Long-Term Mortality in Hospitalized Patients. QJM 2020, 113, 393–398. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Gao, L.; Zhao, Q.; Yang, T.; Zeng, Q.; Huang, Z.; Li, X.; Duan, A.; Wang, Y.; et al. Effects of Malnutrition on Disease Severity and Adverse Outcomes in Idiopathic Pulmonary Arterial Hypertension: A Retrospective Cohort Study. Respir. Res. 2024, 25, 292. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Yang, Y.H.; Zhai, Z.G.; Wang, C.; Wang, J. Red cell distribution width is increased in chronic thromboembolic pulmonary hypertension. Clin. Respir. J. 2016, 10, 54–60. [Google Scholar] [CrossRef] [PubMed]
| Parameter | PAH (n = 148) | CTEPH (n = 127) |
|---|---|---|
| Age (years, median, IQR) | 51 (42.00–67.00) | 67 (57.50–71.00) |
| Sex (female, n, %) | 84 (56.75) | 57 (44.88) |
| BMI (kg/m2, median, IQR) | 28.16 (23.20–32.3) | 26.7 (24.64–31.14) |
| WHO-FC (n, %) | ||
| I | 8 (5.41) | 8 (6.30) |
| II | 50 (33.78) | 53 (41.73) |
| III | 72 (48.65) | 46 (36.22) |
| IV | 18 (12.16) | 20 (15.75) |
| Comorbidities (n, %) | ||
| Cardiovascular Diseases | ||
| Systemic hypertension | 47 (31.76) | 51 (40.16) |
| Coronary artery disease | 11 (22.45) | 19 (14.96) |
| Atrial fibrillation | 49 (33.11) | 24 (18.90) |
| Previous deep vein thrombosis | - | 116 (91.34) |
| Metabolic Disorders | ||
| Excess body weight | 97 (65.54) | 89 (70.08) |
| T2DM | 50 (33.78) | 38 (29.92) |
| Thyroid disease | 40 (27.03) | 23 (18.11) |
| Respiratory Diseases | ||
| Obstructive sleep apnea | 14 (9.46) | 13 (10.24) |
| Asthma | 11 (7.43) | 7 (5.51) |
| COPD | 10 (6.76) | 20 (15.75) |
| Lung disease—other than COPD and asthma | 52 (35.14) | 28 (22.05) |
| History of SARS-CoV-2 infection | 62 (41.90) | 54 (42.52) |
| Length of hospital stay | ||
| LOS (days, median, IQR) | 7 (5.00–9.00) | 7 (5.00–9.00) |
| ELOS (n, %) | 61 (41.22) | 58 (45.67) |
| ELOS (days, median, IQR) | 10 (8.00–15.00) | 14 (8.00–15.75) |
| Mortality (n, %) | ||
| Overall mortality | 15 (10.14) | 7 (5.51) |
| In-hospital mortality | 8 (5.41) | 4 (3.15) |
| 3-month all-cause mortality | 7 (4.73) | 3 (2.36) |
| Parameter | PAH (n = 148) | CTEPH (n = 127) |
|---|---|---|
| Laboratory data | ||
| Neutrophils (×103/µL, mean ± SD) | 4.92 ± 2.44 | 4.88 ± 1.62 |
| Lymphocytes (×103/µL, median, IQR) | 1.56 (1.13–2.13) | 1.39 (0.97–1.77) |
| Creatinine (mg/dL, mean ± SD) | 0.98 ± 0.38 | 1.23 ± 0.52 |
| NT-proBNP (pg/mL, mean ± SD) | 3120.26 ± 3702.10 | 4534.54 ± 4926.93 |
| Prior studied biomarkers | ||
| Albumin (g/dL, median, IQR) | 4.14 (3.82–4.48) | 4.15 (3.83–4.38) |
| NLR (mean ± SD) | 3.57 ± 2.25 | 4.07 ± 2.47 |
| Log NT-proBNP (median, IQR) | 3.22 (2.60–3.68) | 3.4 (2.81–3.84) |
| RDW (fl, mean ± SD) | 51.31 ± 9.75 | 51.88 ± 8.20 |
| eGFR (ml/min/1.73 m2, median, IQR) | 85.1 (63.62–110.32) | 63.19 (47.29–90.01) |
| Proposed biomarkers | ||
| RGR (mean ± SD) | 0.85 ± 2.36 | 0.91 ± 0.50 |
| NTAR (median, IQR) | 2.62 (1.95–3.08) | 2.79 (2.21–3.23) |
| Parameters | Length of Hospital Stay (LOS) | |||
|---|---|---|---|---|
| PAH | CTEPH | |||
| r | p | r | p | |
| Prior studied biomarkers | ||||
| Albumin | −0.13 | 0.122 | −0.24 | 0.007 |
| NLR | 0.23 | 0.004 | 0.33 | <0.001 |
| Log NT-proBNP | 0.23 | 0.005 | 0.48 | <0.001 |
| RDW | 0.21 | 0.009 | 0.19 | 0.028 |
| eGFR | −0.10 | 0.216 | −0.31 | <0.001 |
| Proposed biomarkers | ||||
| RGR | 0.20 | 0.016 | 0.33 | <0.001 |
| NTAR | 0.23 | 0.005 | 0.46 | <0.001 |
| Independent Variables | Extended Length of Hospital Stay (ELOS) | |||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | p | OR (95% CI) | p | Hosmer–Lemeshow Test, p | Associated Criterion to Youden Index J | |
| PAH group | ||||||
| Prior studied biomarkers | ||||||
| Albumin | 0.579 (0.495–0.659) | 0.102 | 0.587 (0.301–1.146) | 0.118 | 0.449 | ≤4.18 |
| NLR | 0.647 (0.564–0.723) | 0.001 | 1.150 (0.989–1.338) | 0.068 | 0.095 | >3.11 |
| Log NT–proBNP | 0.630 (0.547–0.708) | 0.006 | 1.753 (1.098–2.800) | 0.018 | 0.506 | >3.00 |
| RDW | 0.642 (0.559–0.719) | 0.002 | 1.049 (1.011–1.089) | 0.010 | 0.659 | >52.10 |
| eGFR | 0.580 (0.496–0.660) | 0.096 | 0.990 (0.980–1.000) | 0.061 | 0.896 | ≤84.11 |
| Proposed biomarkers | ||||||
| RGR | 0.637 (0.554–0.715) | 0.002 | 3.722 (1.187–11.671) | 0.024 | 0.084 | >0.60 |
| NTAR | 0.627 (0.544–0.705) | 0.007 | 1.773 (1.116–2.817) | 0.015 | 0.800 | >2.37 |
| CTEPH group | ||||||
| Prior studied biomarkers | ||||||
| Albumin | 0.640 (0.550–0.723) | 0.006 | 1.031 (0.890–1.195) | 0.680 | 0.012 | ≤3.95 |
| NLR | 0.645 (0.555–0.727) | 0.003 | 1.208 (1.035–1.410) | 0.016 | 0.667 | >2.77 |
| Log NT-proBNP | 0.748 (0.664–0.821) | <0.001 | 3.947 (2.085–7.471) | <0.001 | 0.510 | >3.47 |
| RDW | 0.551 (0.460–0.639) | 0.333 | 1.030 (0.986–1.075) | 0.180 | 0.139 | >52.20 |
| eGFR | 0.645 (0.555–0.728) | 0.003 | 0.984 (0.972–0.997) | 0.014 | 0.766 | ≤60.00 |
| Proposed biomarkers | ||||||
| RGR | 0.648 (0.559–0.731) | 0.003 | 3.766 (1.582–8.960) | 0.002 | 0.309 | >0.58 |
| NTAR | 0.743 (0.658–0.817) | <0.001 | 3.641 (1.992–6.654) | <0.001 | 0.979 | >3.09 |
| Independent Variables | In-Hospital Mortality | |||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | p | OR (95% CI) | p | Hosmer–Lemeshow Test, p | Associated Criterion to Youden Index J | |
| PAH group | ||||||
| Prior studied biomarkers | ||||||
| Albumin | 0.804 (0.731–0.865) | <0.001 | 0.152 (0.037–0.619) | 0.008 | 0.490 | ≤4.16 |
| NLR | 0.858 (0.791–0.910) | <0.001 | 1.492 (1.163–1.913) | 0.001 | 0.480 | >3.28 |
| Log NT-proBNP | 0.784 (0.709–0.848) | <0.001 | 10.291 (1.490–71.065) | 0.018 | 0.498 | >3.65 |
| RDW | 0.700 (0.619–0.773) | 0.054 | 1.050 (0.993–1.111) | 0.082 | 0.018 | >58.60 |
| eGFR | 0.701 (0.621–0.774) | 0.031 | 0.974 (0.948–1.000) | 0.058 | 0.411 | ≤97.39 |
| Proposed biomarkers | ||||||
| RGR | 0.765 (0.688–0.830) | <0.001 | 1.018 (0.799–1.298) | 0.881 | 0.458 | >0.65 |
| NTAR | 0.815 (0.743–0.874) | <0.001 | 12.083 (1.743–83.748) | 0.011 | 0.093 | >3.08 |
| CTEPH group | ||||||
| Prior studied biomarkers | ||||||
| Albumin | 0.772 (0.690–0.842) | 0.010 | 0.455 (0.179–1.156) | 0.098 | 0.305 | ≤3.77 |
| NLR | 0.706 (0.619–0.784) | 0.079 | 1.197 (0.869–1.649) | 0.268 | 0.372 | >2.96 |
| Log NT-proBNP | 0.799 (0.718–0.865) | 0.005 | 13.824 (0.620–308.177) | 0.097 | 0.738 | >3.40 |
| RDW | 0.578 (0.487–0.665) | 0.569 | 1.016 (0.903–1.144) | 0.780 | 0.214 | >55.1 |
| eGFR | 0.644 (0.555–0.727) | 0.492 | 0.988 (0.952–1.026) | 0.546 | 0.470 | ≤49.46 |
| Proposed biomarkers | ||||||
| RGR | 0.637 (0.547–0.721) | 0.511 | 4.229 (1.172–15.264) | 0.027 | 0.434 | >1.11 |
| NTAR | 0.817 (0.739–0.880) | 0.005 | 7.779 (0.959–63.066) | 0.054 | 0.577 | >3.24 |
| Independent Variables | 3-Month All-Cause Mortality | |||||
|---|---|---|---|---|---|---|
| AUC (95% CI) | p | OR (95% CI) | p | Hosmer–Lemeshow Test, p | Associated Criterion to Youden Index J | |
| PAH group | ||||||
| Prior studied biomarkers | ||||||
| Albumin | 0.782 (0.707–0.846) | 0.006 | 0.135 (0.030–0.604) | 0.008 | 0.686 | ≤3.88 |
| NLR | 0.608 (0.525–0.688) | 0.342 | 1.060 (0.784–1.435) | 0.701 | 0.068 | >3.47 |
| Log NT-proBNP | 0.564 (0.480–0.646) | 0.601 | 1.340 (0.453–3.958) | 0.596 | 0.324 | >3.70 |
| RDW | 0.547 (0.463–0.629) | 0.680 | 0.983 (0.901–1.072) | 0.703 | 0.428 | ≤47.10 |
| eGFR | 0.599 (0.515–0.678) | 0.399 | 1.009 (0.988–1.030) | 0.382 | 0.923 | >74.63 |
| Proposed biomarkers | ||||||
| RGR | 0.598 (0.514–0.677) | 0.396 | 1.578 (0.009–5.800) | 0.374 | 0.387 | ≤0.63 |
| NTAR | 0.578 (0.494–0.658) | 0.508 | 1.506 (0.501–4.527) | 0.465 | 0.897 | >2.36 |
| CTEPH group | ||||||
| Prior studied biomarkers | ||||||
| Albumin | 0.833 (0.757–0.894) | <0.001 | 0.487 (0.169–1.405) | 0.183 | 0.799 | ≤3.98 |
| NLR | 0.875 (0.805–0.927) | <0.001 | 1.491 (1.047–2.123) | 0.026 | 0.925 | >4.83 |
| Log NT-proBNP | 0.743 (0.658–0.817) | 0.253 | 4.929 (0.360–67.448) | 0.232 | 0.117 | >4.16 |
| RDW | 0.581 (0.490–0.668) | 0.654 | 1.033 (0.905–1.180) | 0.625 | 0.512 | >46.8 |
| eGFR | 0.793 (0.712–0.860) | 0.018 | 0.945 (0.879–1.017) | 0.136 | 0.822 | ≤59.21 |
| Proposed biomarkers | ||||||
| RGR | 0.741 (0.655–0.814) | 0.081 | 3.347 (0.807–13.882) | 0.095 | 0.578 | >0.78 |
| NTAR | 0.748 (0.670–0.816) | 0.142 | 4.232 (0.525–34.083) | 0.175 | 0.277 | >3.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iancu, D.G.; Cristescu, L.; Mares, R.G.; Varga, A.; Tilea, I. Evaluating NT-proBNP-to-Albumin (NTAR) and RDW-to-eGFR (RGR) Ratios as Biomarkers for Predicting Hospitalization Duration and Mortality in Pulmonary Arterial Hypertension (PAH) and Chronic Thromboembolic Pulmonary Hypertension (CTEPH). Diagnostics 2025, 15, 2126. https://doi.org/10.3390/diagnostics15172126
Iancu DG, Cristescu L, Mares RG, Varga A, Tilea I. Evaluating NT-proBNP-to-Albumin (NTAR) and RDW-to-eGFR (RGR) Ratios as Biomarkers for Predicting Hospitalization Duration and Mortality in Pulmonary Arterial Hypertension (PAH) and Chronic Thromboembolic Pulmonary Hypertension (CTEPH). Diagnostics. 2025; 15(17):2126. https://doi.org/10.3390/diagnostics15172126
Chicago/Turabian StyleIancu, Dragos Gabriel, Liviu Cristescu, Razvan Gheorghita Mares, Andreea Varga, and Ioan Tilea. 2025. "Evaluating NT-proBNP-to-Albumin (NTAR) and RDW-to-eGFR (RGR) Ratios as Biomarkers for Predicting Hospitalization Duration and Mortality in Pulmonary Arterial Hypertension (PAH) and Chronic Thromboembolic Pulmonary Hypertension (CTEPH)" Diagnostics 15, no. 17: 2126. https://doi.org/10.3390/diagnostics15172126
APA StyleIancu, D. G., Cristescu, L., Mares, R. G., Varga, A., & Tilea, I. (2025). Evaluating NT-proBNP-to-Albumin (NTAR) and RDW-to-eGFR (RGR) Ratios as Biomarkers for Predicting Hospitalization Duration and Mortality in Pulmonary Arterial Hypertension (PAH) and Chronic Thromboembolic Pulmonary Hypertension (CTEPH). Diagnostics, 15(17), 2126. https://doi.org/10.3390/diagnostics15172126










