Abstract
Heart failure (HF) imposes a significant burden on public health, affecting over 56.19 million people worldwide. Right ventricular (RV) dysfunction may occur in HF patients due to various factors, including adverse interventricular interactions, ischemic heart disease, and HF-correlated pulmonary hypertension. Additionally, the deterioration of RV function plays a critical role in the progression of HF, regardless of left ventricular (LV) systolic function, suggesting an unfavorable outcome. Throughout the progression of HF and increasing afterload, the right ventricle undergoes adaptive remodeling to preserve adequate cardiac output. Right ventricular-pulmonary artery (RV-PA) coupling integrates the dynamic adaptation of RV systolic function to afterload and has been considered a stronger predictor of HF prognosis than other conventional parameters. Thus, accurate evaluations of RV-PA coupling are significant in the clinical diagnosis and management of HF patients, along with prognostic speculation. In this review, we summarize the basic principles and measurements of RV-PA coupling and focus on its clinical significance across each subtype of HF.
1. Introduction
Heart failure (HF) is a life-threatening issue associated with substantial morbidity and mortality, affecting over 56.19 million people worldwide [1]. HF has been categorized into three subtypes based on the left ventricular ejection fraction (LVEF): HF with reduced ejection fraction (HFrEF, LVEF < 40%), mildly reduced ejection fraction (HFmrEF, LVEF between 41% and 49%), or preserved ejection fraction (HFpEF, LVEF ≥ 50%) [2,3]. Right ventricular (RV) function carries substantial prognostic significance in HF, regardless of left ventricular (LV) systolic function [4,5,6]. The right ventricle, being a less muscular chamber, is more sensitive to afterload changes. An elevated left-sided filling pressure caused by LV systolic or diastolic dysfunction is transmitted passively to the pulmonary vessels, resulting in pulmonary hypertension (PH) and subsequent RV dysfunction [7,8]. Right ventricular-pulmonary artery (RV-PA) coupling, defined as the ratio of pulmonary arterial elastance and RV end-systolic function, is a comprehensive parameter that quantitatively indicates the dynamic adaptation of RV systolic function and RV afterload. It has emerged as a stronger predictor of prognosis in HF than other conventional RV function parameters [9,10,11]. In the early stage of PH, the right ventricle undergoes an adaptive response to maintain sufficient cardiac output through contractility enhancement characterized by RV hypertrophy, while RV function remains within the normal range and RV-PA coupling is preserved. However, with progressive PH, right ventricle maladaptive remodeling occurs, featuring chamber dilatation, RV volume increases, RV dysfunction, and even right heart failure [12]. RV-PA uncoupling occurs when the right ventricle fails to generate sufficient contractility to match the RV afterload. Progression from RV-PA coupling to uncoupling in the course of progressively increasing afterload and pulmonary vascular resistance caused by HF is shown in Figure 1. Additionally, RV-PA uncoupling indicates an advanced stage of HF and is a sensitive marker of RV dysfunction [13,14]. Thus, early detection of RV-PA uncoupling plays an important role in both guiding clinical decision-making and predicting prognosis. With the growing interest in RV-PA coupling assessment among HF populations, an increasing number of studies have demonstrated the coupling’s essential impact. In this review, we systematically outline the basic principles and measurements of RV-PA coupling and emphasize its clinical significance in patients with each subtype of HF.
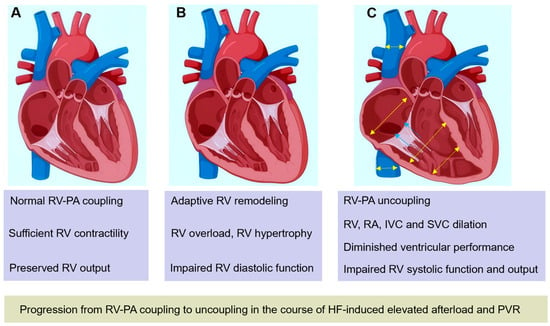
Figure 1.
Progression from RV-PA coupling to uncoupling in the course of progressively increasing afterload and PVR caused by HF. (A) Normal RV-PA coupling. (B) With increasing RV afterload, RV adaptively remodels to maintain sufficient output, RV hypertrophy, diastolic dysfunction, and RV systolic function occur. (C) With progressively increasing afterload and PVR, RV dilates to maintain stroke volume, accompanied by tricuspid regurgitation, RA, IVC, and SVC dilation. RV systolic function and output are impaired when RV-PA uncoupling occurs. HF: heart failure; RV, right ventricle; RV-PA, right ventricular-pulmonary artery; IVC: inferior vena cava; PVR: pulmonary vascular resistance; SVC, superior vena cava.
2. Invasive Assessment of Right Ventricular–Pulmonary Artery Coupling
The quantification of RV-PA coupling is complex since determining both load-independent RV contractility and RV afterload simultaneously in one metric is challenging. An invasive approach through right heart catheterization (RHC) can be conducted to describe pressure and volume based on ‘single-beat’ and ‘multi-beat’ pressure–volume loops and is considered the gold standard for assessing RV-PA coupling [15,16,17,18]. The RV contractility and the RV afterload are assessed separately as the end-systolic elastance (Ees) and effective arterial elastance (Ea), and RV-PA coupling is subsequently calculated as the ratio of Ees to Ea as follows:
Ees, representing RV systolic function, is typically calculated as the ratio of end-systolic pressure (ESP) to end-systolic volume (ESV) minus the hypothetical uncompressed ventricular volume (V0), as follows:
Ea represents a composite of PA resistance, compliance, wave reflection, and RV wall stiffness, which are expressed as the ratio of ESP to stroke volume (SV), as follows:
RV V0 is not defined exactly and is generally considered negligible. Thus, RV-PA coupling can also be simplified to the following:
which turns it into a volumetric index [19,20]. The invasive approach to RV-PA coupling is shown in Figure 2. A coupling ratio of 1.5–2.0 represents the right ventricle maintaining flow output at minimal energy cost [20]. A decrease in Ees/Ea describes relative uncoupling of the RV-PA interaction, indicating the right ventricle failing to adapt to the increased afterload. The Ees/Ea ratio exhibits a certain degree of reserve, indicating that RV volumes may increase until the ratio is 0.8–1.5, but the exact threshold of uncoupling is not defined [18,21,22]. The ratio is preserved in healthy subjects during exercise and at rest but may be impaired in PH or HF populations [23,24,25]. Tello et al. showed that an Ees/Ea cutoff of 0.805 has the ability to detect RV failure in patients with PH [22], while Richter and colleagues reached the conclusion that Ees/Ea < 0.7 is an independent predictor of clinical events [18]. Schmeißer et al. found that Ees/Ea ≥ 0.68 is linked to preserved RV function and mid-term survival in patients with PH due to HFrEF [26]. The invasive ratio of Ees/Ea or SV/ESV is a sensitive and accurate parameter for the detection of the RV adaptation to increasing afterload; however, its clinical application is limited due to its critical technical skill requirements and intrusiveness.
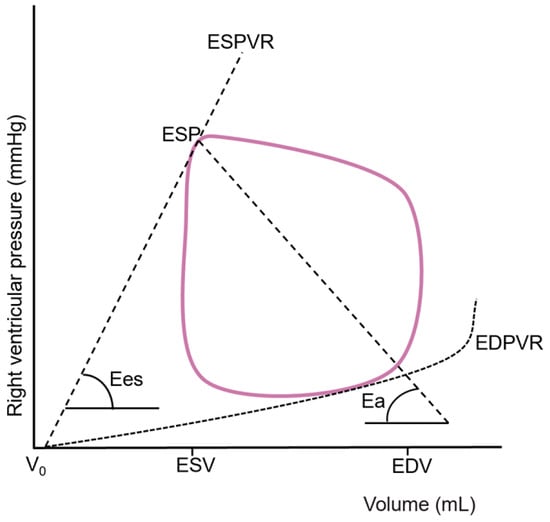
Figure 2.
Right ventricular pressure–volume loop from which effective arterial elastance (Ea) and end-systolic elastance (Ees) are derived. EDV: end-diastolic volume; EDPVR: end-diastolic pressure–volume relationship; ESP: end-systolic pressure; ESPVR: end-systolic pressure–volume relationship; ESV: end-systolic volume; V0: hypothetical uncompressed ventricular volume.
3. Non-Invasive Assessment of Right Ventricular–Pulmonary Artery Coupling
Catheter-based measurement of RV-PA coupling has the unavoidable limitations of being invasive, time-consuming, technically demanding, and expensive; therefore, non-invasive alternative metrics have been introduced, in which RV function and afterload are derived non-intrusively. Non-invasive assessments including cardiac magnetic resonance (CMR) and echocardiography are commonly used surrogates of RHC.
3.1. Cardiac Magnetic Resonance Measurement
CMR has emerged as a reliable modality for diagnosing and predicting prognosis in cardiovascular diseases, particularly in HF and PH [18,27,28]. CMR is recognized as the gold standard for assessing RV volumes and EF [29,30,31,32]. As discussed above, Ees/Ea can be simplified to SV/ESV, which can also be acquired by means of CMR and has been confirmed to be strongly correlated with RHC-derived Ees/Ea in patients with pulmonary arterial hypertension [33,34]. In several studies focusing on the prognostic impact of CMR-derived RV-PA coupling, lower SV/ESV values have been demonstrated to be strongly associated with poor outcomes [19,33,34]. However, it should be noted that the simplified formula may underestimate Ees/Ea because of the omission of V0.
3.2. Echocardiography Measurement
Echocardiography is a well-established tool for qualitatively and quantitatively assessing cardiac structure and function. Owing to its widespread availability, real-time capacity, and clinical convenience, echocardiography has become a frontline imaging modality for assessing RV-PA coupling in cardiovascular diseases. Beyond the commonly used two-dimensional echocardiography (2DE), novel techniques such as three-dimensional echocardiography (3DE) and strain imaging have emerged as reliable tools for evaluating RV-PA coupling.
Tricuspid annular systolic plane excursion (TAPSE) is a common RV systolic function parameter in clinical practice. Pulmonary arterial systolic pressure (PASP) can be acquired through echocardiography and is an estimated index of RV afterload. TAPSE/PASP was first introduced as a surrogate marker for Ees/Ea by Guazzi et al. and has been confirmed to closely correlate with RHC-derived Ees/Ea [35,36]. This indicator has become the most frequently used non-invasive surrogate marker in clinical settings, with multiple studies demonstrating its potent prognostic advantage in tricuspid regurgitation (TR), PH, HF, and congenital heart diseases [18,35,36,37,38,39,40]. The prognostic cutoff value of TAPSE/PASP in HF is about 0.36 mm/mmHg but may differ slightly in different studies and diseases [35,41]. Similarly, other RV systolic function parameters in place of TAPSE are used as non-invasive surrogates, such as the tricuspid annular systolic velocity (S’) and RV functional area change (FAC) measured via 2DE, speckle tracking echocardiography-derived RV free wall longitudinal strain (RVFWS) and RV global longitudinal strain (RVGLS), and 3DE-derived RVEF. All of the above-mentioned indices can be divided by the PASP to serve as non-invasive markers of RV-PA coupling, and they demonstrate predictive value for prognosis in clinical applications [42,43,44,45]. Examples of measurements of RV-PA coupling in HFpEF and HFrEF patients using 2DE and speckle tracking echocardiography are presented in Figure 3. Of note, some recent research suggests that RVFWS/PASP may be a better index, with enhanced diagnostic efficacy, highlighting RVFWS/PASP as a promising indicator for future applications [46].
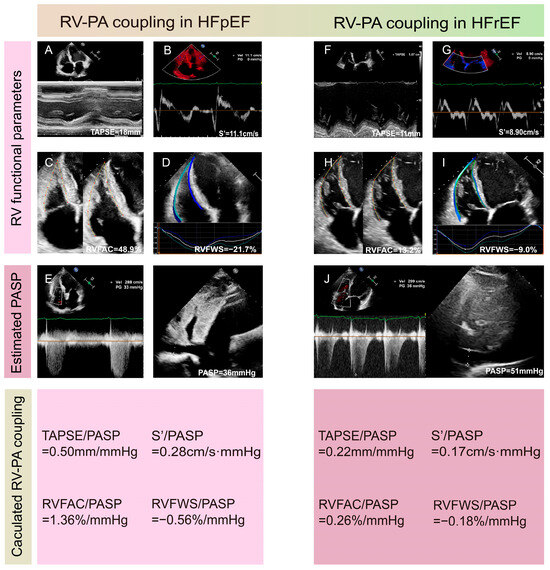
Figure 3.
Examples of measurements of right ventricular-pulmonary artery coupling in HFpEF (A–E) and HFrEF (F–J) patients using 2DE and speckle tracking echocardiography. (A,F) Measurements of TAPSE. (B,G) Measurement of S’. (C,H) Calculation of RVFAC. (D,I) Evaluation of RVFWS. (E,J) Estimation of PASP using peak tricuspid regurgitation velocity, RVSP, and inferior vena cava diameter. 2DE: two-dimensional echocardiography; FAC: functional area change; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; PASP: pulmonary arterial systolic pressure; RVFWS: free wall longitudinal strain; RVSP: right ventricular systolic pressure; S’: tricuspid annular systolic velocity; TAPSE: tricuspid annular plane systolic excursion.
Similar to CMR, 3DE can accurately obtain volume parameters without relying on geometric assumptions of RV morphology. Additionally, 3DE-derived SV/ESV has emerged as a novel parameter of RV-PA coupling, and examples of assessments of SV/ESV using CMR and 3DE are shown in Figure 4. A strong correlation of SV/ESV with Ees/Ea has been confirmed in patients with PH [47]. A few studies have concluded that SV/ESV is a prognostic index in HFpEF and TR [45,48]. Given the limited clinical evidence on 3DE-derived RV-PA coupling in cardiovascular diseases, further studies should explore its diagnostic applicability across various conditions and compare its efficacy with that of other conventional markers. The 2025 ASE guidelines endorse echocardiographic assessments of RV-PA coupling as a valuable adjunct for enhancing risk stratification in clinical practice [49], which emphasizes the need for comprehensive evaluations of RV-PA coupling in the process of evaluating RV function.
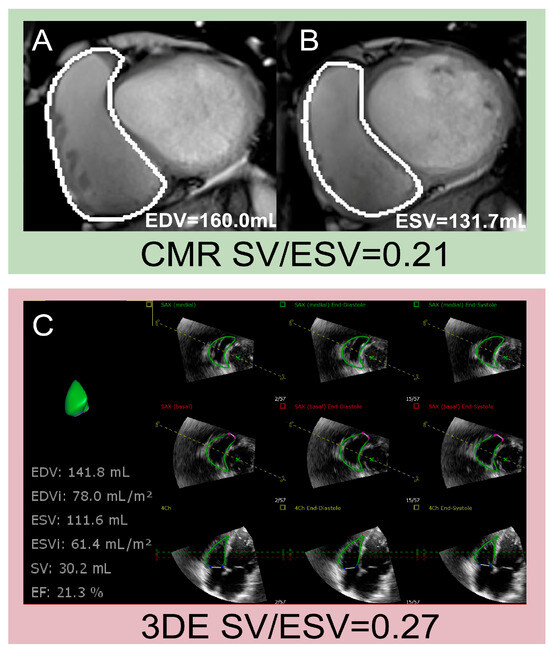
Figure 4.
Examples of assessments of SV/ESV using CMR and 3DE. (A,B) Measurement of EDV, ESV, and SV using CMR; (C) measurement of EDV, ESV and SV using 3DE. 3DE: three-dimensional echocardiography; CMR: cardiac magnetic resonance; EDV: end-diastolic volume; ESV: end-systolic volume; SV: stroke volume.
4. Clinical Usefulness of RV-PA Coupling in Heart Failure
4.1. Heart Failure with Preserved Ejection Fraction (HFpEF)
HFpEF is a common subtype of HF, affecting more than half of HF patients, with its prevalence continuing to rise over recent decades [50,51]. It has long been seen as an isolated abnormality in LV diastolic function due to LV hypertrophy and interstitial fibrosis, but numerous studies in recent years have highlighted the fundamental role of RV function and its prognostic value in HFpEF [52,53]. Obokata et al. [54] found that RV structure and function deteriorated more than LV function in patients with HFpEF during a follow-up period of four years, and the development of RV dysfunction was associated with a nearly two-fold increased risk of death. Prior studies confirmed that HFpEF patients are prone to PH and RV dysfunction, with prevalence of nearly 80% and 20–40%, respectively [23,52,53,54,55,56]. The main pathophysiological outcomes related to RV dysfunction and PH include left atrial hypertension caused by backward transmission of high LV filling pressures, pulmonary vascular disease, RV contractile impairment, interventricular interactions, systemic vascular stiffening, and myocardial ischemia [57,58,59]. Therefore, RV-PA coupling, integrating RV contractility and afterload, may serve as a more comprehensive and sensitive indicator for clinical phenotyping, treatment guidance, and prognosis prediction in cases of HFpEF. Studies concerning the application of RV-PA coupling in patients with HFpEF are listed in Table 1.

Table 1.
Studies concerning the application of right ventricular-pulmonary artery coupling in HFpEF patients.
RV-PA uncoupling represents the terminal stage of HF progression, when a therapeutic approach may be futile. Therefore, identifying these patients in the early stage may be of help in risk stratification and clinical treatment. RV-PA coupling has recently been proposed as a novel parameter to predict outcomes and to help identify different phenotypes. In a study of 528 HFpEF (LVEF ≥ 45%) patients from the PARAGON-HF trial, RVFWS and the RVFWS/PASP ratio were assessed through speckle tracking echocardiography [4]. After a median follow-up of 2.8 years, the researchers concluded that lower absolute RVFWS and RVFWS/PASP ratio values were correlated with higher NT-pro brain natriuretic peptide (BNP) levels and higher prevalence of adverse outcomes of HF hospitalizations and cardiovascular death [4]. Likewise, Nakagawa et al. [67] conducted a multi-center Asian cohort study of 655 individuals, enrolling acute decompensated HFpEF patients and demonstrating that TAPSE/PASP < 0.48 mm/mmHg was independently associated with HF readmission and all-cause mortality. Their study further identified that RV-PA uncoupling in HFpEF patients was correlated with renal dysfunction, higher NT-pro BNP levels, impaired exercise tolerance, and shortened 6 min walk distances [67]. Similarly, emerging evidence underscores the critical role of RV-PA coupling in HFpEF phenotyping to guide therapeutic strategies [74,75]. Guazzi and colleagues analyzed 387 HFpEF patients (of which 219 individuals underwent RHC) [63] to validate TAPSE/PASP against invasive RV-PA coupling measures (Ees/Ea). They observed that, from the highest to the lowest tertiles of the TAPSE/PASP ratio, there was a gradual increase in the levels of BNP, deterioration of systemic and pulmonary hemodynamics, abnormal exercise oxygen consumption, and reduced ventilation efficiency [63]. Moreover, the lowest tertile of TAPSE/PASP had lower PA compliance, higher pulmonary vascular resistance, and a higher incidence of clinical worsening, which supports the assertion that the impact of PH on adverse outcomes is related to RV-PA coupling, regardless of LV systolic function [63]. The strong correlation between TAPSE/PASP and Ees/Ea supports its utility as a reliable non-invasive marker of RV-PA coupling. This study emphasizes the possible application of the non-invasive index TAPSE/PASP as a convenient method for stratifying HFpEF phenotypes and predicting prognosis. Considering that HFpEF patients may experience normal hemodynamics but suffer multiple cardiac abnormalities during exercise [52], another recent study by Saito and colleagues aimed to identify HFpEF phenogroups through exercise echocardiography-based machine learning cluster analysis [76]. Two hundred and sixty-five enrolled HFpEF patients were categorized into three phenogroups: phenogroup 1, with preserved biventricular systolic reserve, the highest cardiac output, and RV-PA coupling during exercise; phenogroup 2, with a higher prevalence of atrial fibrillation, increased PA and RV pressure, impaired RV functional reserve, the most severe left atrial remodeling, RV-PA uncoupling, and RV failure during exercise; and phenogroup 3 with ventricular and arterial stiffness, impaired LV diastolic reserve, and impaired exercise capacity [76]. Phenogroups 2 and 3 had a three-fold increased risk of composite outcomes when compared with phenogroup 1. The study further demonstrated that the cluster analysis based on exercise echocardiography had incremental prognostic value over the conventional 2DE parameters and may be a promising tool not only in diagnosis but as a risk stratification tool in HFpEF. The studies by Guazzi and Saito et al. both indicated a higher prevalence of atrial fibrillation in HFpEF patients with the lowest TAPSE/PASP values [63,76]. Gorter et al. compared RV function and RV-PA coupling in HFpEF patients with sinus rhythm and atrial fibrillation through 2DE and RHC [65]. Their conclusion that RV-PA coupling was lower in patients with HFpEF combined with atrial fibrillation was consistent with the findings of the studies above [65].
Since PH is common in HFpEF patients and is a strong predictor of poor outcomes, early detection and differentiation of PH may be beneficial to enhancing the prognosis of HFpEF. Chen and colleagues conducted a trial on HFpEF using the calculated TAPSE/PASP and S’/PASP values both during exercise and at rest [70]. Lower resting/exercise RV-PA coupling was found in HFpEF patients with PH compared with those without PH. Both TAPSE/PASP and S’/PASP were associated with mPAP and pulmonary capillary wedge pressure. The researchers concluded that the cutoff points to differentiate HFpEF patients with PH from those without PH l were TAPSE/PASP ≤ 0.62 mm/mmHg and S’/PASP ≤ 0.47 cm/s per mmHg [70]. Thus, a decline in TAPSE/PASP and S’/PASP in the context of HFpEF may be suggestive of appropriate diagnostic procedures to guide clinical treatment.
Other than helping to stratify phenotypes and predict outcomes in HFpEF patients, RV-PA coupling is a powerful tool to guide clinical management. A prospective trial conducted by Andersen et al. enrolled 39 HFpEF patients and 18 controls, all of whom underwent comprehensive hemodynamic assessments by means of both RHC and echocardiography at rest and during dobutamine infusion [60]. Compared with the control group, dobutamine infusion in HFpEF patients led to a more pronounced reduction in pulmonary arterial resistance, increased pulmonary arterial compliance, and a more negative slope in the PA pressure–flow relationship, which indicated that the pulmonary vascular damage in the early stage was reversible [60]. The dynamic RV-PA coupling represented by S’/mean PA pressure (mPAP) revealed that the improvement in RV ejection during dobutamine infusion in HFpEF was caused by afterload relief rather than contractility enhancement. The study then summarized that the favorable pulmonary vascular response to dobutamine in early-stage HFpEF may improve RV-PA coupling and provide evidence for β-stimulation as a potential treatment approach for HFpEF [60]. Another study by Reddy et al. enrolled 37 randomized participants to evaluate the effect of dapagliflozin on RV-PA coupling and pulmonary vascular load at rest and during exercise [71]. Dapagliflozin was able to improve the prognosis and health condition by decreasing the abnormal pulmonary capillary wedge pressure of HFpEF patients during exercise [77]. Former studies saw the role of dapagliflozin in ameliorating metabolic dysfunction and inflammation, but the exact mechanisms remained unknown. The researchers then hypothesized that dapagliflozin could improve the pulmonary vascular load and RV-PA coupling during exercise to achieve this therapeutic goal. The conclusion made after 24 weeks of dapagliflozin treatment were consistent with this hypothesis. During exercise, the pulmonary vascular load was reduced, while pulmonary arterial compliance and RV-PA coupling were enhanced; these enhancements were correlated with reductions in right atrial and pulmonary capillary wedge pressure, together with improvement in load-independent RV functional reserve [71]. The improvement in RV-PA coupling may partially explain the benefits observed with dapagliflozin in HFpEF treatment beyond the left heart. Therefore, monitoring dynamic changes in RV-PA coupling during clinical treatment could predict therapeutic response, since it may be useful in identifying a patient cohort that is sensitive to a certain treatment.
4.2. Heart Failure with Mildly Reduced Ejection Fraction (HFmrEF)
HFmrEF is a type of HF that has gradually attracted more attention in recent years as its prevalence has been increasing to 10–25% among the overall population of patients with HF [78]. The pathophysiological mechanisms in HFmrEF patients are relatively complex and may include mild impairment of LV systolic function, diastolic dysfunction, or abnormalities in right heart function and pulmonary circulation [79,80]. Patients with HFmrEF may have a similar or higher risk of non-cardiovascular adverse events to patients with HFrEF and higher rates of cardiovascular mortality than those with HFpEF [79]. This indicates that HFmrEF patients have an elevated risk of disease progression and require more aggressive management and treatment [79].
RV-PA coupling indices can serve as independent predictors for the prognostic assessment of HFmrEF patients, facilitating early identification of high-risk patients and enabling timely therapeutic strategy optimization, ultimately improving clinical outcomes. Multiple studies have shown that RV-PA coupling indices are closely related to the prognosis of HFmrEF patients [6,79,80,81]. A retrospective investigation that enrolled 400 outpatients with chronic HF aged over 70 years and a reduced mid-range ejection fraction (LVEF ≤ 50%) showed that TAPSE/PASP outperformed LV function parameters in predicting the prognosis of these HF patients, with an optimal threshold of 0.34 mm/mmHg [81]. A study conducted by Ghio et al. [6] involving 1663 HF patients (1123 with HFrEF, 156 with HFmrEF, and 384 with HFpEF) reported that the TAPSE/PASP ratio was an independent predictor of prognosis for all types of HF patients, regardless of the degree of left ventricular systolic dysfunction.
Although a growing number of studies have demonstrated the prognostic value of RV-PA coupling in patients with HFmrEF, research on the usefulness of RV-PA coupling in HFmrEF is still in the preliminary stage. Further in-depth studies on its mechanisms and clinical application are needed in the future to better guide clinical decision-making.
4.3. Heart Failure with Reduced Ejection Fraction (HFrEF)
HFrEF is a common type of HF, with complex pathophysiological mechanisms involving multiple structural and functional abnormalities. RV-PA coupling, as an important physiological mechanism of cardiopulmonary circulation, plays a significant role in the progression and prognosis of HFrEF. Studies concerning the application of RV-PA coupling in patients with HFrEF are listed in Table 2.

Table 2.
Studies concerning the application of right ventricular-pulmonary artery coupling in HFrEF patients.
RV-PA coupling is of great significance in assessing the clinical progression and prognosis of HFrEF. Preserved RV-PA coupling is essential for maintaining stable cardiac function, while RV-PA uncoupling indicates pathological remodeling, disease progression, and adverse clinical outcomes. A study involving 97 patients with HFrEF showed that although the patients in group B (those with TAPSE at rest of < 16 mm and median TAPSE ≥ 15.5 mm at peak exercise) and group C (those with TAPSE at rest of < 16 mm and median TAPSE < 15.5 mm at peak exercise) had similar LVEFs and resting RV impairment, those in group B showed some degree of right ventricular exercise contractile reserve (RVECR) (upward shift of the length–force relationship), better RV-PA coupling (lower mean pulmonary artery pressure vs. cardiac output slope), and greater ventilator efficiency (lower slope of minute ventilation vs. carbon dioxide output) [83]. Thus, an unfavorable RV contractile adaptive response to exercise might not always be caused by impaired RV function at rest. Evaluating the degree of RVECR and RV-PA coupling during exercise could be useful and unmask various clinical phenotypes and different levels of risk even in the more advanced stages of HF. Legris et al. [84]. demonstrated that RV function rather than LV function was closely associated with exercise capacity in ambulatory HFrEF patients, providing new evidence of the importance of RV function and RV-PA coupling in exercise tolerance in HFrEF. The TAPSE/PASP ratio was the only echocardiographic parameter associated with peak VO2; specifically, a ratio threshold of ≤0.45 mm/mmHg predicted peak VO2 ≤ 14 mL/kg/min and a threshold of ≤ 0.39 mm/mmHg predicted peak VO2 ≤ 12 mL/kg/min. This study indicated that TAPSE/PASP could be used to identify patients with reduced exercise capacity.
Multiple studies have shown that impaired RV-PA coupling is closely related to poor prognosis in patients with HFrEF [92,93]. In a 2.39-year follow-up study of 456 patients with ventricular–secondary mitral regurgitation caused by HFrEF, RV-PA coupling calculated as the TAPSE/PASP ratio was used to evaluate the compensatory increase in pulmonary pressure. The study showed that RV-PA uncoupling (TAPSE/PASP ratio < 0.37 mm/mmHg) was associated with reduced survival across all severities of mitral regurgitation [85]. Similarly, Hădăreanu et al. found that RVFWS/PASP is a robust prognostic marker in HFrEF–secondary mitral regurgitation, with a cutoff of 0.46%/mmHg [94]. An investigation that involved 4312 patients with HF showed that the RVGLS/PASP ratio was a significant predictor of all-cause mortality, including HFrEF (HR, 2.124; p = 0.002), HFmrEF (HR, 2.733; p = 0.021), and HFpEF (HR, 2.134; p = 0.006), and an RVGLS/PASP ratio of ≤0.32%/mmHg was associated with an increased risk of mortality among all the HF phenotypes [87]. A study that involved 315 patients with an LVEF of <45% showed that RVGLS/PASP and RVFWS/PASP were significantly and independently associated with increased mortality risk. Moreover, the accuracy of the combined assessment of RVGLS/PASP and RVFWS/PASP was greater than that of the parameters evaluated separately, which could be used to identify high-risk patients. The prognostic accuracy of RVGLS/PASP and RVFWS/PASP was higher than that of TAPSE/PASP [89].
Although TAPSE/PASP is a convenient index to evaluate RV-PA coupling, it also has limitations that cannot be ignored. Under circumstances of inadequate visualization of the inferior vena cava and/or the tricuspid regurgitation velocity (TRV) Doppler signal, TAPSE/PASP may not be feasible. A cohort study of 200 hospitalized HF patients used alternative measures of RV-PA coupling parameters, namely the product of TAPSE and pulmonary acceleration time (TAPSE × pACT) and the ratio of TAPSE to the peak tricuspid regurgitation velocity (TAPSE/TRV), for prognostic assessment in HF [86]. They demonstrated that TAPSE/PASP was the most powerful predictor of mortality, followed by TAPSE × pACT and TAPSE/TRV, and both had strong correlations with the TAPSE/PASP ratio [86].
As TAPSE exclusively quantifies longitudinal contraction, the TAPSE/PASP ratio might serve as a suboptimal surrogate for RV-PA coupling in patients with advanced RV structural remodeling. A prospective study enrolling 105 outpatients with dilated cardiomyopathy (DCM) and a mean LVEF of 28 ± 7% showed that the RV SV/ESV ratio was independently correlated with severe HF symptoms in patients with DCM, and the cutoff value was 0.54 (area under the curve = 0.712, p < 0.001) for predicting severely symptomatic status; thus, it might be a useful risk stratification tool for these patients [88]. Aura et al. assessed RV-PA coupling in 60 patients with DCM and LVEF < 40% using five echocardiographic parameters: TAPSE/PASP, RVGLS/PASP, RVFWS/PASP, 3D RVEF/PASP, and RV SV/ESV. They found that RVFWS/PASP and RVEF/PASP were independent predictors of HF rehospitalization in patients with DCM. Cutoff values of RVFWS/PASP > −0.40%/mmHg and RVEF/PASP < 1.30%/mmHg were proposed to predict a high risk of HF rehospitalization [90]. Therefore, early identification and intervention in cases of abnormal RV-PA coupling have potential clinical value in improving the prognosis of patients with HFrEF.
In addition, RV-PA coupling can be used to guide treatment decisions. Currently, the treatment strategies in patients with HFrEF mainly include pharmacological therapy and device-based therapy. In terms of pharmacological therapy, sacubitril/valsartan is a novel angiotensin receptor neprilysin inhibitor (ARNI), which can improve the contractile function of the right ventricle, ameliorate RV relaxation, and reduce pulmonary artery pressure, thereby partially restoring RV-PA coupling. Daniele Masarone et al. found that sacubitril/valsartan could improve RV-PA coupling in patients with HFrEF, as evidenced by an increase in TAPSE and a decrease in PASP. The improvement in RV-PA coupling was related not to LV reverse remodeling but only to reduction in the left atrial volume index [82]. This suggests that when treating HFrEF, it is possible to consider using this type of drug to optimize the treatment plan based on the patient’s RV-PA coupling status to improve RV function and alleviate PH. Regarding device-based therapy, such as cardiac resynchronization therapy (CRT), this method can optimize the electrical activity and mechanical contraction of the heart, which may have a positive impact on RV-PA coupling, but more than 1/3 of patients do not respond to CRT [95]. Therefore, there is a strong need to identify new factors to predict and/or influence the response of HFrEF to CRT. In a prospective investigation including 54 patients with HFrEF undergoing CRT, a TAPSE/PASP ratio of ≥ 0.58 mm/mmHg displayed good sensitivity (90%) and specificity (81.8%) for predicting CRT response. Moreover, a TAPSE/PASP ratio of < 0.58 mm/mmHg was associated with a higher incidence of death and HF hospitalizations during the follow-up period [91]. Hence, by monitoring changes in RV-PA coupling after treatment, the therapeutic effect can be evaluated, and treatment strategies can be adjusted accordingly.
5. Summary and Prospects
The application of RV-PA coupling in different types of HF holds significant clinical importance. RV-PA coupling can be used to assess exercise tolerance, guide treatments, monitor treatment efficacy, and stratify risk in HF patients.
Despite the advancements made in understanding RV-PA coupling within the context of HF, several challenges and future research directions remain. First, the current methods for assessing RV-PA coupling are diverse, including both invasive and non-invasive approaches, each with its own advantages and disadvantages. Future efforts should focus on further optimizing these assessment methods to improve their accuracy and reproducibility, thereby facilitating better application in clinical practice. Second, the research on RV-PA coupling in HFmrEF is relatively limited at present. More studies are needed to better understand its role and value in this type of HF. In addition, the potential applications of RV-PA coupling in HF treatment warrant further exploration. For instance, interventions targeting RV-PA coupling may offer new therapeutic strategies for HF patients. Future research could investigate how to improve RV-PA coupling through pharmacological therapy, device-based therapy, or other interventions, thereby improving the prognosis of HF patients. Lastly, long-term follow-up studies examining changes in RV-PA coupling among HF patients hold considerable importance. By monitoring these changes over time, we can better understand their relationship with the progression and prognosis of HF, which will help in formulating more personalized treatment plans and improving the long-term survival and quality of life of HF patients.
Author Contributions
Conceptualization, M.Y., Z.W., S.Q., M.X. and Y.L. (Yuman Li); methodology, Z.W., S.Q., M.X., L.Z. and Y.L. (Yixia Lin); software, Z.W., Q.H., S.Q., Y.L. (Yixia Lin), M.Y. and Y.L. (Yuman Li); writing—original draft preparation M.Y., Z.W., Q.H., S.Q. and Y.L. (Yuman Li).; writing—review and editing, Z.W., M.J., S.Q., Y.L. (Yixia Lin) and Y.L. (Yuman Li); visualization, Z.W., S.Q. and M.J.; supervision, Y.L. (Yuman Li); funding acquisition, L.Z., M.X. and Y.L. (Yuman Li). All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from National Natural Science Foundation of China (grant numbers 82371991, 82230066, 82201408, 82302225, 82402002); Key Research and Development Program of Hubei (grant numbers 2021BCA138).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data were generated or analyzed for or in support of this paper. We did not use artificial intelligence (AI)-assisted technologies in the production of the submitted work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ARNI | angiotensin receptor neprilysin inhibitor |
| Ea | effective arterial elastance |
| Ees | end-systolic elastance |
| HFmrEF | heart failure with mildly reduced ejection fraction |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| pACT | pulmonary acceleration time |
| PASP | pulmonary arterial systolic pressure |
| PH | pulmonary hypertension |
| RHC | right heart catheterization |
| RVECR | right ventricular exercise contractile reserve |
| RV-PA coupling | right ventricular-pulmonary artery coupling |
| RVFWS | right ventricular free wall longitudinal strain |
| RVGLS | right ventricular global longitudinal strain |
| TRV | tricuspid regurgitation velocity |
References
- Yan, T.; Zhu, S.; Yin, X.; Xie, C.; Xue, J.; Zhu, M.; Weng, F.; Zhu, S.; Xiang, B.; Zhou, X.; et al. Burden, Trends, and Inequalities of Heart Failure Globally, 1990 to 2019: A Secondary Analysis Based on the Global Burden of Disease 2019 Study. J. Am. Heart Assoc. 2023, 12, e027852. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Inciardi, R.M.; Abanda, M.; Shah, A.M.; Cikes, M.; Claggett, B.; Skali, H.; Vaduganathan, M.; Prasad, N.; Litwin, S.; Merkely, B.; et al. Right Ventricular Function and Pulmonary Coupling in Patients With Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2023, 82, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Jakstaite, A.M.; Mueller-Leisse, J.; Hillmann, H.A.K.; Hohmann, S.; Eiringhaus, J.; Bavendiek, U.; Kempf, T.; Veltmann, C.; Bauersachs, J.; Duncker, D.; et al. Right Ventricular Dysfunction for Prediction of Long-Term Recovery in de Novo HFrEF: A PROLONG-II Substudy. ESC Heart Fail. 2025, 12, 2166–2176. [Google Scholar] [CrossRef]
- Ghio, S.; Guazzi, M.; Scardovi, A.B.; Klersy, C.; Clemenza, F.; Carluccio, E.; Temporelli, P.L.; Rossi, A.; Faggiano, P.; Traversi, E.; et al. Different Correlates but Similar Prognostic Implications for Right Ventricular Dysfunction in Heart Failure Patients with Reduced or Preserved Ejection Fraction. Eur. J. Heart Fail. 2017, 19, 873–879. [Google Scholar] [CrossRef]
- Girerd, N.; Seronde, M.-F.; Coiro, S.; Chouihed, T.; Bilbault, P.; Braun, F.; Kenizou, D.; Maillier, B.; Nazeyrollas, P.; Roul, G.; et al. Integrative Assessment of Congestion in Heart Failure Throughout the Patient Journey. JACC Heart Fail. 2018, 6, 273–285. [Google Scholar] [CrossRef]
- Vachiéry, J.-L.; Tedford, R.J.; Rosenkranz, S.; Palazzini, M.; Lang, I.; Guazzi, M.; Coghlan, G.; Chazova, I.; De Marco, T. Pulmonary Hypertension Due to Left Heart Disease. Eur. Respir. J. 2019, 53, 1801897. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Cartocci, A.; Pirrotta, F.; Vannuccini, F.; Campora, A.; Martini, L.; Dini, F.L.; Carluccio, E.; Ruocco, G. Different Right Ventricular Dysfunction and Pulmonary Coupling in Acute Heart Failure According to the Left Ventricular Ejection Fraction. Prog. Cardiovasc. Dis. 2023, 81, 89–97. [Google Scholar] [CrossRef]
- Houston, B.A.; Brittain, E.L.; Tedford, R.J. Right Ventricular Failure. N. Engl. J. Med. 2023, 388, 1111–1125. [Google Scholar] [CrossRef]
- Parasca, C.A.; Calin, A.; Cadil, D.; Mateescu, A.; Rosca, M.; Botezatu, S.B.; Enache, R.; Beladan, C.; Ginghina, C.; Deleanu, D.; et al. Right Ventricle to Pulmonary Artery Coupling after Transcatheter Aortic Valve Implantation-Determinant Factors and Prognostic Impact. Front. Cardiovasc. Med. 2023, 10, 1150039. [Google Scholar] [CrossRef] [PubMed]
- Kubba, S.; Davila, C.D.; Forfia, P.R. Methods for Evaluating Right Ventricular Function and Ventricular–Arterial Coupling. Prog. Cardiovasc. Dis. 2016, 59, 42–51. [Google Scholar] [CrossRef]
- Nochioka, K.; Querejeta Roca, G.; Claggett, B.; Biering-Sørensen, T.; Matsushita, K.; Hung, C.-L.; Solomon, S.D.; Kitzman, D.; Shah, A.M. Right Ventricular Function, Right Ventricular–Pulmonary Artery Coupling, and Heart Failure Risk in 4 US Communities: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2018, 3, 939–948. [Google Scholar] [CrossRef]
- Suzuki, M.; Matsumoto, K.; Tanaka, Y.; Yamashita, K.; Shono, A.; Sumimoto, K.; Shibata, N.; Yokota, S.; Suto, M.; Dokuni, K.; et al. Preoperative Coupling between Right Ventricle and Pulmonary Vasculature Is an Important Determinant of Residual Symptoms after the Closure of Atrial Septal Defect. Int. J. Cardiovasc. Imaging 2021, 37, 2931–2941. [Google Scholar] [CrossRef]
- Sagawa, K. The End-Systolic Pressure-Volume Relation of the Ventricle: Definition, Modifications and Clinical Use. Circulation 1981, 63, 1223–1227. [Google Scholar] [CrossRef]
- Kelly, R.P.; Ting, C.T.; Yang, T.M.; Liu, C.P.; Maughan, W.L.; Chang, M.S.; Kass, D.A. Effective Arterial Elastance as Index of Arterial Vascular Load in Humans. Circulation 1992, 86, 513–521. [Google Scholar] [CrossRef]
- Inuzuka, R.; Hsu, S.; Tedford, R.J.; Senzaki, H. Single-Beat Estimation of Right Ventricular Contractility and Its Coupling to Pulmonary Arterial Load in Patients With Pulmonary Hypertension. J. Am. Heart Assoc. 2018, 7, e007929. [Google Scholar] [CrossRef]
- Richter, M.J.; Peters, D.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Sommer, N.; Gall, H.; Grimminger, F.; Seeger, W.; Tello, K. Evaluation and Prognostic Relevance of Right Ventricular–Arterial Coupling in Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2020, 201, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; García-Alvarez, A.; Fernández-Friera, L.; Nair, A.; Mirelis, J.G.; Sawit, S.T.; Pinney, S.; Fuster, V. Right Ventriculo-Arterial Coupling in Pulmonary Hypertension: A Magnetic Resonance Study. Heart 2012, 98, 238–243. [Google Scholar] [CrossRef]
- Vanderpool, R.R.; Rischard, F.; Naeije, R.; Hunter, K.; Simon, M.A. Simple Functional Imaging of the Right Ventricle in Pulmonary Hypertension: Can Right Ventricular Ejection Fraction Be Improved? Int. J. Cardiol. 2016, 223, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Naeije, R. Right Heart Phenotype in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2021, 14, e007840. [Google Scholar] [CrossRef]
- Tello, K.; Dalmer, A.; Axmann, J.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Seeger, W.; Sommer, N.; Wilhelm, J.; et al. Reserve of Right Ventricular-Arterial Coupling in the Setting of Chronic Overload. Circ. Heart Fail. 2019, 12, e005512. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Rahaghi, F.N.; Naeije, R.; Oliveira, R.K.F.; Systrom, D.M.; Waxman, A.B. Right Ventricular-Arterial Uncoupling During Exercise in Heart Failure With Preserved Ejection Fraction: Role of Pulmonary Vascular Dysfunction. Chest 2019, 156, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Oliveira, R.K.F.; Heerdt, P.M.; Pari, R.; Systrom, D.M.; Waxman, A.B. Sex-Related Differences in Dynamic Right Ventricular-Pulmonary Vascular Coupling in Heart Failure With Preserved Ejection Fraction. Chest 2021, 159, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Pagnamenta, A.; Dewachter, C.; McEntee, K.; Fesler, P.; Brimioulle, S.; Naeije, R. Early Right Ventriculo-Arterial Uncoupling in Borderline Pulmonary Hypertension on Experimental Heart Failure. J. Appl. Physiol. 2010, 109, 1080–1085. [Google Scholar] [CrossRef]
- Schmeißer, A.; Rauwolf, T.; Groscheck, T.; Fischbach, K.; Kropf, S.; Luani, B.; Tanev, I.; Hansen, M.; Meißler, S.; Schäfer, K.; et al. Predictors and Prognosis of Right Ventricular Function in Pulmonary Hypertension Due to Heart Failure with Reduced Ejection Fraction. ESC Heart Fail. 2021, 8, 2968–2981. [Google Scholar] [CrossRef]
- Kanagala, P.; Arnold, J.R.; Singh, A.; Khan, J.N.; Gulsin, G.S.; Gupta, P.; Squire, I.B.; Ng, L.L.; McCann, G.P. Prevalence of Right Ventricular Dysfunction and Prognostic Significance in Heart Failure with Preserved Ejection Fraction. Int. J. Cardiovasc. Imaging 2021, 37, 255–266. [Google Scholar] [CrossRef]
- Kanagala, P.; Cheng, A.S.H.; Singh, A.; Khan, J.N.; Gulsin, G.S.; Patel, P.; Gupta, P.; Arnold, J.R.; Squire, I.B.; Ng, L.L.; et al. Relationship Between Focal and Diffuse Fibrosis Assessed by CMR and Clinical Outcomes in Heart Failure With Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2019, 12, 2291–2301. [Google Scholar] [CrossRef]
- van Wolferen, S.A.; Marcus, J.T.; Boonstra, A.; Marques, K.M.J.; Bronzwaer, J.G.F.; Spreeuwenberg, M.D.; Postmus, P.E.; Vonk-Noordegraaf, A. Prognostic Value of Right Ventricular Mass, Volume, and Function in Idiopathic Pulmonary Arterial Hypertension. Eur. Heart J. 2007, 28, 1250–1257. [Google Scholar] [CrossRef]
- Knight, D.S.; Kotecha, T.; Martinez-Naharro, A.; Brown, J.T.; Bertelli, M.; Fontana, M.; Muthurangu, V.; Coghlan, J.G. Cardiovascular Magnetic Resonance-Guided Right Heart Catheterization in a Conventional CMR Environment-Predictors of Procedure Success and Duration in Pulmonary Artery Hypertension. J. Cardiovasc. Magn. Reson. 2019, 21, 57. [Google Scholar] [CrossRef]
- Moledina, S.; Pandya, B.; Bartsota, M.; Mortensen, K.H.; McMillan, M.; Quyam, S.; Taylor, A.M.; Haworth, S.G.; Schulze-Neick, I.; Muthurangu, V. Prognostic Significance of Cardiac Magnetic Resonance Imaging in Children with Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2013, 6, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Swift, A.J.; Rajaram, S.; Campbell, M.J.; Hurdman, J.; Thomas, S.; Capener, D.; Elliot, C.; Condliffe, R.; Wild, J.M.; Kiely, D.G. Prognostic Value of Cardiovascular Magnetic Resonance Imaging Measurements Corrected for Age and Sex in Idiopathic Pulmonary Arterial Hypertension. Circ. Cardiovasc. Imaging 2014, 7, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Truong, U.; Patel, S.; Kheyfets, V.; Dunning, J.; Fonseca, B.; Barker, A.J.; Ivy, D.; Shandas, R.; Hunter, K. Non-Invasive Determination by Cardiovascular Magnetic Resonance of Right Ventricular-Vascular Coupling in Children and Adolescents with Pulmonary Hypertension. J. Cardiovasc. Magn. Reson. 2015, 17, 81. [Google Scholar] [CrossRef]
- Breeman, K.T.N.; Dufva, M.; Ploegstra, M.J.; Kheyfets, V.; Willems, T.P.; Wigger, J.; Hunter, K.S.; Ivy, D.D.; Berger, R.M.F.; Truong, U. Right Ventricular-Vascular Coupling Ratio in Pediatric Pulmonary Arterial Hypertension: A Comparison between Cardiac Magnetic Resonance and Right Heart Catheterization Measurements. Int. J. Cardiol. 2019, 293, 211–217. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid Annular Plane Systolic Excursion and Pulmonary Arterial Systolic Pressure Relationship in Heart Failure: An Index of Right Ventricular Contractile Function and Prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef]
- Brener, M.I.; Lurz, P.; Hausleiter, J.; Rodés-Cabau, J.; Fam, N.; Kodali, S.K.; Rommel, K.-P.; Muntané-Carol, G.; Gavazzoni, M.; Nazif, T.M.; et al. Right Ventricular-Pulmonary Arterial Coupling and Afterload Reserve in Patients Undergoing Transcatheter Tricuspid Valve Repair. J. Am. Coll. Cardiol. 2022, 79, 448–461. [Google Scholar] [CrossRef]
- Sandeep, B.; Huang, X.; Xu, F.; Su, P.; Wang, T.; Sun, X. Etiology of Right Ventricular Restrictive Physiology Early after Repair of Tetralogy of Fallot in Pediatric Patients. J. Cardiothorac. Surg. 2019, 14, 84. [Google Scholar] [CrossRef]
- Gorter, T.M.; van Veldhuisen, D.J.; Voors, A.A.; Hummel, Y.M.; Lam, C.S.P.; Berger, R.M.F.; van Melle, J.P.; Hoendermis, E.S. Right Ventricular-Vascular Coupling in Heart Failure with Preserved Ejection Fraction and Pre- vs. Post-Capillary Pulmonary Hypertension. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 425–432. [Google Scholar] [CrossRef]
- Fernández Ruiz, A.; Ruiz Ortiz, M.; Fernández-Avilés Irache, C.; Rodríguez Almodóvar, A.M.; Delgado Ortega, M.; Esteban Martínez, F.; Resúa Collazo, A.; Heredia Campos, G.; González Manzanares, R.; López Aguilera, J.; et al. Right Ventricular-Pulmonary Arterial Coupling as a Predictor of Death or Heart Failure Admission in Patients with Severe Tricuspid Regurgitation. Rev. Esp. Cardiol. (Engl. Ed.) 2025, in press. [Google Scholar] [CrossRef]
- Shechter, A.; Vaturi, M.; Kaewkes, D.; Koren, O.; Koseki, K.; Solanki, A.; Natanzon, S.S.; Patel, V.; Skaf, S.; Makar, M.; et al. Prognostic Value of Baseline Tricuspid Annular Plane Systolic Excursion to Pulmonary Artery Systolic Pressure Ratio in Mitral Transcatheter Edge-to-Edge Repair. J. Am. Soc. Echocardiogr. 2023, 36, 391–401.e19. [Google Scholar] [CrossRef]
- Brener, M.I.; Grayburn, P.; Lindenfeld, J.; Burkhoff, D.; Liu, M.; Zhou, Z.; Alu, M.C.; Medvedofsky, D.A.; Asch, F.M.; Weissman, N.J.; et al. Right Ventricular-Pulmonary Arterial Coupling in Patients With HF Secondary MR: Analysis From the COAPT Trial. JACC Cardiovasc. Interv. 2021, 14, 2231–2242. [Google Scholar] [CrossRef]
- Eleid, M.F.; Padang, R.; Pislaru, S.V.; Greason, K.L.; Crestanello, J.; Nkomo, V.T.; Pellikka, P.A.; Jentzer, J.C.; Gulati, R.; Sandhu, G.S.; et al. Effect of Transcatheter Aortic Valve Replacement on Right Ventricular–Pulmonary Artery Coupling. JACC Cardiovasc. Interv. 2019, 12, 2145–2154. [Google Scholar] [CrossRef]
- Egbe, A.C.; Kothapalli, S.; Miranda, W.R.; Pislaru, S.; Ammash, N.M.; Borlaug, B.A.; Pellikka, P.A.; Najam, M.; Connolly, H.M. Assessment of Right Ventricular-Pulmonary Arterial Coupling in Chronic Pulmonary Regurgitation. Can. J. Cardiol. 2019, 35, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Gavazzoni, M.; Badano, L.P.; Cascella, A.; Heilbron, F.; Tomaselli, M.; Caravita, S.; Baratto, C.; Perelli, F.; Radu, N.; Perger, E.; et al. Clinical Value of a Novel Three-Dimensional Echocardiography-Derived Index of Right Ventricle-Pulmonary Artery Coupling in Tricuspid Regurgitation. J. Am. Soc. Echocardiogr. 2023, 36, 1154–1166.e3. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, S.; Bézy, S.; Cvijic, M.; Duchenne, J.; Delcroix, M.; Voigt, J.-U. Right Ventricular Strain Related to Pulmonary Artery Pressure Predicts Clinical Outcome in Patients with Pulmonary Arterial Hypertension. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Jone, P.-N.; Schäfer, M.; Pan, Z.; Ivy, D.D. Right Ventricular-Arterial Coupling Ratio Derived From 3-Dimensional Echocardiography Predicts Outcomes in Pediatric Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e008176. [Google Scholar] [CrossRef]
- Kuwajima, K.; Ogawa, M.; Ruiz, I.; Yamane, T.; Hasegawa, H.; Yagi, N.; Rader, F.; Siegel, R.J.; Shiota, T. Comparison of Prognostic Value among Echocardiographic Surrogates of Right Ventricular-Pulmonary Arterial Coupling: A Three-Dimensional Echocardiographic Study. Echocardiography 2024, 41, e15717. [Google Scholar] [CrossRef]
- Mukherjee, M.; Rudski, L.G.; Addetia, K.; Afilalo, J.; D’Alto, M.; Freed, B.H.; Friend, L.B.; Gargani, L.; Grapsa, J.; Hassoun, P.M.; et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults and Special Considerations in Pulmonary Hypertension: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 141–186. [Google Scholar] [CrossRef]
- Redfield, M.M.; Borlaug, B.A. Heart Failure With Preserved Ejection Fraction: A Review. JAMA 2023, 329, 827–838. [Google Scholar] [CrossRef]
- Campbell, P.; Rutten, F.H.; Lee, M.M.; Hawkins, N.M.; Petrie, M.C. Heart Failure with Preserved Ejection Fraction: Everything the Clinician Needs to Know. Lancet 2024, 403, 1083–1092. [Google Scholar] [CrossRef]
- Singh, I.; Oliveira, R.K.F.; Naeije, R.; Rahaghi, F.N.; Oldham, W.M.; Systrom, D.M.; Waxman, A.B. Pulmonary Vascular Distensibility and Early Pulmonary Vascular Remodeling in Pulmonary Hypertension. Chest 2019, 156, 724–732. [Google Scholar] [CrossRef]
- Melenovsky, V.; Hwang, S.-J.; Lin, G.; Redfield, M.M.; Borlaug, B.A. Right Heart Dysfunction in Heart Failure with Preserved Ejection Fraction. Eur. Heart J. 2014, 35, 3452–3462. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Pislaru, S.; Borlaug, B.A. Deterioration in Right Ventricular Structure and Function over Time in Patients with Heart Failure and Preserved Ejection Fraction. Eur. Heart J. 2019, 40, 689–697. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, I.; Ezzeddine, O.F.A.; Takahama, H.; Kwon, S.H.; Forfia, P.; Roger, V.L.; Redfield, M.M. Right Ventricular Function in Heart Failure with Preserved Ejection Fraction: A Community Based Study. Circulation 2014, 130, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Hamo, C.E.; DeJong, C.; Hartshorne-Evans, N.; Lund, L.H.; Shah, S.J.; Solomon, S.; Lam, C.S.P. Heart Failure with Preserved Ejection Fraction. Nat. Rev. Dis. Primers 2024, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Omote, K.; Sorimachi, H.; Obokata, M.; Reddy, Y.N.V.; Verbrugge, F.H.; Omar, M.; DuBrock, H.M.; Redfield, M.M.; Borlaug, B.A. Pulmonary Vascular Disease in Pulmonary Hypertension Due to Left Heart Disease: Pathophysiologic Implications. Eur. Heart J. 2022, 43, 3417–3431. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Inciardi, R.M.; Abanda, M.; Shah, A.M.; Cikes, M.; Claggett, B.L.; Prasad, N.; Lam, C.S.P.; Redfield, M.; McMurray, J.J.V.; et al. Multiparametric Assessment of Right Ventricular Dysfunction in Heart Failure: An Analysis From PARAGON-HF. J. Am. Heart Assoc. 2025, 14, e037380. [Google Scholar] [CrossRef]
- Gorter, T.M.; Hoendermis, E.S.; van Veldhuisen, D.J.; Voors, A.A.; Lam, C.S.P.; Geelhoed, B.; Willems, T.P.; van Melle, J.P. Right Ventricular Dysfunction in Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Eur. J. Heart Fail. 2016, 18, 1472–1487. [Google Scholar] [CrossRef]
- Andersen, M.J.; Hwang, S.-J.; Kane, G.C.; Melenovsky, V.; Olson, T.P.; Fetterly, K.; Borlaug, B.A. Enhanced Pulmonary Vasodilator Reserve and Abnormal Right Ventricular: Pulmonary Artery Coupling in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2015, 8, 542–550. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Kane, G.C.; Melenovsky, V.; Olson, T.P. Abnormal Right Ventricular-Pulmonary Artery Coupling with Exercise in Heart Failure with Preserved Ejection Fraction. Eur. Heart J. 2016, 37, 3293–3302. [Google Scholar] [CrossRef]
- Hussain, I.; Mohammed, S.F.; Forfia, P.R.; Lewis, G.D.; Borlaug, B.A.; Gallup, D.S.; Redfield, M.M. Impaired Right Ventricular-Pulmonary Arterial Coupling and Effect of Sildenafil in Heart Failure With Preserved Ejection Fraction: An Ancillary Analysis From the Phosphodiesterase-5 Inhibition to Improve Clinical Status And Exercise Capacity in Diastolic Heart Failure (RELAX) Trial. Circ. Heart Fail. 2016, 9, e002729. [Google Scholar] [CrossRef]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV Contractile Function and Its Coupling to Pulmonary Circulation in Heart Failure With Preserved Ejection Fraction: Stratification of Clinical Phenotypes and Outcomes. JACC Cardiovasc. Imaging 2017, 10, 1211–1221. [Google Scholar] [CrossRef]
- Bosch, L.; Lam, C.S.P.; Gong, L.; Chan, S.P.; Sim, D.; Yeo, D.; Jaufeerally, F.; Leong, K.T.G.; Ong, H.Y.; Ng, T.P.; et al. Right Ventricular Dysfunction in Left-Sided Heart Failure with Preserved versus Reduced Ejection Fraction. Eur. J. Heart Fail. 2017, 19, 1664–1671. [Google Scholar] [CrossRef]
- Gorter, T.M.; van Melle, J.P.; Rienstra, M.; Borlaug, B.A.; Hummel, Y.M.; van Gelder, I.C.; Hoendermis, E.S.; Voors, A.A.; van Veldhuisen, D.J.; Lam, C.S.P. Right Heart Dysfunction in Heart Failure With Preserved Ejection Fraction: The Impact of Atrial Fibrillation. J. Card. Fail. 2018, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Santas, E.; Palau, P.; Guazzi, M.; de la Espriella, R.; Miñana, G.; Sanchis, J.; Bayes-Genís, A.; Lupón, J.; Chorro, F.J.; Núñez, J. Usefulness of Right Ventricular to Pulmonary Circulation Coupling as an Indicator of Risk for Recurrent Admissions in Heart Failure With Preserved Ejection Fraction. Am. J. Cardiol. 2019, 124, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Yasumura, Y.; Yoshida, C.; Okumura, T.; Tateishi, J.; Yoshida, J.; Abe, H.; Tamaki, S.; Yano, M.; Hayashi, T.; et al. Prognostic Importance of Right Ventricular-Vascular Uncoupling in Acute Decompensated Heart Failure With Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2020, 13, e011430. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, N.R.; Mazzola, M.; Madonna, R.; Gargani, L.; De Biase, N.; Dini, F.L.; Taddei, S.; De Caterina, R.; Masi, S. Exercise-Induced Pulmonary Hypertension in HFpEF and HFrEF: Different Pathophysiologic Mechanism behind Similar Functional Impairment. Vascul Pharmacol. 2022, 144, 106978. [Google Scholar] [CrossRef]
- Jia, H.; Liu, L.; Bi, X.; Li, X.; Cong, H. Right Ventricular-Arterial Uncoupling as an Independent Prognostic Factor in Acute Heart Failure with Preserved Ejection Fraction Accompanied with Coronary Artery Disease. Chin. Med. J. 2023, 136, 1198–1206. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Chung, Y.-W.; Cheng, J.-F.; Huang, C.-Y.; Chen, S.-Y.; Lin, L.-Y.; Lai, H.-C.; Wu, C.-K. Right Ventricular-Vascular Uncoupling Predicts Pulmonary Hypertension in Clinically Diagnosed Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2024, 13, e030025. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Carter, R.E.; Sorimachi, H.; Omar, M.; Popovic, D.; Alogna, A.; Jensen, M.D.; Borlaug, B.A. Dapagliflozin and Right Ventricular–Pulmonary Vascular Interaction in Heart Failure With Preserved Ejection Fraction: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 843–851. [Google Scholar] [CrossRef]
- Lechuga, C.G.; Raza, F.; Colebank, M.J.; Korcarz, C.E.; Broman, A.T.; Eickhoff, J.C.; Chesler, N.C. Characteristic Pulmonary Impedance With Exercise Detects Abnormal Pulmonary Vascular Response and Uncoupling in Pulmonary Hypertension Resulting From Heart Failure With Preserved Ejection Fraction. Chest 2025, 168, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Decotto, S.; Fernandez Villar, G.; Rossi, E.; Iroulart, J.M.; Bergier, M.; Del Castillo, S.; Perez de Arenaza, D.; Lillo, E.; Bluro, I.M.; Falconi, M.L.; et al. Prognostic Value of Right Ventricle–Pulmonary Artery Uncoupling in Elderly Patients Hospitalized for Heart Failure with Preserved Ejection Fraction. Curr. Probl. Cardiol. 2025, 50, 103126. [Google Scholar] [CrossRef] [PubMed]
- Segar, M.W.; Patel, K.V.; Ayers, C.; Basit, M.; Tang, W.H.W.; Willett, D.; Berry, J.; Grodin, J.L.; Pandey, A. Phenomapping of Patients with Heart Failure with Preserved Ejection Fraction Using Machine Learning-Based Unsupervised Cluster Analysis. Eur. J. Heart Fail. 2020, 22, 148–158. [Google Scholar] [CrossRef]
- Kyodo, A.; Kanaoka, K.; Keshi, A.; Nogi, M.; Nogi, K.; Ishihara, S.; Kamon, D.; Hashimoto, Y.; Nakada, Y.; Ueda, T.; et al. Heart Failure with Preserved Ejection Fraction Phenogroup Classification Using Machine Learning. ESC Heart Fail. 2023, 10, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Omae, Y.; Harada, T.; Sorimachi, H.; Yuasa, N.; Kagami, K.; Murakami, F.; Naito, A.; Tani, Y.; Kato, T.; et al. Exercise Stress Echocardiography-Based Phenotyping of Heart Failure With Preserved Ejection Fraction. J. Am. Soc. Echocardiogr. 2024, 37, 759–768. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Reddy, Y.N.V.; Braun, A.; Sorimachi, H.; Omar, M.; Popovic, D.; Alogna, A.; Jensen, M.D.; Carter, R. Cardiac and Metabolic Effects of Dapagliflozin in Heart Failure With Preserved Ejection Fraction: The CAMEO-DAPA Trial. Circulation 2023, 148, 834–844. [Google Scholar] [CrossRef]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.-P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and One-Year Outcomes in Patients with Chronic Heart Failure and Preserved, Mid-Range and Reduced Ejection Fraction: An Analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef]
- Savarese, G.; Stolfo, D.; Sinagra, G.; Lund, L.H. Heart Failure with Mid-Range or Mildly Reduced Ejection Fraction. Nat. Rev. Cardiol. 2022, 19, 100–116. [Google Scholar] [CrossRef]
- Koh, A.S.; Tay, W.T.; Teng, T.H.K.; Vedin, O.; Benson, L.; Dahlstrom, U.; Savarese, G.; Lam, C.S.P.; Lund, L.H. A Comprehensive Population-Based Characterization of Heart Failure with Mid-Range Ejection Fraction. Eur. J. Heart Fail. 2017, 19, 1624–1634. [Google Scholar] [CrossRef]
- Rosa, G.M.; D’Agostino, A.; Giovinazzo, S.; La Malfa, G.; Fontanive, P.; Miccoli, M.; Dini, F.L. Echocardiography of Right Ventricular-Arterial Coupling Predicts Survival of Elderly Patients with Heart Failure and Reduced to Mid-Range Ejection Fraction. Monaldi Arch. Chest Dis. 2020, 90, 224–230. [Google Scholar] [CrossRef]
- Masarone, D.; Errigo, V.; Melillo, E.; Valente, F.; Gravino, R.; Verrengia, M.; Ammendola, E.; Vastarella, R.; Pacileo, G. Effects of Sacubitril/Valsartan on the Right Ventricular Arterial Coupling in Patients with Heart Failure with Reduced Ejection Fraction. J. Clin. Med. 2020, 9, 3159. [Google Scholar] [CrossRef]
- Guazzi, M.; Villani, S.; Generati, G.; Ferraro, O.E.; Pellegrino, M.; Alfonzetti, E.; Labate, V.; Gaeta, M.; Sugimoto, T.; Bandera, F. Right Ventricular Contractile Reserve and Pulmonary Circulation Uncoupling During Exercise Challenge in Heart Failure: Pathophysiology and Clinical Phenotypes. JACC Heart Fail. 2016, 4, 625–635. [Google Scholar] [CrossRef]
- Legris, V.; Thibault, B.; Dupuis, J.; White, M.; Asgar, A.W.; Fortier, A.; Pitre, C.; Bouabdallaoui, N.; Henri, C.; O’Meara, E.; et al. Right Ventricular Function and Its Coupling to Pulmonary Circulation Predicts Exercise Tolerance in Systolic Heart Failure. ESC Heart Fail. 2022, 9, 450–464. [Google Scholar] [CrossRef]
- Watson, W.D.; Burrage, M.K.; Ong, L.P.; Bhagra, S.; Garbi, M.; Pettit, S. Right Ventricular-Pulmonary Arterial Uncoupling and Ventricular-Secondary Mitral Regurgitation: Relationship with Outcomes in Advanced Heart Failure. JHLT Open 2024, 4, 100080. [Google Scholar] [CrossRef] [PubMed]
- Pestelli, G.; Fiorencis, A.; Trevisan, F.; Luisi, G.A.; Smarrazzo, V.; Mele, D. New Measures of Right Ventricle-Pulmonary Artery Coupling in Heart Failure: An All-Cause Mortality Echocardiographic Study. Int. J. Cardiol. 2021, 329, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kim, M.; Park, J.J.; Park, J.-B.; Cho, G.-Y. Prognostic Role of RVGLS/PASP Ratio, a New Echocardiographic Parameter of the Right Ventricle-Pulmonary Artery Coupling, in Patients With Acute Heart Failure. Int. J. Heart Fail. 2024, 6, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Vîjîiac, A.; Onciul, S.; Deaconu, S.; Vătășescu, R.; Guzu, C.; Verinceanu, V.; Scărlătescu, A.; Zamfir, D.; Petre, I.; Scafa-Udriște, A.; et al. Three-Dimensional Right Ventriculo-Arterial Coupling as an Independent Determinant of Severe Heart Failure Symptoms in Patients with Dilated Cardiomyopathy. Echocardiography 2022, 39, 194–203. [Google Scholar] [CrossRef]
- Iacoviello, M.; Monitillo, F.; Citarelli, G.; Leone, M.; Grande, D.; Antoncecchi, V.; Rizzo, C.; Terlizzese, P.; Romito, R.; Caldarola, P.; et al. Right Ventriculo-Arterial Coupling Assessed by Two-Dimensional Strain: A New Parameter of Right Ventricular Function Independently Associated with Prognosis in Chronic Heart Failure Patients. Int. J. Cardiol. 2017, 241, 318–321. [Google Scholar] [CrossRef]
- Vîjîiac, A.; Bătăilă, V.; Onciul, S.; Verinceanu, V.; Guzu, C.; Deaconu, S.; Petre, I.; Scărlătescu, A.; Zamfir, D.; Dorobanţu, M. Non-Invasive Right Ventriculo-Arterial Coupling as a Rehospitalization Predictor in Dilated Cardiomyopathy: A Comparison of Five Different Methods. Kardiol. Pol. 2022, 80, 182–190. [Google Scholar] [CrossRef]
- Deaconu, S.; Deaconu, A.; Scarlatescu, A.; Petre, I.; Onciul, S.; Vijiac, A.; Onut, R.; Zamfir, D.; Marascu, G.; Iorgulescu, C.; et al. Right Ventricular-Arterial Coupling—A New Perspective for Right Ventricle Evaluation in Heart Failure Patients Undergoing Cardiac Resynchronization Therapy. Echocardiography 2021, 38, 1157–1164. [Google Scholar] [CrossRef]
- Schmeisser, A.; Rauwolf, T.; Groscheck, T.; Kropf, S.; Luani, B.; Tanev, I.; Hansen, M.; Meißler, S.; Steendijk, P.; Braun-Dullaeus, R.C. Pressure-Volume Loop Validation of TAPSE/PASP for Right Ventricular Arterial Coupling in Heart Failure with Pulmonary Hypertension. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 168–176. [Google Scholar] [CrossRef]
- Sciaccaluga, C.; D’Ascenzi, F.; Mandoli, G.E.; Rizzo, L.; Sisti, N.; Carrucola, C.; Cameli, P.; Bigio, E.; Mondillo, S.; Cameli, M. Traditional and Novel Imaging of Right Ventricular Function in Patients with Heart Failure and Reduced Ejection Fraction. Curr. Heart Fail. Rep. 2020, 17, 28–33. [Google Scholar] [CrossRef]
- Hădăreanu, C.-D.; Hădăreanu, D.-R.; Toader, D.-M.; Iovănescu, M.-L.; Florescu, C.; Raicea, V.-C.; Donoiu, I. Prognostic Value of the Ratio between Right Ventricular Free Wall Longitudinal Strain and Systolic Pulmonary Artery Pressure in Patients with Heart Failure with Reduced Ejection Fraction and Ventricular Secondary Mitral Regurgitation. Front. Cardiovasc. Med. 2025, 12, 1611772. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).