Anorectal Malignant Melanoma: Diagnostic Pitfalls and Prognostic Insights from a Single-Center Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Research Design
2.2. Statistical Analysis
2.3. Ethical Considerations
3. Results
3.1. Baseline Characteristics
3.2. Disease Management and Outcomes
4. Discussion
4.1. Clinical Features and Diagnostic Challenges
4.2. Treatment Outcomes and Prognostic Factors
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Chen, H.; Cai, Y.; Liu, Y.; He, J.; Hu, Y.; Xiao, Q.; Hu, W.; Ding, K. Incidence, Surgical Treatment, and Prognosis of Anorectal Melanoma From 1973 to 2011: A Population-Based SEER Analysis. Medicine 2016, 95, e2770. [Google Scholar] [CrossRef]
- Bello, D.M.; Smyth, E.; Perez, D.; Khan, S.; Temple, L.K.; Ariyan, C.E.; Weiser, M.R.; Carvajal, R.D. Anal versus rectal melanoma: Does site of origin predict outcome? Dis. Colon. Rectum. 2013, 56, 150–157. [Google Scholar] [CrossRef]
- Menon, H.; Patel, R.R.; Cushman, T.R.; Amini, A.; Seyedin, S.N.; Adams, A.C.; Lin, C.; Verma, V. Management and outcomes of primary anorectal melanoma in the United States. Future Oncol. 2020, 16, 329–338. [Google Scholar] [CrossRef]
- Dodds, T.J.; Wilmott, J.S.; Jackett, L.A.; Lo, S.N.; Long, G.V.; Thompson, J.F.; Scolyer, R.A. Primary anorectal melanoma: Clinical, immunohistology and DNA analysis of 43 cases. Pathology 2019, 51, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Aytac, B.; Adim, S.B.; Yerci, O.; Yilmazlar, T. Anorectal malignant melanomas: Experience of Uludag University. Kaohsiung J. Med. Sci. 2010, 26, 658–662. [Google Scholar] [CrossRef]
- Chute, D.J.; Cousar, J.B.; Mills, S.E. Anorectal malignant melanoma: Morphologic and immunohistochemical features. Am. J. Clin. Pathol. 2006, 126, 93–100. [Google Scholar] [CrossRef]
- Fastner, S.; Hieken, T.J.; McWilliams, R.R.; Hyngstrom, J. Anorectal melanoma. J. Surg. Oncol. 2023, 128, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Prichard, R.S.; Hill, A.D.; Skehan, S.J.; O’Higgins, N.J. Positron emission tomography for staging and management of malignant melanoma. Br. J. Surg. 2002, 89, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Miyashita, M.; Matsumoto, S.; Takahashi, G.; Matsutani, T.; Yamada, T.; Kishi, T.; Uchida, E. Abdominoperineal resection provides better local control but equivalent overall survival to local excision of anorectal malignant melanoma: A systematic review. Ann. Surg. 2015, 261, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Spencer, S.A.; Lydiatt, W. Mucosal melanoma: A clinically and biologically unique disease entity. J. Natl. Compr. Cancer Netw. 2012, 10, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, A.J. Malignant melanoma of the skin of the head and neck. An analysis of 405 cases. Am. J. Surg. 1970, 120, 425–431. [Google Scholar] [CrossRef]
- Falch, C.; Stojadinovic, A.; Hann-von-Weyhern, C.; Protic, M.; Nissan, A.; Faries, M.B.; Daumer, M.; Bilchik, A.J.; Itzhak, A.; Brücher, B.L. Anorectal malignant melanoma: Extensive 45-year review and proposal for a novel staging classification. J. Am. Coll. Surg. 2013, 217, 324–335. [Google Scholar] [CrossRef]
- Paolino, G.; Podo Brunetti, A.; De Rosa, C.; Cantisani, C.; Rongioletti, F.; Carugno, A.; Zerbinati, N.; Valenti, M.; Mascagni, D.; Tosti, G.; et al. Anorectal melanoma: Systematic review of the current literature of an aggressive type of melanoma. Melanoma Res. 2024, 34, 487–496. [Google Scholar] [CrossRef]
- Smith, H.G.; Bagwan, I.; Board, R.E.; Capper, S.; Coupland, S.E.; Glen, J.; Lalondrelle, S.; Mayberry, A.; Muneer, A.; Nugent, K.; et al. Ano-uro-genital mucosal melanoma UK national guidelines. Eur. J. Cancer 2020, 135, 22–30. [Google Scholar] [CrossRef]
- Bleicher, J.; Cohan, J.N.; Huang, L.C.; Peche, W.; Pickron, T.B.; Scaife, C.L.; Bowles, T.L.; Hyngstrom, J.R.; Asare, E.A. Trends in the management of anorectal melanoma: A multi-institutional retrospective study and review of the world literature. World J. Gastroenterol. 2021, 27, 267–280. [Google Scholar] [CrossRef]
- Buyucek, S.; Gamsizkan, M.; Coskun, S.K.; Naldemir, A.; Karagun, E.; Gamsizkan, Z.; Onal, B. National Review of Melanomas in Turkey and Comparison with Clinicopathological Features of Melanomas Diagnosed at a Northwestern Academic Tertiary Center. SN Compr. Clin. Med. 2021, 3, 104–116. [Google Scholar] [CrossRef]
- Solak, M.; Kılıçkap, S.; Celık, İ. Retrospective evaluation of malignant melanoma patients: A single-center experience. Fam. Pract. Palliat. Care 2021, 6, 98–104. [Google Scholar] [CrossRef]
- Çınkır, H.Y.; Yıldız, F.; Aslan, F.; Dogan, I.; Arslan, U.Y.; Sönmez, Ö. Primary Anorectal Malignant Melanoma: A Single Center Experience. Acta Haematol. Oncol. Turc. 2020, 53, 68–73. [Google Scholar] [CrossRef]
- Sönmez, Ö.; Üyetürk, Ü.; Helvaci, K.; Türker, I.; Köş, F.T.; Doğan, L.; Budakoğlu, B.; Arslan, Ü.Y.; Öksüzoğlu, Ö.B.Ç. Primary anorectal malignant melanoma: Rare but highly lethal malignancy. Turk. J. Med. Sci. 2012, 42, 1513–1518. [Google Scholar] [CrossRef]
- Kolosov, A.; Leskauskaitė, J.; Dulskas, A. Primary melanoma of the anorectal region: Clinical and histopathological review of 17 cases. A retrospective cohort study. Color. Dis. 2021, 23, 2706–2713. [Google Scholar] [CrossRef]
- Garg, V.; Rastogi, S.; Aswar, H.; Shamim, S.A.; Dhamija, E.; Barwad, A.; Pandey, R.; Panwar, R.; Upadhyay, A. Clinicopathological profile and outcomes of anorectal melanoma from a tertiary care center in India. Future Sci. O.A. 2022, 8, FSO786. [Google Scholar] [CrossRef]
- Das, G.; Gupta, S.; Shukla, P.J.; Jagannath, P. Anorectal melanoma: A large clinicopathologic study from India. Int. Surg. 2003, 88, 21–24. [Google Scholar] [PubMed]
- Kahl, A.R.; Gao, X.; Chioreso, C.; Goffredo, P.; Hassan, I.; Charlton, M.E.; Lin, C. Presentation, Management, and Prognosis of Primary Gastrointestinal Melanoma: A Population-Based Study. J. Surg. Res. 2021, 260, 46–55. [Google Scholar] [CrossRef]
- Brady, M.S.; Kavolius, J.P.; Quan, S.H. Anorectal melanoma. A 64-year experience at Memorial Sloan-Kettering Cancer Center. Dis. Colon. Rectum. 1995, 38, 146–151. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, F.; Wan, D. Effect of misdiagnosis on the prognosis of anorectal malignant melanoma. J. Cancer Res. Clin. Oncol. 2010, 136, 1401–1405. [Google Scholar] [CrossRef]
- Nam, S.; Kim, C.W.; Baek, S.J.; Hur, H.; Min, B.S.; Baik, S.H.; Kim, N.K. The clinical features and optimal treatment of anorectal malignant melanoma. Ann. Surg. Treat. Res. 2014, 87, 113–117. [Google Scholar] [CrossRef]
- Yeh, J.J.; Weiser, M.R.; Shia, J.; Hwu, W.J. Response of stage IV anal mucosal melanoma to chemotherapy. Lancet Oncol. 2005, 6, 438–439. [Google Scholar] [CrossRef]
- Ballo, M.T.; Gershenwald, J.E.; Zagars, G.K.; Lee, J.E.; Mansfield, P.F.; Strom, E.A.; Bedikian, A.Y.; Kim, K.B.; Papadopoulos, N.E.; Prieto, V.G.; et al. Sphincter-sparing local excision and adjuvant radiation for anal-rectal melanoma. J. Clin. Oncol. 2002, 20, 4555–4558. [Google Scholar] [CrossRef]
- Heppt, M.V.; Roesch, A.; Weide, B.; Gutzmer, R.; Meier, F.; Loquai, C.; Kähler, K.C.; Gesierich, A.; Meissner, M.; von Bubnoff, D.; et al. Prognostic factors and treatment outcomes in 444 patients with mucosal melanoma. Eur. J. Cancer 2017, 81, 36–44. [Google Scholar] [CrossRef]
- Ragnarsson-Olding, B.K.; Nilsson, P.J.; Olding, L.B.; Nilsson, B.R. Primary ano-rectal malignant melanomas within a population-based national patient series in Sweden during 40 years. Acta Oncol. 2009, 48, 125–131. [Google Scholar] [CrossRef]
| Characteristics | n (%) |
|---|---|
| Age (years) * | 58 ± 12 |
| Gender | |
| Male | 8 (57.1) |
| Female | 6 (42.9) |
| Tumor anatomic site | |
| Rectal | 2 (14.3) |
| Anorectal | 6 (42.9) |
| Anal canal | 6 (42.9) |
| Presentation symptoms | |
| Rectal bleeding | 10 (71.4) |
| Weight loss | 6 (42.9) |
| Anorectal mass | 5 (35.7) |
| Change in bowel habits | 5 (35.7) |
| Perianal pain | 2 (14.3) |
| Misdiagnosis | |
| No | 8 (57.1) |
| Hemorrhoids | 2 (14.3) |
| Polyp | 1 (7.1) |
| Normal colonoscopic findings | 3 (21.4) |
| Endoscopic evaluation of tumor | |
| Polypoid | 5 (35.7) |
| Luminal mass | 9 (64.3) |
| Ulceration | |
| Yes | 4 (28.6) |
| No | 4 (28.6) |
| Unknown | 6 (42.9) |
| Pigmentation | |
| Melanotic | 3 (21.4) |
| Amelanotic | 2 (14.3) |
| Unknown | 9 (64.3) |
| Lymphovascular invasion | |
| Yes | 3 (21.4) |
| No | 2 (14.3) |
| Unknown | 2 (14.3) |
| BRAF mutation | |
| Negative | 12 (85.7) |
| Mutated | 0 (0) |
| Unknown | 2 (14.3) |
| Characteristics | n (%) |
|---|---|
| Stage at diagnosis | |
| Localized (Stage I) | 7 (50.0) |
| Regional (Stage II) | 1 (7.1) |
| Distant (Stage III) | 6 (42.9) |
| Distant metastasis pattern | |
| Liver | 1 (7.1) |
| Liver and bone | 1 (7.1) |
| Liver and lung | 2 (14.3) |
| Liver and lymph node | 1 (7.1) |
| Lung and lymph node | 1 (7.1) |
| Lymph node | 1 (7.1) |
| Non-regional lymph node metastasis | |
| Yes | 4 (28.6) |
| No | 10 (71.4) |
| Metastasis to liver | |
| Yes | 5 (35.7) |
| No | 9 (64.3) |
| Characteristics | n (%) |
|---|---|
| Initial treatment modality | |
| Surgery | 2 (14.3) |
| Surgery + adjuvant therapy | 5 (35.7) |
| Palliative chemotherapy | 7 (50.0) |
| Type of surgery * | |
| Abdominoperineal resection | 6 (85.7) |
| Local excision | 1 (14.3) |
| Adjuvant interferon * | |
| Yes | 5 (71.4) |
| No | 2 (28.6) |
| Adjuvant radiotherapy * | |
| Yes | 4 (57.1) |
| No | 3 (42.9) |
| Ostomy status | |
| Yes | 5 (35.7) |
| No | 9 (64.3) |
| First-line palliative chemotherapy | |
| Temozolomide | 9 (64.3) |
| DTIC + Carboplatin | 4 (28.6) |
| Paclitaxel + Carboplatin | 1 (7.1) |
| Second-line palliative therapy † | |
| Temozolomide | 2 (22.2) |
| Nivolumab | 5 (55.6) |
| Ipilimumab | 2 (22.2) |
| Third-line palliative therapy ‡ | |
| Temozolomide | 1 (25.0) |
| Paclitaxel + Carboplatin | 1 (25.0) |
| DTIC + Ipilimumab | 2 (50.0) |
| Fourth-line palliative therapy | |
| DTIC + Ipilimumab | 1 (100) |
| Outcome | |
| Alive | 0 (0) |
| Deceased | 14 (100) |
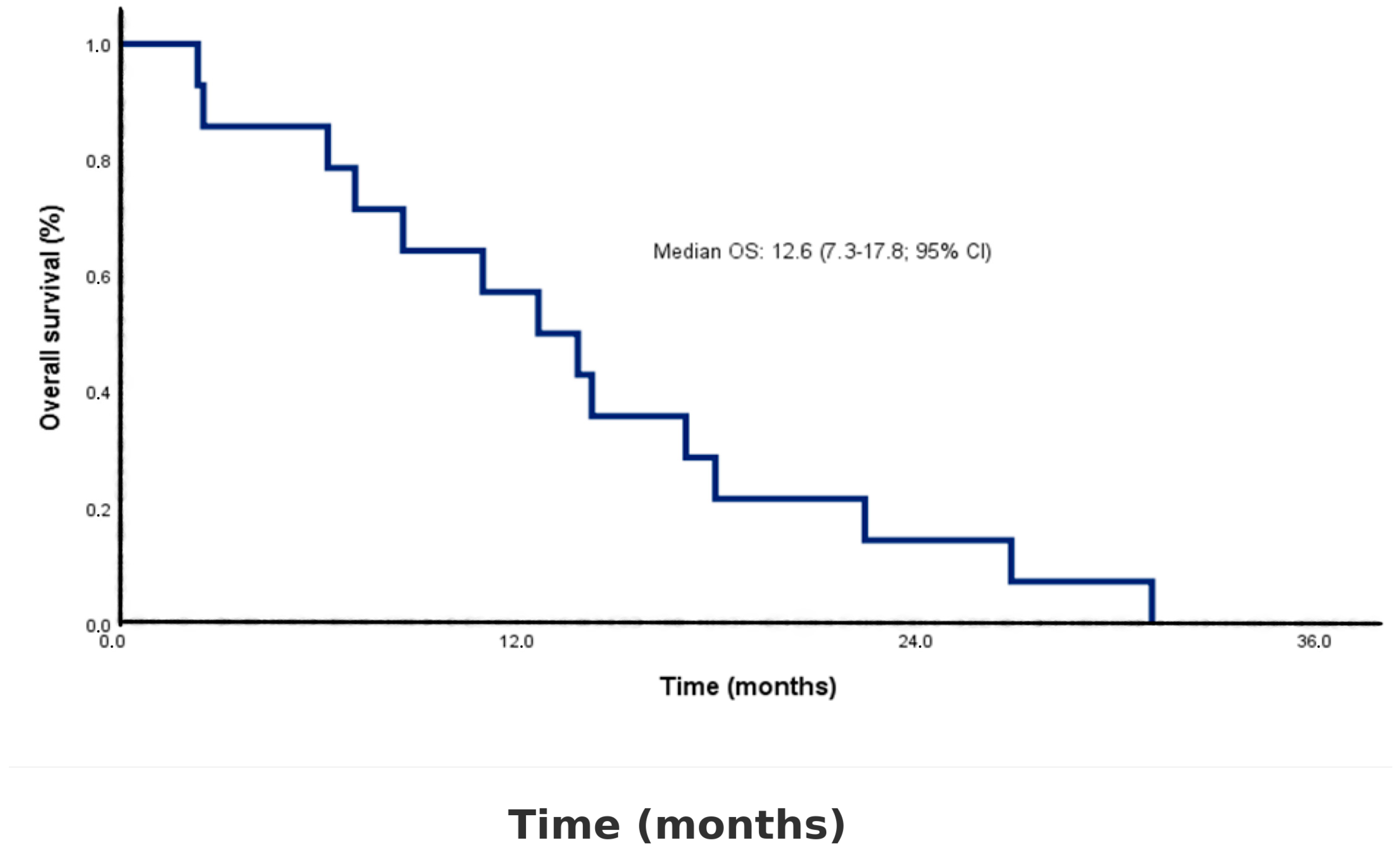
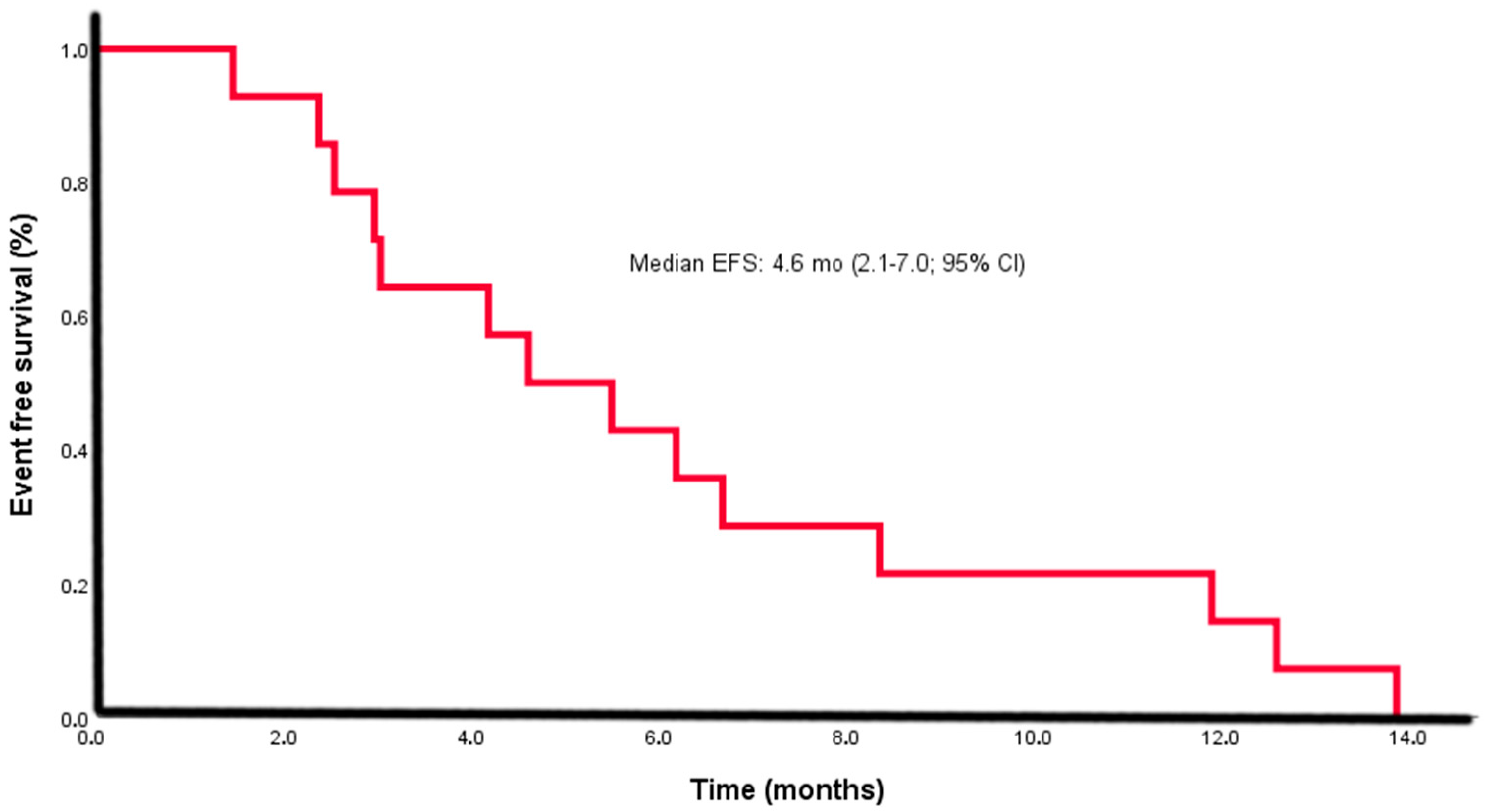
| Follow-up time, median (range), months | 13.3 (2.4–31.1) |
| Time to event (relapse/progression), median (IQR), months (EFS) | 4.6 (2.1–7.0) |
| Time to death, median (IQR), months (OS) | 12.6 (7.3–17.8) |
| Variable | n (%) | Median OS (95% CI), Months | Univariate Analysis HR (95% CI), p-Value | Multivariate Analysis HR (95% CI), p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 8 (57.1) | 7.8 (2.4–14.2) | 0.056 (0.01–0.48), 0.008 | 0.11 (0.01–1.23), 0.07 |
| Female | 6 (42.9) | 20.2 (14.1–31.1) | — | — |
| Age at diagnosis | ||||
| <58 years | 7 (50.0) | 12.6 (2.4–22.4) | 1.86 (0.58–5.99), 0.293 | — |
| ≥58 years | 7 (50.0) | 14.2 (2.5–31.1) | — | — |
| ECOG PS | ||||
| 0 | 8 (57.1) | 14.2 (8.0–20.3) | 0.98 (0.31–3.10), 0.976 | — |
| 1 | 6 (42.9) | 7.0 (4.3–9.8) | — | — |
| Tumor site | ||||
| Anorectal | 6 (42.9) | 6.7 (2.4–11.0) | 0.34 (0.08–1.36), 0.168 | 0.86 (0.01–11.03), 0.936 |
| Rectum/anal canal | 8 (57.1) | 17.5 (12.6–31.1) | — | — |
| Endoscopic evaluation | ||||
| Polypoid | 5 (35.7) | 14.2 (12.6–18.1) | 1.00 (0.29–3.38), 0.99 | — |
| Luminal mass | 9 (64.3) | 8.5 (2.4–31.1) | — | — |
| Stage | ||||
| Stage I | 7 (50.0) | 14.2 (13.1–15.3) | 1.39 (0.46–4.22), 0.55 | — |
| Stage II–III | 7 (50.0) | 7.0 (4.9–9.2) | — | — |
| Liver metastasis | ||||
| Yes | 9 (64.3) | 6.2 (2.4–11.0) | 2.24 (0.48–10.25), 0.06 | 1.68 (0.17–16.12), 0.65 |
| No | 5 (35.7) | 17.1 (8.5–31.1) | — | — |
| Pigmentation | ||||
| Melanotic | 3 (21.4) | 6.3 (2.4–22.4) | 0.73 (0.38–1.42), 0.364 | — |
| Amelanotic | 2 (14.3) | 13.3 (12.6–14.1) | — | — |
| Unknown | 9 (64.3) | 14.2 (2.5–31.1) | — | — |
| Ulceration | ||||
| Yes | 4 (28.6) | 15.6 (7.1–22.4) | 1.01 (0.53–1.92), 0.96 | — |
| No | 4 (28.6) | 7.4 (2.5–31.1) | — | — |
| Unknown | 6 (42.9) | 13.4 (2.4–26.8) | — | — |
| Ostomy | ||||
| Yes | 5 (35.7) | 12.6 (6.3–22.4) | 1.52 (0.47–4.98), 0.48 | — |
| No | 9 (64.3) | 14.2 (2.4–31.1) | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafızoğlu, E.; Bardakçı, M.; Ergun, Y.; Karahan, I.; Bayram, D.; Kos, F.T.; Algın, E.; Bal, O.; Uncu, D. Anorectal Malignant Melanoma: Diagnostic Pitfalls and Prognostic Insights from a Single-Center Retrospective Analysis. Diagnostics 2025, 15, 2086. https://doi.org/10.3390/diagnostics15162086
Hafızoğlu E, Bardakçı M, Ergun Y, Karahan I, Bayram D, Kos FT, Algın E, Bal O, Uncu D. Anorectal Malignant Melanoma: Diagnostic Pitfalls and Prognostic Insights from a Single-Center Retrospective Analysis. Diagnostics. 2025; 15(16):2086. https://doi.org/10.3390/diagnostics15162086
Chicago/Turabian StyleHafızoğlu, Emre, Murat Bardakçı, Yakup Ergun, Irfan Karahan, Doğan Bayram, Fahriye Tugba Kos, Efnan Algın, Oznur Bal, and Dogan Uncu. 2025. "Anorectal Malignant Melanoma: Diagnostic Pitfalls and Prognostic Insights from a Single-Center Retrospective Analysis" Diagnostics 15, no. 16: 2086. https://doi.org/10.3390/diagnostics15162086
APA StyleHafızoğlu, E., Bardakçı, M., Ergun, Y., Karahan, I., Bayram, D., Kos, F. T., Algın, E., Bal, O., & Uncu, D. (2025). Anorectal Malignant Melanoma: Diagnostic Pitfalls and Prognostic Insights from a Single-Center Retrospective Analysis. Diagnostics, 15(16), 2086. https://doi.org/10.3390/diagnostics15162086






