Accuracy of Analog and Digital Full-Arch Mandibular Impressions: In Vitro and In Vivo Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- -
- Inclusion criteria: young patients (18–30 years), intact dental arches, healthy periodontal status, no edentulous spaces or prosthetic works, no current orthodontic treatment.
- -
- Exclusion criteria: exaggerated vomiting reflex, poor oral hygiene, malpositioned teeth, small mouth opening.
2.2. Sample Size
2.3. Transfer Aid
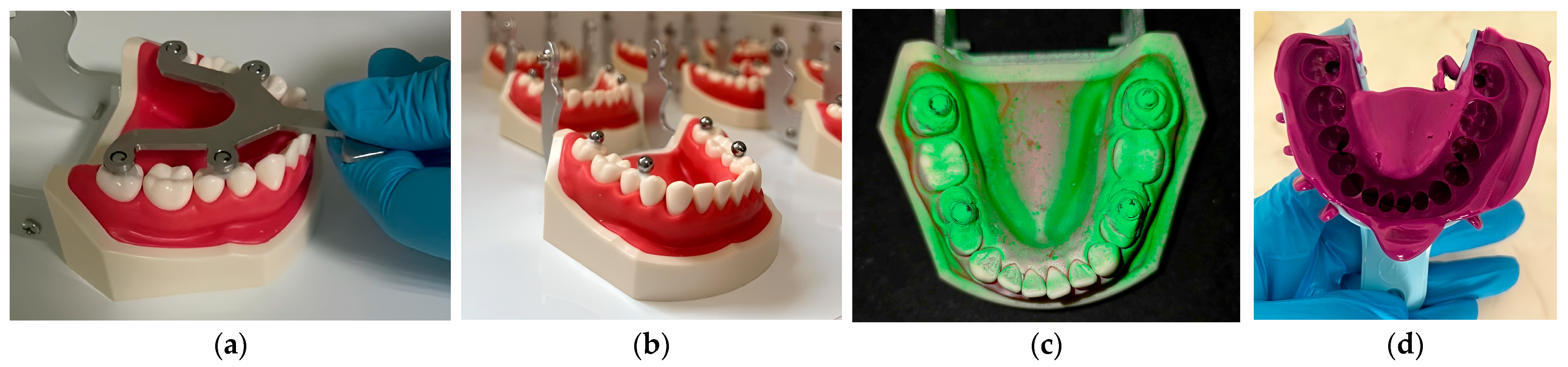
2.4. In Vitro Methodology
2.5. In Vivo Methodology
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brintha, J.J.; Anitha, K.V. Evolution of Impression Tray and Materials—A Literature Review. J. Clin. Prosthodont. Implantol. 2021, 3, 37–41. [Google Scholar] [CrossRef]
- Pinsathanon, P.; Bhattarai, B.P.; Suphangul, S.; Wongsirichat, N.; Aimjirakul, N. Penetration and Tensile Strength of Various Impression Materials of Vinylsiloxanether, Polyether, and Polyvinylsiloxane Impression Materials. Eur. J. Dent. 2022, 16, 339–345. [Google Scholar] [CrossRef]
- Theocharidou, A.; Tzimas, K.; Tolidis, K.; Tortopidis, D. Evaluation of Elastomeric Impression Materials’ Hydrophilicity: An In Vitro Study. Acta Stomatol. Croat. 2021, 55, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Stumpel, L.J. An Analog Intermezzo in a Digital Workflow. Compend. Contin. Educ. Dent. 2020, 41, 536–542. [Google Scholar]
- Albayrak, B.; Sukotjo, C.; Wee, A.G.; Korkmaz, İ.H.; Bayındır, F. Three-Dimensional Accuracy of Conventional Versus Digital Complete Arch Implant Impressions. J. Prosthodont. 2021, 30, 163–170. [Google Scholar] [CrossRef]
- Rose, S.; Aravindakshan, S.; Mohamed Usman, J.A.; Mohamed, R.; Menon, S.; Shafiullah, R.S.; Salloum, M.G. Comparative Evaluation of Surface Detail Reproduction and Dimensional Stability of Poly Ether, Vinyl Siloxane, and Vinyl Siloxane Ether Impression Materials: An In vitro Study. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. S1), S851–S856. [Google Scholar] [CrossRef] [PubMed]
- Parize, H.; Dias Corpa Tardelli, J.; Bohner, L.; Sesma, N.; Muglia, V.A.; Cândido Dos Reis, A. Digital Versus Conventional Workflow for the Fabrication of Physical Casts for Fixed Prosthodontics: A Systematic Review of Accuracy. J. Prosthet. Dent. 2022, 128, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lakhia, S.; Turkyilmaz, I.; Romanos, G. Challenges of Integrating Intraoral Optical Scanners into High-Volume Dental Facilities. Compend. Contin. Educ. Dent. 2020, 41, 554–556. [Google Scholar]
- Ural, Ç.; Kaleli, N. Direct Digitalization Devices in Today’s Dental Practice: Intraoral Scanners. J. Exp. Clin. Med. 2021, 38, 136–142. [Google Scholar] [CrossRef]
- Huettig, F.; Klink, A.; Kohler, A.; Mutschler, M.; Rupp, F. Flowability, Tear Strength, and Hydrophilicity of Current Elastomers for Dental Impressions. Materials 2021, 14, 2994. [Google Scholar] [CrossRef]
- Imburgia, M.; Logozzo, S.; Hauschild, U.; Veronesi, G.; Mangano, C.; Mangano, F.G. Accuracy of Four Intraoral Scanners in Oral Implantology: A Comparative In Vitro Study. BMC Oral Health 2017, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Punj, A.; Bompolaki, D.; Garaicoa, J. Dental Impression Materials and Techniques. Dent. Clin. N. Am. 2017, 61, 779–796. [Google Scholar] [CrossRef]
- Tallarico, M. Computerization and Digital Workflow in Medicine: Focus on Digital Dentistry. Materials 2020, 13, 2172. [Google Scholar] [CrossRef]
- Arcas, L.; Tribst, J.; Baroudi, K.; Amaral, M.; Silva-Concilio, L.; Vitti, R. Dimensional Accuracy Comparison of Physical Models Generated by Digital Impression/3D-Printing or Analog Impression/Plaster Methods. Int. J. Odontostomatol. 2021, 15, 562–568. [Google Scholar] [CrossRef]
- Nulty, A.B. A Comparison of Full Arch Trueness and Precision of Nine Intra-Oral Digital Scanners and Four Lab Digital Scanners. Dent. J. 2021, 9, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keul, C.; Güth, J.F. Accuracy of Full-Arch Digital Impressions: An In Vitro and In Vivo Comparison. Clin. Oral Investig. 2020, 24, 735–745. [Google Scholar] [CrossRef]
- Güth, J.F.; Edelhoff, D.; Schweiger, J.; Keul, C. A New Method for the Evaluation of the Accuracy of Full-Arch Digital Impressions In Vitro. Clin. Oral Investig. 2016, 20, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Schlenz, M.A.; Stillersfeld, J.M.; Wöstmann, B.; Schmidt, A. Update on the Accuracy of Conventional and Digital Full-Arch Impressions of Partially Edentulous and Fully Dentate Jaws in Young and Elderly Subjects: A Clinical Trial. J. Clin. Med. 2022, 11, 3723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schlenz, M.A.; Klaus, K.; Schmidt, A.; Wöstmann, B.; Mersmann, M.; Ruf, S.; Bock, N.C. The Transfer Accuracy of Digital and Conventional Full-Arch Impressions Influenced by Fixed Orthodontic Appliances: A Reference Aid–Based In Vitro Study. Clin. Oral Investig. 2023, 27, 273–283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt, A.; Klussmann, L.; Wöstmann, B.; Schlenz, M.A. Accuracy of Digital and Conventional Full-Arch Impressions in Patients: An Update. J. Clin. Med. 2020, 9, 688. [Google Scholar] [CrossRef]
- Kuhr, F.; Schmidt, A.; Rehmann, P.; Wöstmann, B. A New Method for Assessing the Accuracy of Full Arch Impressions in Patients. J. Dent. 2016, 55, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Sayed, M.E.; Khawaji, R.A.A.; Hakami, G.A.J.; Solan, E.H.M.; Daish, M.A.; Jokhadar, H.F.; AlResayes, S.S.; Altoman, M.S.; Alshehri, A.H.; et al. Accuracy of 3 Intraoral Scanners in Recording Impressions for Full Arch Dental Implant-Supported Prosthesis: An In Vitro Study. Med. Sci. Monit. 2024, 30, e946624. [Google Scholar] [CrossRef]
- Negucioiu, M.; Buduru, S.; Coman, D.; Condor, A.M.; Berar, A.; Condor, D.; Buduru, R. Digital impressions versus traditional impressions: An overview on accuracy, time and patient preferences. Rom. J. Oral Rehabil. 2024, 16, 755–769. [Google Scholar] [CrossRef]
- Passos, L.; Meiga, S.; Brigagão, V.; Street, A. Impact of Different Scanning Strategies on the Accuracy of Two Current Intraoral Scanning Systems in Complete-Arch Impressions: An In Vitro Study. Int. J. Comput. Dent. 2019, 22, 307–319. [Google Scholar] [PubMed]
- Vogel, A.B.; Kilic, F.; Schmidt, F.; Rübel, S.; Lapatki, B.G. Dimensional Accuracy of Jaw Scans Performed on Alginate Impressions or Stone Models: A Practice-Oriented Study. J. Orofac. Orthop. 2015, 76, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Ender, A.; Zimmermann, M.; Mehl, A. Accuracy of Complete- and Partial-Arch Impressions of Actual Intraoral Scanning Systems In Vitro. Int. J. Comput. Dent. 2019, 22, 11–19. [Google Scholar] [PubMed]
- Ender, A.; Zimmermann, M.; Attin, T.; Mehl, A. In Vivo Precision of Conventional and Digital Methods for Obtaining Quadrant Dental Impressions. Clin. Oral Investig. 2016, 20, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Ireland, A.J.; McNamara, C.; Clover, M.J.; House, K.; Wenger, N.; Barbour, M.E.; Alemzadeh, K.; Zhang, L.; Sandy, J.R. 3D Surface Imaging in Dentistry—What We Are Looking at. Br. Dent. J. 2008, 205, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Giachetti, L.; Sarti, C.; Cinelli, F.; Russo, D.S. Accuracy of Digital Impressions in Fixed Prosthodontics: A Systematic Review of Clinical Studies. Int. J. Prosthodont. 2020, 33, 192–201. [Google Scholar] [CrossRef]
- Tsirogiannis, P.; Neophytou, S.; Reul, A.; Heydecke, G.; Reissmann, D.R. Can We Measure Patients’ Perception During Dental Impressions? The Burdens in Dental Impression-Making Questionnaire—BiDIM-Q. J. Prosthodont. Res. 2017, 61, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bandiaky, O.N.; Le Bars, P.; Gaudin, A.; Hardouin, J.B.; Cheraud-Carpentier, M.; Mbodj, E.B.; Soueidan, A. Comparative Assessment of Complete-Coverage, Fixed Tooth-Supported Prostheses Fabricated from Digital Scans or Conventional Impressions: A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2022, 127, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hasanzade, M.; Shirani, M.; Afrashtehfar, K.I.; Naseri, P.; Alikhasi, M. In Vivo and In Vitro Comparison of Internal and Marginal Fit of Digital and Conventional Impressions for Full-Coverage Fixed Restorations: A Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2019, 19, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Jánosi, K.M.; Cerghizan, D.; Mártha, K.I.; Elekes, É.; Szakács, B.; Elekes, Z.; Kovács, A.; Szász, A.; Mureșan, I.; Hănțoiu, L.G. Evaluation of Intraoral Full-Arch Scan versus Conventional Preliminary Impression. J. Clin. Med. 2023, 12, 5508. [Google Scholar] [CrossRef] [PubMed]
- Kontis, P.; Güth, J.F.; Keul, C. Accuracy of full-arch digitalization for partially edentulous jaws—A laboratory study on basis of coordinate-based data analysis. Clin. Oral Investig. 2022, 26, 3651–3662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, A.V.; Vo, N.T.N.; Nguyen, T.T.; Tong, S.M. Evaluation of the trueness and precision of ceramic laminate veneers fabricated with four computer-aided manufacturing methods. Adv. Appl. Ceram. 2024, 123, 48–55. [Google Scholar] [CrossRef]
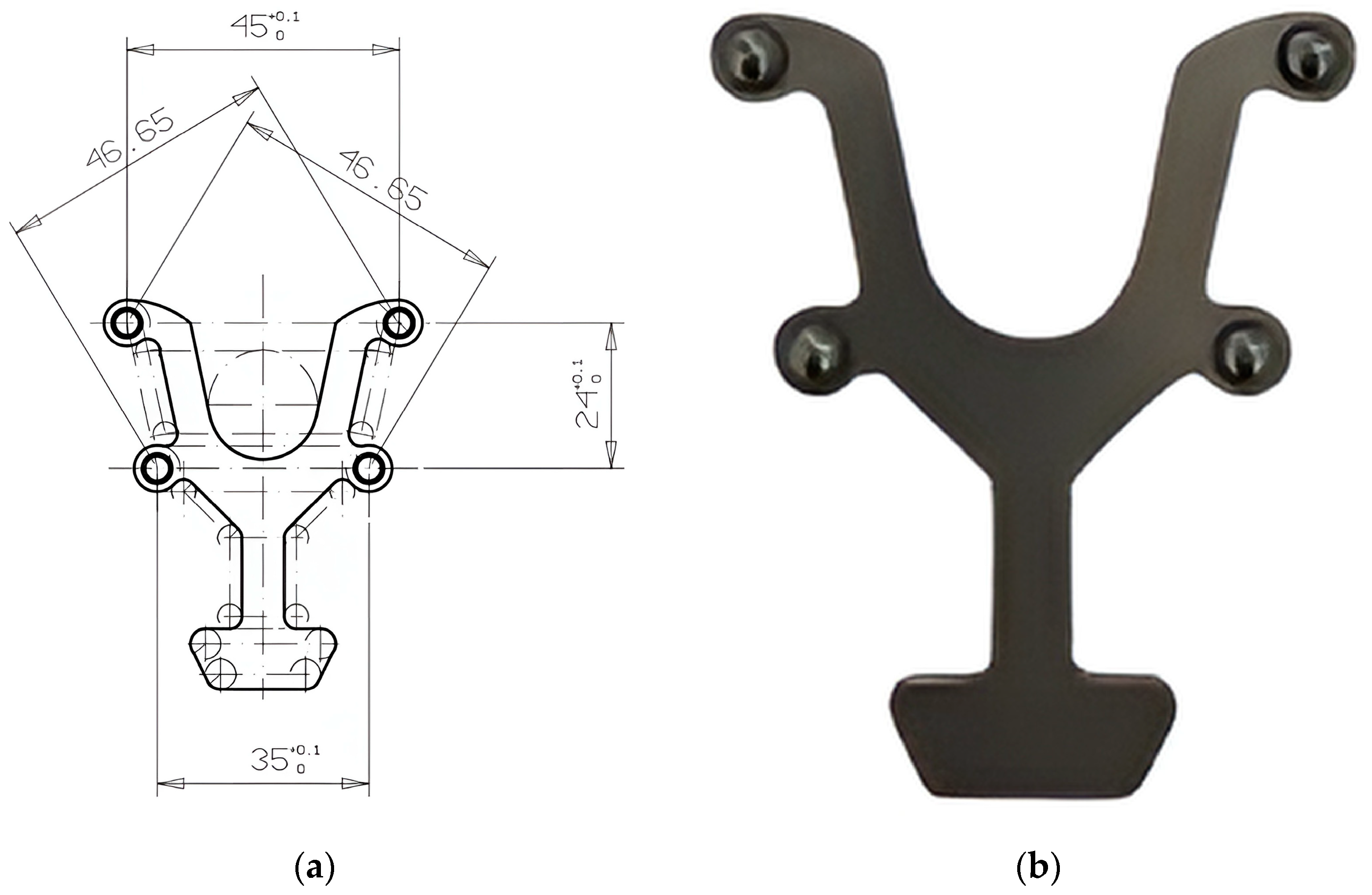
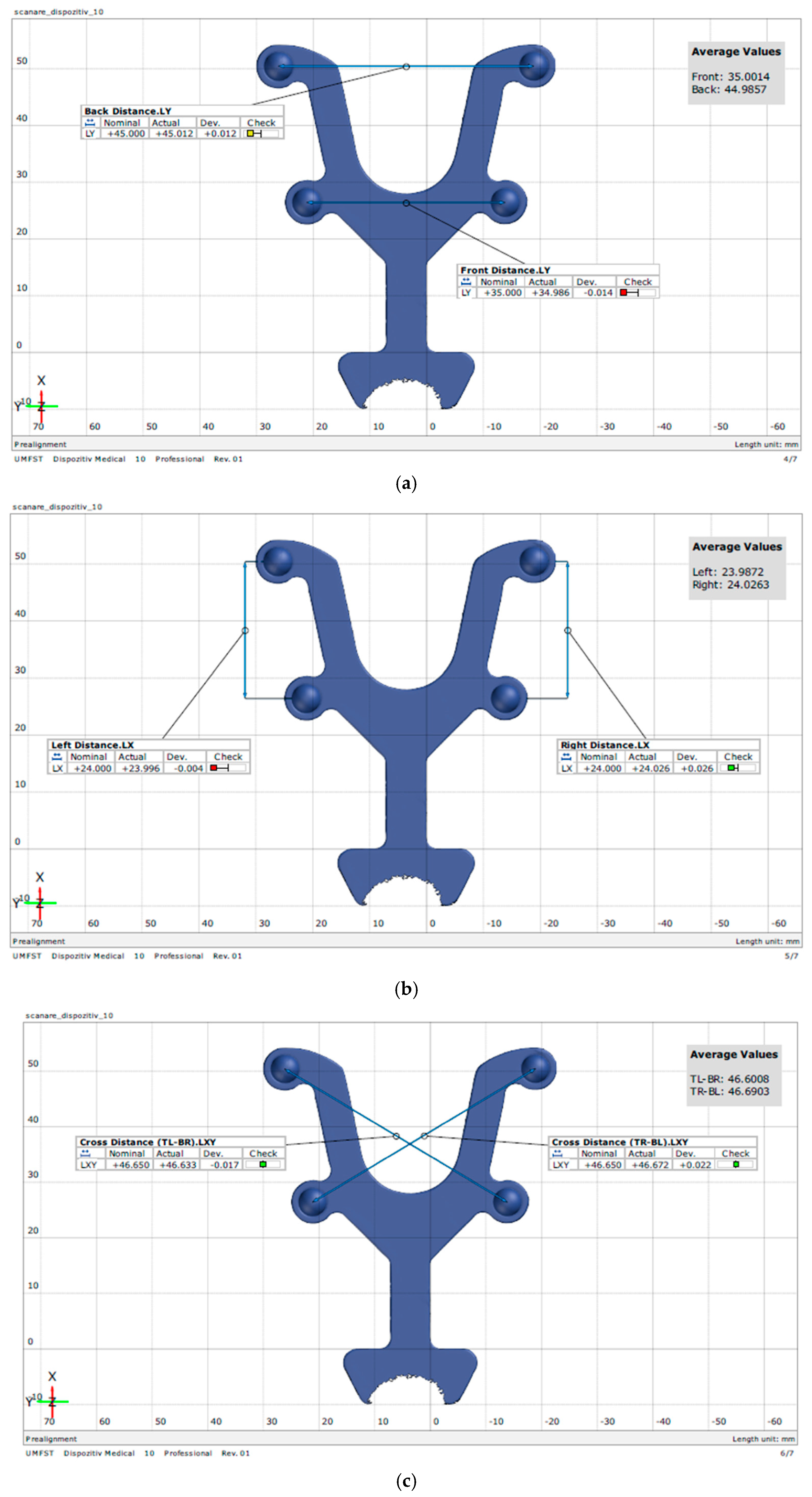
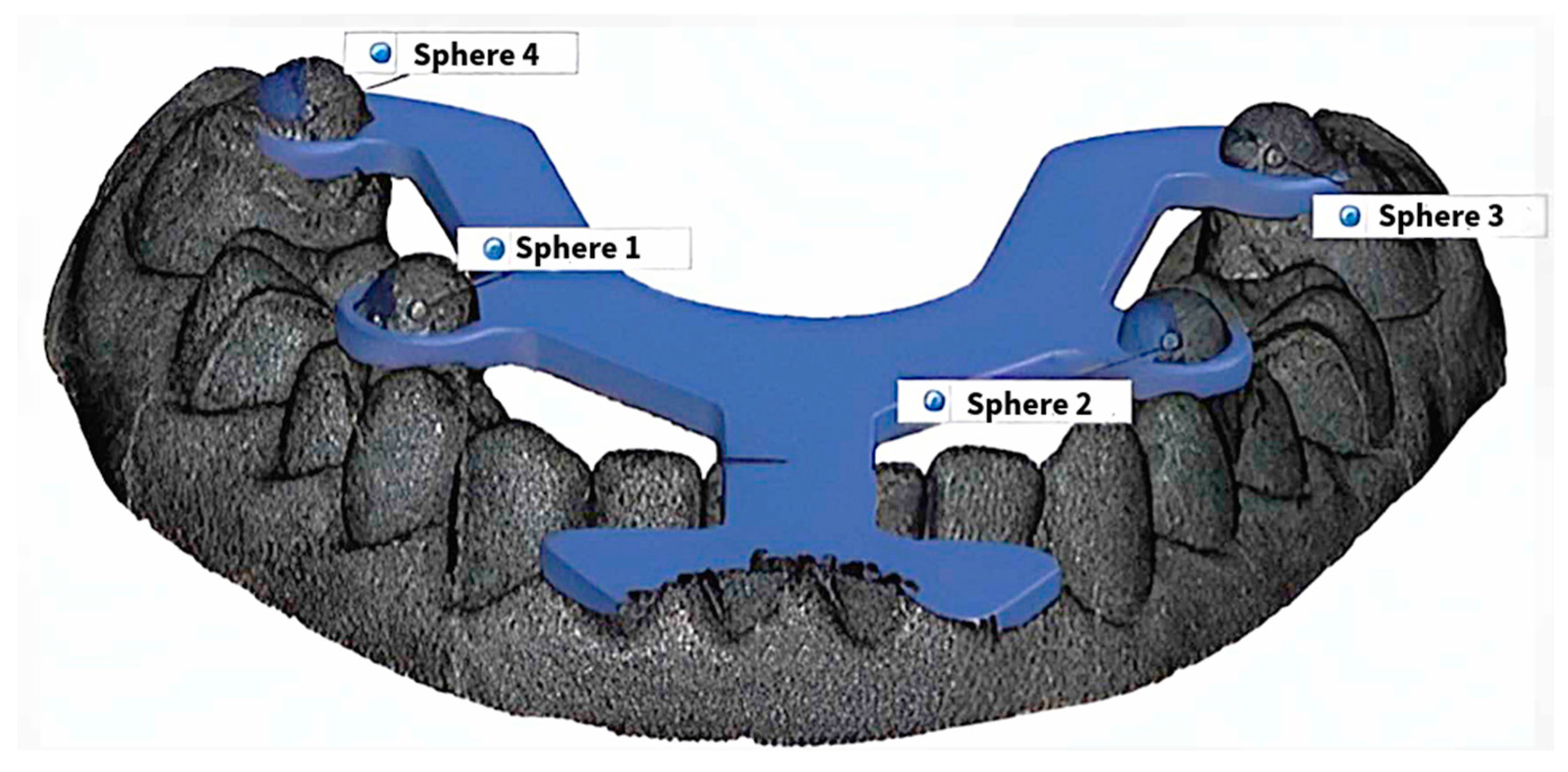
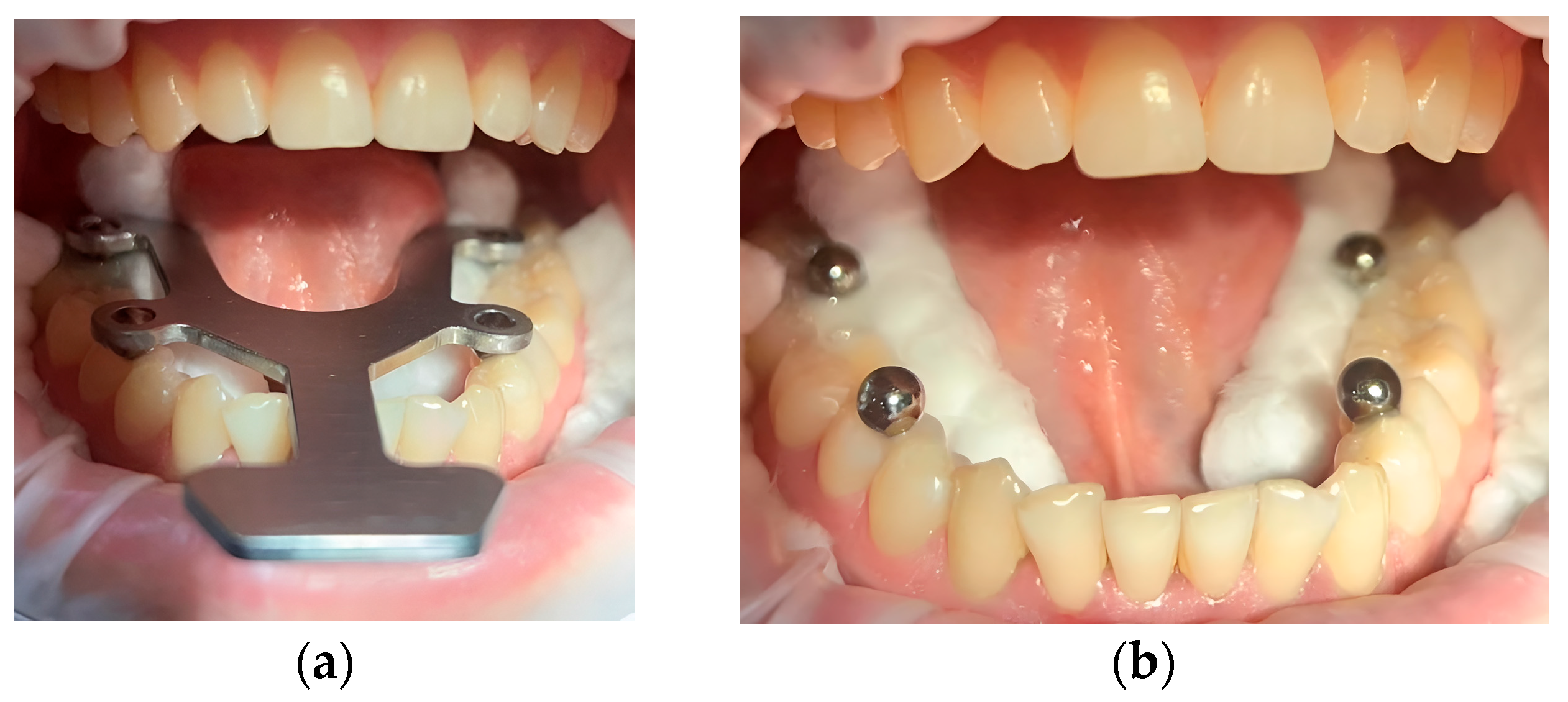
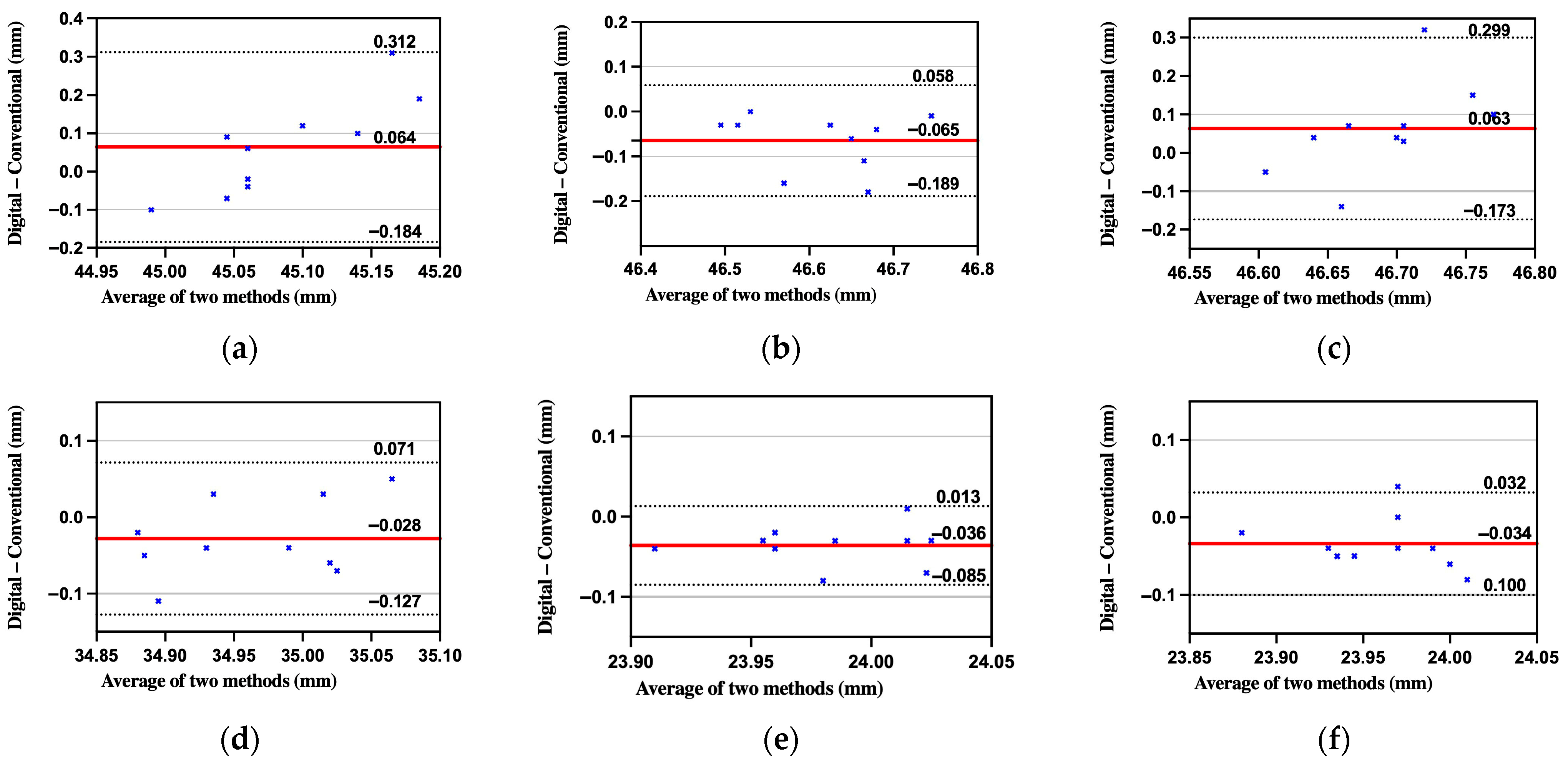
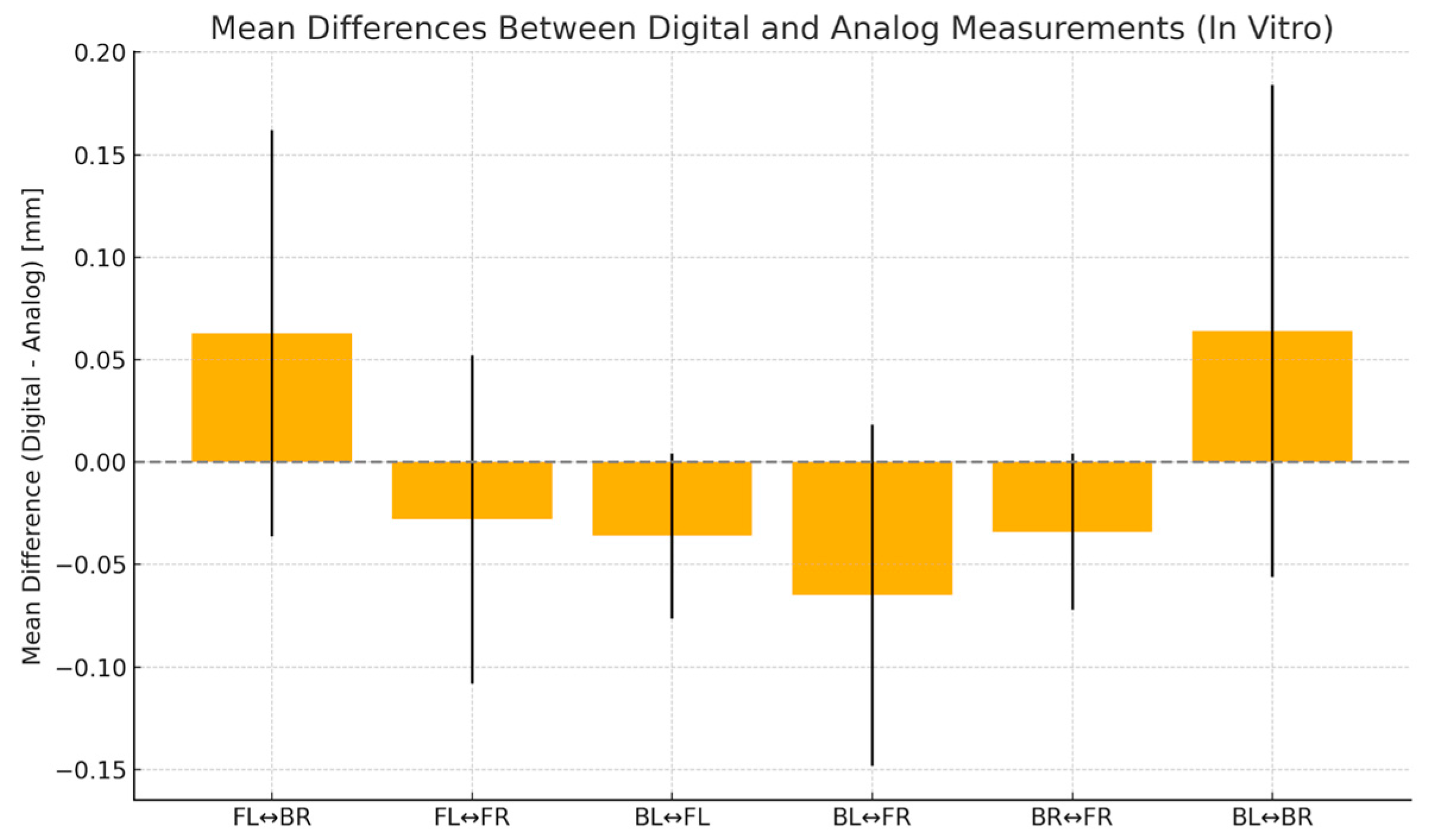

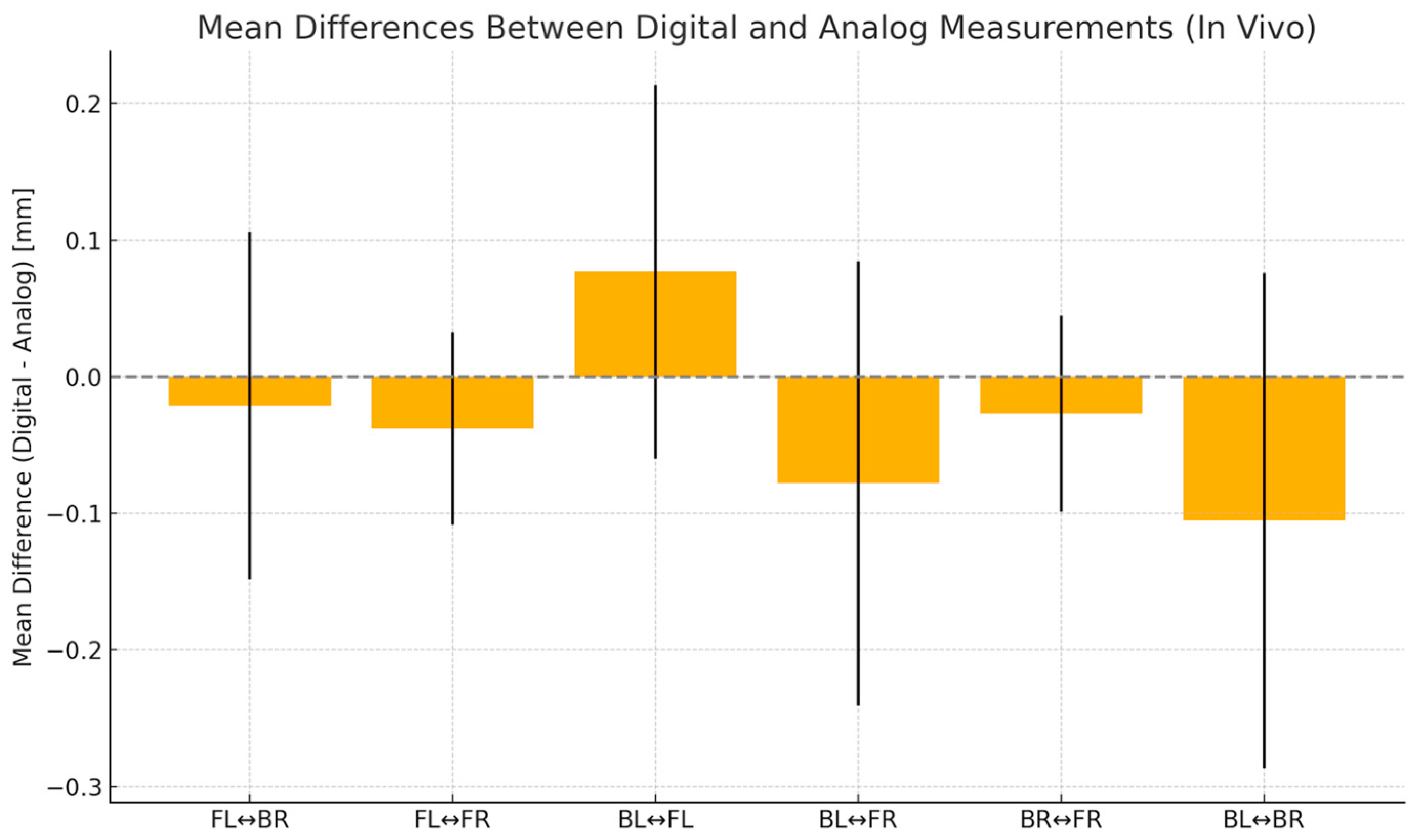
| BL-BR | BL-FR | FL-BR | FL-FR | BL-FL | BR-FR | |
|---|---|---|---|---|---|---|
| Number of values | 10 | 10 | 10 | 10 | 10 | 10 |
| Minimum | 45.00 | 46.51 | 46.56 | 34.89 | 23.93 | 23.89 |
| Maximum | 45.09 | 46.76 | 46.73 | 35.06 | 24.06 | 24.05 |
| Mean | 45.05 | 46.65 | 46.66 | 34.98 | 24.00 | 23.98 |
| Std. Deviation | 0.03335 | 0.09358 | 0.05131 | 0.06197 | 0.03900 | 0.04572 |
| Lower 95% CI of mean | 45.03 | 46.58 | 46.62 | 34.93 | 23.97 | 23.94 |
| Upper 95% CI of mean | 45.08 | 46.71 | 46.70 | 35.02 | 24.03 | 24.01 |
| BL-BR | BL-FR | FL-BR | FL-FR | BL-FL | BR-FR | |
|---|---|---|---|---|---|---|
| Number of values | 10 | 10 | 10 | 10 | 10 | 10 |
| Minimum | 44.94 | 46.48 | 46.58 | 34.84 | 23.89 | 23.87 |
| Maximum | 45.32 | 46.74 | 46.88 | 35.09 | 24.02 | 23.99 |
| Mean | 45.12 | 46.58 | 46.72 | 34.95 | 23.97 | 23.94 |
| Std. Deviation | 0.1200 | 0.08324 | 0.09913 | 0.08014 | 0.04035 | 0.03831 |
| Lower 95% CI of mean | 45.03 | 46.52 | 46.65 | 34.89 | 23.94 | 23.92 |
| Upper 95% CI of mean | 45.20 | 46.64 | 46.79 | 35.01 | 23.99 | 23.97 |
| Distance | Absolute Mean Error (mm) | RMSE (mm) | Dahlberg Error (mm) | Paired t-Test p | ICC |
|---|---|---|---|---|---|
| BL-BR | 0.110 | 0.136 | 0.096 | 0.144 | −0.064 |
| BL-FR | 0.065 | 0.088 | 0.062 | 0.010 | 0.751 |
| FL-BR | 0.101 | 0.131 | 0.092 | 0.133 | −0.208 |
| FL-FR | 0.050 | 0.056 | 0.039 | 0.115 | 0.774 |
| BL-FL | 0.038 | 0.043 | 0.030 | 0.001 | 0.801 |
| BR-FR | 0.042 | 0.047 | 0.033 | 0.011 | 0.691 |
| BL-BR | BL-FR | FL-BR | FL-FR | BL-FL | BR-FR | |
|---|---|---|---|---|---|---|
| Theoretical mean | 45.00 | 46.65 | 46.65 | 35.00 | 24.00 | 24.00 |
| Actual mean | 45.05 | 46.65 | 46.66 | 34.98 | 24.00 | 23.98 |
| p value (two tailed) | 0.0007 | 0.9215 | 0.5148 | 0.2906 | 0.9372 | 0.1461 |
| p value summary | *** | ns | ns | ns | ns | ns |
| Significant (alpha = 0.05)? | Yes | No | No | No | No | No |
| Discrepancy (trueness) | 0.0530 | −0.0030 | 0.01100 | −0.02200 | 0.001000 | −0.02300 |
| SD of discrepancy (precision) | 0.03335 | 0.09358 | 0.05131 | 0.06197 | 0.03900 | 0.04572 |
| SEM of discrepancy | 0.01055 | 0.02959 | 0.01622 | 0.01960 | 0.01233 | 0.01446 |
| 95% confidence interval | 0.02914 to 0.07686 | −0.06994 to 0.06394 | −0.02570 to 0.04770 | −0.06633 to 0.02233 | −0.02690 to 0.02890 | −0.05570 to 0.009704 |
| BL-BR | BL-FR | FL-BR | FL-FR | BL-FL | BR-FR | |
|---|---|---|---|---|---|---|
| Theoretical mean | 45.00 | 46.65 | 46.65 | 46.65 | 24.00 | 24.00 |
| Actual mean | 45.12 | 46.58 | 46.72 | 46.72 | 23.97 | 23.94 |
| p value (two tailed) | 0.0131 | 0.0295 | 0.0426 | 0.0426 | 0.0227 | 0.0011 |
| p value summary | * | * | * | * | * | ** |
| Significant (alpha = 0.05)? | Yes | Yes | Yes | Yes | Yes | Yes |
| Discrepancy (trueness) | 0.1170 | −0.06800 | 0.07400 | 0.07400 | −0.03500 | −0.05700 |
| SD of discrepancy (precision) | 0.1200 | 0.08324 | 0.09913 | 0.09913 | 0.04035 | 0.03831 |
| SEM of discrepancy | 0.03795 | 0.02632 | 0.03135 | 0.03135 | 0.01276 | 0.01212 |
| 95% confidence interval | 0.03115 to 0.2028 | −0.1275 to −0.008454 | 0.003087 to 0.1449 | 0.003087 to 0.1449 | −0.06386 to −0.006138 | −0.08441 to −0.02959 |
| BL-BR | BL-FR | FL-BR | FL-FR | BL-FL | BR-FR | |
|---|---|---|---|---|---|---|
| Number of values | 10 | 10 | 10 | 10 | 10 | 10 |
| Minimum | 44.94 | 46.58 | 46.39 | 34.89 | 23.26 | 23.92 |
| Maximum | 45.19 | 46.96 | 46.95 | 35.09 | 24.36 | 24.36 |
| Mean | 45.09 | 46.74 | 46.69 | 35.01 | 24.01 | 24.09 |
| Std. Deviation | 0.08873 | 0.1287 | 0.1484 | 0.05547 | 0.3079 | 0.1312 |
| Lower 95% CI of mean | 0.02806 | 0.04069 | 0.04691 | 0.01754 | 0.09738 | 0.04149 |
| Upper 95% CI of mean | 45.02 | 46.65 | 46.58 | 34.97 | 23.79 | 23.99 |
| BL-BR | BL-FR | FL-BR | FL-FR | BL-FL | BR-FR | |
|---|---|---|---|---|---|---|
| Number of values | 10 | 10 | 10 | 10 | 10 | 10 |
| Minimum | 44.76 | 46.42 | 46.49 | 34.8 | 23.88 | 23.97 |
| Maximum | 45.4 | 46.91 | 46.86 | 35.05 | 24.34 | 24.22 |
| Mean | 44.98 | 46.66 | 46.67 | 34.97 | 24.09 | 24.06 |
| Std. Deviation | 0.1814 | 0.1627 | 0.1273 | 0.07047 | 0.1368 | 0.07203 |
| Lower 95% CI of mean | 44.85 | 46.55 | 46.58 | 34.92 | 23.99 | 24.01 |
| Upper 95% CI of mean | 45.11 | 46.78 | 46.76 | 35.02 | 24.19 | 24.11 |
| Distance | Absolute Mean Error (mm) | RMSE (mm) | Dahlberg Error (mm) | Paired t-Test p | ICC |
|---|---|---|---|---|---|
| BL-BR | 0.175 | 0.200 | 0.142 | 0.098 | 0.263 |
| BL-FR | 0.110 | 0.125 | 0.088 | 0.040 | 0.776 |
| FL-BR | 0.113 | 0.139 | 0.098 | 0.657 | 0.457 |
| FL-FR | 0.046 | 0.052 | 0.037 | 0.011 | 0.847 |
| BL-FL | 0.133 | 0.289 | 0.205 | 0.429 | 0.322 |
| BR-FR | 0.051 | 0.067 | 0.047 | 0.219 | 0.964 |
| BL-BR | BL-FR | FL-BR | FL-FR | BL-FL | BR-FR | |
|---|---|---|---|---|---|---|
| Theoretical mean | 45.00 | 46.65 | 46.65 | 35 | 24 | 24 |
| Actual mean | 45.09 | 46.74 | 46.69 | 35.01 | 24.01 | 24.09 |
| p value (two tailed) | 0.0143 | 0.0543 | 0.4049 | 0.6202 | 0.8967 | 0.063 |
| p value summary | * | ns | ns | ns | ns | ns |
| Significant (alpha = 0.05)? | Yes | No | No | No | No | No |
| Discrepancy (trueness) | 0.085 | 0.09 | 0.041 | 0.009 | 0.013 | 0.088 |
| SD of discrepancy (precision) | 0.08873 | 0.1287 | 0.1484 | 0.05547 | 0.3079 | 0.1312 |
| SEM of discrepancy | 0.02806 | 0.04069 | 0.04691 | 0.01754 | 0.09738 | 0.04149 |
| 95% confidence interval | 0.02153 to 0.1485 | −0.002044 to 0.1820 | −0.06513 to 0.1471 | −0.03068 to 0.04868 | −0.2073 to 0.2333 | −0.005867 to 0.1819 |
| BL-BR | BL-FR | FL-BR | FL-FR | BL-FL | BR-FR | |
|---|---|---|---|---|---|---|
| Theoretical mean | 45 | 46.65 | 46.65 | 35 | 24 | 24 |
| Actual mean | 44.98 | 46.66 | 46.67 | 34.97 | 24.09 | 24.06 |
| p value (two tailed) | 0.7354 | 0.8208 | 0.6312 | 0.2254 | 0.0672 | 0.0253 |
| p value summary | ns | ns | ns | ns | ns | * |
| Significant (alpha = 0.05)? | No | No | No | No | No | Yes |
| Discrepancy (trueness) | −0.02 | 0.012 | 0.02 | −0.029 | 0.09 | 0.061 |
| SD of discrepancy (precision) | 0.1814 | 0.1627 | 0.1273 | 0.07047 | 0.1368 | 0.07203 |
| SEM of discrepancy | 0.05737 | 0.05146 | 0.04025 | 0.02228 | 0.04326 | 0.02278 |
| 95% confidence interval | −0.1498 to 0.1098 | −0.1044 to 0.1284 | −0.07105 to 0.1111 | −0.07941 to 0.02141 | −0.007853 to 0.1879 | 0.009476 to 0.1125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerghizan, D.; Jánosi, K.M.; Farcas, A.; Bojan, M.M.; Muntean, M.H.; Nechiti, A.A.M.; Mureșan, I.É.; Pop, S.I.; Marada, G. Accuracy of Analog and Digital Full-Arch Mandibular Impressions: In Vitro and In Vivo Evaluation. Diagnostics 2025, 15, 2077. https://doi.org/10.3390/diagnostics15162077
Cerghizan D, Jánosi KM, Farcas A, Bojan MM, Muntean MH, Nechiti AAM, Mureșan IÉ, Pop SI, Marada G. Accuracy of Analog and Digital Full-Arch Mandibular Impressions: In Vitro and In Vivo Evaluation. Diagnostics. 2025; 15(16):2077. https://doi.org/10.3390/diagnostics15162077
Chicago/Turabian StyleCerghizan, Diana, Kinga Mária Jánosi, Alexandra Farcas, Marcel Mihai Bojan, Mircea Horia Muntean, Andreea Ana Maria Nechiti, Izabella Éva Mureșan, Silvia Izabella Pop, and Gyula Marada. 2025. "Accuracy of Analog and Digital Full-Arch Mandibular Impressions: In Vitro and In Vivo Evaluation" Diagnostics 15, no. 16: 2077. https://doi.org/10.3390/diagnostics15162077
APA StyleCerghizan, D., Jánosi, K. M., Farcas, A., Bojan, M. M., Muntean, M. H., Nechiti, A. A. M., Mureșan, I. É., Pop, S. I., & Marada, G. (2025). Accuracy of Analog and Digital Full-Arch Mandibular Impressions: In Vitro and In Vivo Evaluation. Diagnostics, 15(16), 2077. https://doi.org/10.3390/diagnostics15162077






