Abstract
Background: Acute Kidney Injury (AKI) is a pivotal concern in neurocritical care, impacting patient survival and quality of life. This study harnesses machine learning (ML) techniques to predict the occurrence of AKI in patients receiving hyperosmolar therapy, aiming to optimize patient outcomes in neurocritical settings. Methods: We conducted a retrospective cohort study of 4886 patients who underwent hyperosmolar therapy in the neurosurgical intensive care unit (ICU). Comparative predictive analyses were carried out using advanced ML algorithms—eXtreme Gradient Boosting (XGBoost), Gradient Boosting Machine (GBM), Random Forest (RF)—against standard multivariate logistic regression. Predictive performance was assessed using an 8:2 training-testing data split, with model fine-tuning through cross-validation. Results: The RF with KNN imputation showed slightly better performance than other approaches in predicting AKI. When applied to an independent test set, it achieved a sensitivity of 79% (95% CI: 70–87%) and specificity of 85% (95% CI: 82–88%), with an overall accuracy of 84% (95% CI: 81–87%) and AUROC of 0.86 (95% CI: 0.82–0.91). The multivariate logistic regression analysis, while informative, showed less predictive strength compared to the ML models. Delta chloride levels and serum osmolality proved to be the most influential predictors, with additional significant variables including pH, age, bicarbonate, and the osmolar gap. Conclusions: The prominence of delta chloride and serum osmolality among the predictive variables underscores its potential as a biomarker for AKI risk in this patient population.
1. Background
Acute kidney injury (AKI) is a crucial determinant of prognosis in critically ill patients, significantly impacting mortality and the length of hospital stays in the intensive care unit (ICU) [1,2]. The occurrence of AKI, particularly in conjunction with other organ dysfunctions, escalates the mortality rate substantially, often reaching 60 to 80% [3]. This underlines the critical importance of early identification and management of AKI in the ICU setting.
In neurocritically ill patients, AKI emerges as a particularly critical issue due to unique pathophysiological challenges and treatment modalities [4,5]. These patients frequently require hyperosmolar therapy, including mannitol and hypertonic saline, for managing cerebral edema following severe neurological injuries. While these therapies are crucial for reducing intracranial pressure, they have been consistently linked to an increased incidence of AKI [4,5,6,7,8]. Studies have shown that AKI affects 18–46% of neurocritical care patients, with significantly higher mortality rates and longer ICU stays compared to those without AKI [7,8].
Traditional AKI prediction models have predominantly relied on clinical scoring systems and logistic regression approaches incorporating established risk factors such as baseline creatinine, comorbidities, and severity scores [6,9]. However, these conventional models demonstrate significant limitations when applied to neurocritical care populations. They often lack specificity for the unique brain-kidney interactions and inadequately account for the complex effects of hyperosmolar therapy on renal function [10,11]. Furthermore, traditional statistical approaches may fail to capture non-linear relationships and complex interactions between multiple variables that characterize AKI development in this setting [12,13].
Recent studies have highlighted the importance of previously underrecognized risk factors, particularly electrolyte disturbances such as hyperchloremia and acid-base imbalances, in patients receiving hyperosmolar therapy [14,15]. However, comprehensive studies specifically examining AKI prediction in neurocritical care populations using advanced analytical approaches remain limited [16,17].
Machine learning (ML) techniques have emerged as promising tools for improving clinical prediction models, excelling at identifying complex patterns in high-dimensional datasets that traditional statistical methods might miss [18,19,20,21,22,23,24]. Several studies have demonstrated the superior performance of ML approaches compared to conventional methods in various AKI prediction scenarios [25,26]. However, most existing ML-based AKI prediction models have been developed for general ICU populations, with limited focus on neurocritical care settings receiving hyperosmolar therapy [27,28].
In light of these challenges, our study employed ML techniques to predict AKI in neurocritically ill patients undergoing hyperosmolar therapy. Utilizing historical patient data and sophisticated algorithmic models, ML can offer a novel approach to uncover complex interactions and risk factors not easily identifiable through traditional analysis [18,19,20,21,22,23,24]. Our goal is to harness ML to offer insights that could significantly improve patient management in neurocritical care settings.
2. Literature Review
AKI is a crucial determinant of prognosis in critically ill patients, significantly impacting mortality and ICU length of stay [1,2]. In neurocritically ill patients, AKI emerges as a particularly critical issue affecting 18–46% of patients, with mortality rates reaching 60–80% [3,7,8]. These patients frequently require hyperosmolar therapy for managing cerebral edema, which paradoxically increases AKI risk despite being essential for reducing intracranial pressure [4,5,6,7,8].
Traditional AKI prediction models have relied on clinical scoring systems and logistic regression incorporating established risk factors such as baseline creatinine and severity scores [6,9]. However, these conventional models demonstrate significant limitations in neurocritical care populations, lacking specificity for brain-kidney interactions and inadequately accounting for hyperosmolar therapy effects [10,11]. Recent studies have highlighted previously underrecognized risk factors, particularly electrolyte disturbances such as hyperchloremia, though comprehensive studies using advanced analytical approaches remain limited [14,15,16,17]. ML techniques have emerged as promising tools for improving clinical prediction models, demonstrating superior performance compared to conventional methods in various AKI prediction scenarios [18,19,20,21,22,23,24]. However, most existing ML-based models have been developed for general ICU populations, with limited focus on neurocritical care settings [27,28]. Existing literature reveals several studies addressing AKI prediction in neurocritical care, though significant gaps remain (Table 1). Studies by Buttner et al. and others have achieved modest performance (AUC 0.69–0.78) using traditional approaches, but were limited by small sample sizes, single-center designs, and reliance on conventional statistical methods [7,14,16,29,30,31,32]. This comprehensive review identifies critical needs for larger cohorts, systematic ML algorithm comparison, and novel biomarker exploration in neurocritical care AKI prediction.

Table 1.
Summary of Previous Studies on Acute Kidney Injury Prediction in Critical Care Settings.
This table summarizes existing studies on AKI prediction in neurocritical care and general ICU populations to highlight research gaps that our study addresses. The table includes studies focusing on neurocritical care patients, general ICU populations using ML approaches, and studies specifically examining hyperosmolar therapy-related AKI. Key limitations identified include small sample sizes in neurocritical care studies, limited exploration of ML algorithms in this population, insufficient focus on hyperosmolar therapy-induced AKI, and lack of comprehensive biomarker analysis, including novel predictors such as delta chloride levels. Our study addresses these gaps through a large-scale cohort (n = 4886), systematic comparison of multiple ML algorithms, and identification of delta chloride as a novel predictive biomarker in neurocritical care patients receiving hyperosmolar therapy.
This comprehensive review reveals several critical gaps in the existing literature. First, most studies focusing specifically on neurocritical care populations have employed relatively small sample sizes, limiting the generalizability and statistical power of their findings. Second, while ML approaches have shown promise in general ICU populations, their systematic application and comparison in neurocritical care settings remain underexplored. Third, although several studies have identified the importance of electrolyte disturbances, particularly hyperchloremia, a comprehensive investigation of novel biomarkers such as delta chloride levels has been limited. Finally, most existing research has focused on specific neurological conditions rather than the broader neurocritical care population receiving hyperosmolar therapy.
3. Study Contributions and Objectives
Building upon the identified gaps in existing literature, this study makes several key contributions to the field of AKI prediction in neurocritical care. We present the largest single-center cohort of neurocritical care patients receiving hyperosmolar therapy (n = 4886), providing substantially greater statistical power than previous studies. Additionally, we systematically evaluate seven ML algorithms specifically optimized for this population, filling a critical gap in advanced analytics applications. Most importantly, we identify and validate delta chloride as a novel, clinically actionable biomarker that advances beyond traditional static chloride measures to capture dynamic changes over time.
Our primary objective is to develop and validate superior ML-based prediction models for early AKI detection in neurocritical care settings. Specifically, we aim to compare ML algorithms against traditional approaches, identify the most relevant predictors of AKI in patients receiving hyperosmolar therapy, and develop a robust, clinically implementable model with optimal performance metrics. Through comprehensive validation and feature importance analysis, we seek to provide both practical clinical tools and new insights into hyperosmolar therapy-induced AKI pathophysiology.
4. Methods
4.1. Study Population
This retrospective observational study was carried out at a single center, the Samsung Medical Center, a tertiary hospital in Seoul, Republic of Korea. It encompassed adult patients admitted to the neurosurgical ICU from January 2013 to September 2019. The Institutional Review Board (IRB) of Samsung Medical Center approved the study (approval number SMC 2020-09-082), and due to its retrospective nature, the requirement for informed consent was waived by the IRB. The study’s criteria included patients admitted to the neurosurgical ICU who received hyperosmolar therapy and had comprehensive initial laboratory tests—serum sodium, chloride, osmolality, urea, glucose, creatinine, glomerular filtration rate (GFR), and arterial blood gas analysis—performed within the first 12 h following ICU admission, along with subsequent follow-up levels. Exclusion criteria were patients below 18 years, those with end-stage renal disease on renal replacement therapy, those with inadequate medical records, a ‘do not resuscitate’ order, admissions to departments other than neurosurgery, transfers to other hospitals, or an uncertain prognosis.
4.2. Definitions and Outcomes
In this study, we collected baseline characteristics such as comorbidities, behavioral risk factors, ICU management, and laboratory data retrospectively using our center’s “Clinical Data Warehouse Darwin-C”. This data warehouse was designed specifically for investigators to search and retrieve de-identified medical records from electronic archives. Laboratory data were collected at least once daily from all neurosurgical patients admitted to the ICU. To assess kidney function, we used the Modification of Diet in Renal Disease 4-variable formula to calculate the GFR [33]. Perioperative acute renal dysfunction was defined according to the Acute Kidney Injury Network (AKIN) criteria [34]. “Stage 1” was defined as an increase of creatinine by ≥0.3 mg/dL or 1.5–1.9 times, “stage 2” as an increase of creatinine 2.0–2.9 times, and “stage 3” as an increase of creatinine 3 times [34]. We calculated osmolality (mOsm/kg) using the following formula: serum sodium level (mEq/L) × 2 + (glucose [mg/dL]/18) + (blood urea nitrogen [mg/dL]/2.3) [35]. The difference between the measured osmolarity and the calculated osmolality was defined as the osmolar gap (OG) [35]. End-stage renal disease was defined as being on dialysis or having a GFR < 30 mL/min/1.73 m2 for at least 12 weeks [36]. Delta chloride was defined as the difference between the initial chloride level and the highest chloride level observed during the patient’s stay in the ICU. Sodium level, serum osmolality, and chloride level were defined as the highest values recorded during the patient’s ICU stay. On the other hand, pH and bicarbonate level were defined as the lowest values recorded during the same period. The primary endpoint of this study was the occurrence of AKI, which was defined as a ≥0.3-mg/dL increase in serum creatinine level above baseline or a change of ≥50%, according to the AKIN criteria (Stage 1, 2, or 3) [2].
4.3. ML Models
In this study, 7 ML algorithms were developed and evaluated for their ability to predict the occurrence of AKI in a binary classification task. These algorithms included random forest (RF), AdaBoost Classification Trees (AdaBoost), Bagged CART (Bagging), Stochastic Gradient Boosting (GBM), eXtreme Gradient Boosting (XGBoost), Multivariate Adaptive Regression Spline (MARS), and Support Vector Machines with Radial Basis Function Kernel (SVM). Univariate analysis was performed to select the most relevant variables for model development. To ensure that the imbalanced outcome variable (AKI: 16.6% vs. non-AKI:83.4%) was equally represented across data subsets, we split the dataset into training (80%) and testing (20%) sets using stratified partitioning. During model training and hyperparameter optimization, stratified 10-fold cross-validation was employed using AKI status as the stratification variable. Final model performance was evaluated exclusively on the independent testing set (20% holdout) that remained completely separate from any training or hyperparameter tuning processes [37,38,39]. To address missing values in the dataset, two preprocessing strategies were employed: exclusion of incomplete cases and k-nearest neighbors (KNN) imputation. These approaches were compared in the development of ML models to assess their predictive performance. Data preprocessing included scaling of continuous variables and one-hot encoding of categorical variables (such as cause of ICU admission) to convert them into binary indicator variables for each category level, enabling proper incorporation into ML algorithms. For the target variable, we employed a binary classification approach with patients classified as either “AKI” or “non-AKI” based on the AKIN criteria, where AKI was defined as meeting any stage of the AKIN criteria (≥0.3 mg/dL increase in serum creatinine above baseline or ≥50% change), coded as 1 for AKI and 0 for non-AKI. Each ML algorithm was trained with its respective optimal hyperparameters until convergence on the training set. To address the class imbalance during model training, we additionally applied synthetic oversampling techniques, including SMOTE (Synthetic Minority Oversampling Technique) and ROSE (Random Over-Sampling Examples), using the trainControl function in the caret package. These methods were used to assess their impact on classifier performance during cross-validation within the training data. Separately, for diagnostic performance evaluation on the independent validation set, we employed optimal threshold selection strategies such as the closest-to-top-left method on ROC analysis. This allowed us to determine the best cutoff values for binary classification and to report sensitivity, specificity, accuracy, and AUROC based on clinically relevant thresholds. We systematically optimized hyperparameters via grid search combined with 10-fold cross-validation, using AUROC as the objective function. For each algorithm, a comprehensive tuning grid was defined to explore key hyperparameter combinations. The final model performance was then evaluated on an independent holdout test set (20%), separate from the training and tuning processes, to avoid overfitting and ensure generalizability. For each ML algorithm, we defined comprehensive search ranges for key hyperparameters and evaluated all possible combinations to identify optimal settings. Final model performance was evaluated on a completely independent test set (20% holdout) that was not used during the hyperparameter tuning process, ensuring unbiased performance estimation. While cutoff optimization was not the primary objective, we employed the holdout validation set to determine model-specific thresholds using the Youden index. This facilitated meaningful comparisons of diagnostic metrics—including sensitivity, specificity, PPV, NPV, and accuracy—across different ML models under a binary classification framework.
4.4. Statistical Analyses
In the study, continuous variables were reported as means with their respective standard deviations, and categorical variables were described as counts and percentages. For the comparison of data, the Student’s t-test was employed for continuous variables and the Chi-square test for categorical variables. A stepwise multiple logistic regression analysis was conducted to identify significant predictors of AKI, and these results were then compared to those obtained from the ML algorithms. The statistical tests were two-sided, with a p-value threshold of less than 0.05 set for determining statistical significance. All statistical analyses were performed using R Statistical Software (version 4.2.0; R Foundation for Statistical Computing, Vienna, Austria).
5. Results
5.1. Baseline Characteristics and Clinical Outcomes
This study analyzed 4886 patients (Figure 1), with a mean age of 52.0 ± 16.6 years, of whom 2044 (41.8%) were male. Malignancy (54.7%) and hypertension (29.7%) were the most common comorbidities, while brain tumor (44.9%) was the most frequent cause of ICU admission (Table 2). Patients with AKI had significantly higher rates of in-hospital mortality, 28-day mortality, ICU mortality, and ICU readmission within 48 h compared to those without AKI (all p < 0.001). Additionally, the length of hospital and ICU stay was longer for patients with AKI compared to those without AKI (both p < 0.001) (Table 3).
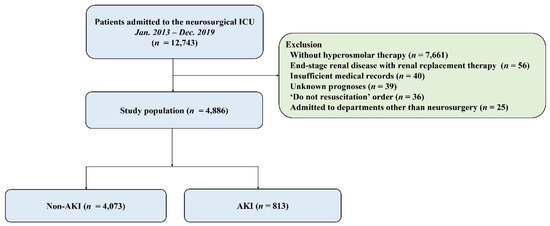
Figure 1.
Study flow chart. ICU, intensive care unit; AKI, acute kidney injury.

Table 2.
Baseline characteristics of patients.

Table 3.
Clinical outcomes according to acute kidney injury (AKI).
5.2. Univariate and Multivariate Logistic Analysis of Risk Factors
In the univariable analysis, significant differences were observed in most variables between the two groups, except for age, gender, malignancy, behavioral risk factors, and duration of continuous renal replacement therapy (Table 2). However, in the multivariable analysis, several factors were found to be significantly associated with AKI (C-statistic: 0.814, 95% CI: 0.797–0.814), including male gender (adjusted odds ratio [OR]: 0.70, 95% confidence interval [CI]: 0.57–0.85), hypertension (adjusted OR: 1.16, 95% CI: 1.05–1.28), intracranial hemorrhage (adjusted OR: 3.25, 95% CI: 2.31–4.56), subarachnoid hemorrhage (adjusted OR: 3.30, 95% CI: 2.31–4.68), traumatic brain injury (adjusted OR: 3.76, 95% CI: 2.57–5.48), spinal surgery (adjusted OR: 2.77, 95% CI: 1.67–4.46), central nervous system infection (adjusted OR: 5.48, 95% CI: 2.72–10.90), APACHE II score on ICU admission (adjusted OR: 1.16, 95% CI: 1.05–1.29), mechanical ventilation (adjusted OR: 1.32, 95% CI: 1.21–1.44), ICP monitoring (adjusted OR: 1.20, 95% CI: 1.11–1.29), use of vasopressor (adjusted OR: 1.18, 95% CI: 1.09–1.27), initial chloride level (adjusted OR: 5.21, 95% CI: 1.01–49.10), delta chloride (adjusted OR: 5.08, 95% CI: 1.22–35.61), and serum osmolality (adjusted OR: 1.36, 95% CI: 1.21–1.53) (Table 4).

Table 4.
Multivariable analysis of factors associated with acute kidney injury.
5.3. Machine Learning-Based Prediction of Acute Kidney Injury
After initial analysis using ML models, AdaBoost, Bagging, MARS, and SVM were excluded in the final analysis because of low predictive power (ROC < 0.78 compared to the top three algorithms achieving ROC > 0.80). Finally, we utilized the top three algorithms, XGBoost, GBM, and RF, with the highest accuracy from the 7 ML algorithms. The hyperparameter optimization process yielded 10-fold cross-validation AUROC values ranging from 0.77 to 0.89 across different algorithms, with optimal models achieving AUROC values of 0.83–0.86 on the independent test set (Table 5). Comprehensive performance evaluation of ML models across different preprocessing approaches is shown in Table 5 and Figure 2. Overall, all three models demonstrated excellent proficiency in predicting AKI, with accuracy scores ranging between 0.78 and 0.84. RF with KNN imputation achieved the highest overall performance among all ML algorithms (AUROC: 0.86, 95% CI: 0.82–0.91; sensitivity: 0.79, 95% CI: 0.70–0.87; specificity: 0.85, 95% CI: 0.82–0.88; accuracy: 0.84, 95% CI: 0.81–0.87) (Table 5). The top 10 critical features predicting AKI in the RF model across different preprocessing approaches are shown in Figure 3. The feature importance rankings remained remarkably consistent between the two preprocessing methods, demonstrating the robustness of key predictive variables regardless of the missing data handling approach. In the comparative analysis of ML models, delta chloride consistently ranked as the most significant predictor, particularly within the XGBoost model, where it was followed by variables such as pH, age, serum osmolality, bicarbonate, and osmolar gap. The models demonstrated robust generalization capability, maintaining consistent performance across different preprocessing approaches, including missing data exclusion and KNN imputation, with AUROC values consistently above 0.83 for all three algorithms. The robustness of our models was further validated through a comprehensive 10-fold cross-validation analysis (Table 6). All algorithms demonstrated consistent performance across folds, with mean AUROC values ranging from 0.824 to 0.836 and standard deviations below 0.042, indicating excellent stability and reliability. The RF exclude missing approach achieved the highest cross-validation performance (AUROC: 0.836 ± 0.035), followed closely by RF with KNN imputation (AUROC: 0.832 ± 0.041). The low variability across folds confirms the generalizability of our models and supports their potential for clinical implementation.

Table 5.
Model Performance on Independent Test Set Across Different Preprocessing Approaches.
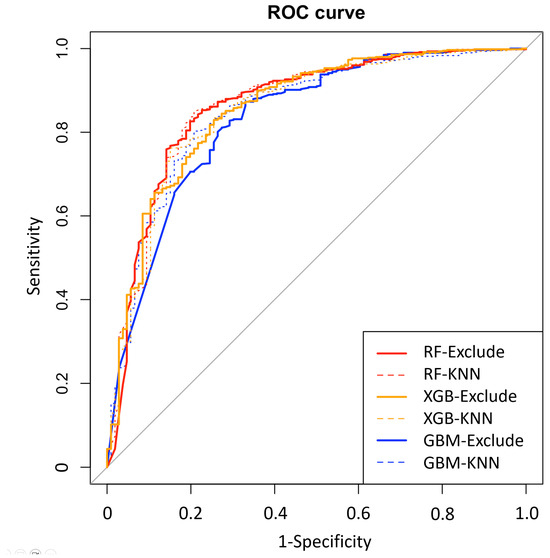
Figure 2.
Receiver operating characteristic (ROC) curves demonstrating model generalization across different missing data handling approaches. Performance comparison of eXtreme Gradient Boosting (XGB), Gradient Boosting Machine (GBM), and Random Forest (RF) models using exclude (excluding subjects with missing data) and k-nearest neighbors (KNN) imputation approaches. The consistent performance across preprocessing methods demonstrates model robustness and generalization capability.
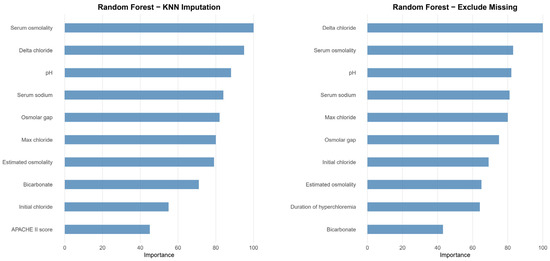
Figure 3.
Variable importance ranking for the top 10 predictors in RF models across different preprocessing approaches. The left panel shows variable importance for RF with KNN imputation, while the right panel displays results for RF with missing data exclusion. Both preprocessing methods demonstrate consistent identification of key predictive variables, with delta chloride and serum osmolality emerging as the most important predictors. The magnitude of importance is scaled so that the maximum value is 100. The remarkable consistency in variable rankings between the two approaches demonstrates the robustness of these predictive features regardless of missing data handling methodology, supporting the clinical relevance of these biomarkers for AKI prediction in neurocritical care patients receiving hyperosmolar therapy.

Table 6.
10-Fold Cross-Validation Area Under the Receiver Operating Characteristic Curve (AUROC) Results for All Algorithm-Preprocessing Combinations.
All performance metrics were calculated on the independent test set (20% holdout) that was not used during model training or hyperparameter tuning. The exclude missing approach used only complete cases, while KNN imputation filled missing values using the k-nearest neighbors algorithm. SMOTE was applied only to RF and XGBoost for comparison of balancing techniques.
All AUROC values were obtained through stratified 10-fold cross-validation on the training set (80% of the total dataset). The consistent performance across folds demonstrates the robustness and generalizability of all models, regardless of the preprocessing approach.
6. Discussion
In this study, neurocritically ill patients with AKI had significantly higher rates of in-hospital mortality, 28-day mortality, ICU mortality, and ICU readmission within 48 h when compared to those without AKI. In our investigation into the utility of ML for predicting AKI in neurocritically ill patients, we have uncovered a pivotal role for advanced analytics in the realm of neurocritical care. By deploying a suite of sophisticated ML algorithms—notably XGBoost, GBM, and RF—our study has demonstrated an improvement in predictive accuracy over traditional logistic regression methods. This superior performance underscores the potential of ML to transform current predictive models for AKI, especially in patients undergoing hyperosmolar therapy. Our findings have identified delta chloride levels as a particularly potent predictive variable, alongside other factors such as patient age, serum osmolality, bicarbonate levels, and the osmolar gap. The XGBoost model emerged as the most accurate, offering a robust tool for clinicians to anticipate and strategize against the development of AKI. Utilizing ML to identify risk factors for AKI in neurocritical care could be instrumental in reducing its incidence.
In neurocritical care, AKI stands out as a particularly critical condition due to its significant impact on patient outcomes [10]. Studies have consistently shown that AKI in this patient population is associated with increased mortality rates, extended lengths of stay in the ICU, and potential long-term neurological impairments [8]. These consequences are not just a reflection of the severity of the primary neurological condition but also indicative of the complex interplay between renal and brain function [8,10]. The development of AKI can exacerbate cerebral edema, potentially worsening the patient’s neurological status. Moreover, AKI’s impact on other organ systems can complicate the overall clinical management of these patients, often leading to a cascade of interventions that further extend hospitalization and recovery times [11]. Thus, understanding and addressing AKI in neurocritically ill patients is paramount, as its occurrence can be both a marker of severity and a contributor to adverse outcomes. This underscores the need for advanced diagnostic and therapeutic strategies aimed at early detection and effective management of AKI to improve overall patient prognosis in neurocritical care.
In the context of AKI among neurocritically ill patients, traditional predictors have included baseline renal function, illness severity, nephrotoxic drug use, hemodynamic instability, sepsis, and chronic kidney disease [7,8,10]. Our study, however, brings to light novel predictive factors such as delta chloride levels, pH imbalances, and specific serum osmolality aspects. We discovered a significant association between changes in chloride levels and AKI occurrence, a factor traditionally underemphasized. This novel finding underscores the need to pay closer attention to chloride level fluctuations, recognizing their potential as indicators of AKI risk. Hyperchloremia, which can lead to intravascular dehydration and vasoconstriction, emerges as a crucial modifiable risk factor [29]. Understanding the intricate relationship between chloride levels and kidney injury is vital. It necessitates vigilant monitoring and proactive management to mitigate AKI risks and enhance patient outcomes in neurocritical care. In addition, the occurrence of hyperchloremia-induced metabolic acidosis may also be a contributing factor to the development of AKI in neurocritically ill patients [31,40].
In this study, the application of ML algorithms has shown an interesting ability to detect complex patterns in clinical data that traditional statistical methods might overlook. The strength of ML lies in its capacity to process large datasets, revealing intricate relationships that can enhance the accuracy and effectiveness of diagnostics [41]. This is especially relevant in neurocritical care, where early and accurate detection of conditions like AKI is crucial for patient outcomes [16]. The use of ML in medical diagnostics represents a significant step forward, offering clinicians an additional tool for managing complex patient scenarios. This approach not only holds promise for improving patient care but also has the potential to streamline healthcare processes, reduce costs, and minimize medical errors [17].
Our study builds upon and extends previous valuable research in neurocritical care AKI prediction. While earlier studies have made important contributions to understanding AKI in neurocritical populations [7,14,29], opportunities remain for further advancement in several key areas. Previous research has primarily utilized traditional statistical approaches, particularly logistic regression, which have provided foundational insights into AKI risk factors [14,30,31,32]. However, the complex nature of neurocritical care settings may benefit from more sophisticated analytical approaches capable of capturing non-linear relationships and multivariable interactions. Additionally, while some studies have explored the role of electrolyte disturbances in AKI development [30,31,32], a comprehensive investigation of novel biomarkers such as delta chloride levels represents an area for continued exploration. Our study contributes to this evolving field by: (1) leveraging a substantial cohort of neurocritical care patients receiving hyperosmolar therapy, (2) systematically evaluating multiple ML algorithms to optimize predictive performance, (3) identifying and validating delta chloride as a highly predictive biomarker, and (4) developing a practical XGBoost model with robust performance for potential clinical implementation. These findings complement existing literature and represent a step forward in developing more precise and clinically applicable AKI prediction tools for neurocritical care settings.
This study has significant practical and theoretical implications for neurocritical care. From a practical standpoint, our XGBoost model can be integrated into electronic health record systems to provide real-time AKI risk assessment, enabling early identification of high-risk patients and facilitating timely nephroprotective interventions [24]. The identification of delta chloride as the most significant predictor offers clinicians a readily available biomarker for continuous monitoring, potentially reducing healthcare costs through preventive rather than reactive approaches [42]. From a theoretical perspective, our findings advance understanding of AKI pathophysiology by highlighting the critical role of chloride dynamics in kidney injury development [15]. The prominence of delta chloride provides new insights into hyperosmolar therapy-induced AKI mechanisms and supports the brain-kidney interaction paradigm [8]. Furthermore, our successful application of ML demonstrates the potential for advanced analytics to uncover previously unrecognized patterns in complex clinical datasets, contributing to precision medicine approaches in critical care [28].
The balance between sensitivity and specificity represents a critical consideration for clinical implementation of AKI prediction models. While high sensitivity is essential for early detection and prevention of AKI, adequate specificity is equally important to minimize false positive predictions that could lead to unnecessary interventions, increased healthcare costs, and alert fatigue among clinicians. Our final models demonstrate well-balanced performance with both sensitivity (79–81%) and specificity (78–85%) at clinically acceptable levels. The choice of optimal threshold should ultimately be guided by clinical context, with consideration for the relative costs of false positives versus false negatives in the specific care setting.
This study had several limitations. First, this was a nonrandomized cohort study. This design inherently carries potential biases, such as selection bias and limitations in data completeness and accuracy. Second, the study’s focus on a single-center cohort might affect the generalizability of the findings. Third, the determination of appropriate sensitivity and specificity values was guided by the Youden Index. However, it’s important to note a limitation in our findings due to the relatively low specificity observed. This aspect necessitates caution in interpreting the results, as it could affect the precision of AKI predictions. Fourth, our study did not systematically assess creatine supplementation history, which could potentially influence baseline creatinine levels, though this is unlikely to be significant in our critically ill neurosurgical population. For future research, it would be beneficial to conduct prospective studies that could validate and extend our findings. Exploring additional predictive variables, possibly through multicenter collaborations, could enhance the robustness and applicability of the ML models. This approach would not only strengthen the predictive accuracy for AKI in neurocritically ill patients but also contribute to the broader understanding and management of this complex condition.
7. Conclusions
This study establishes the strong association of AKI with critical outcomes such as in-hospital mortality, 28-day mortality, ICU mortality, and readmission rates in neurocritically ill patients. Leveraging ML algorithms, particularly XGBoost, we’ve demonstrated enhanced predictive capabilities for AKI in patients undergoing hyperosmolar therapy. Significantly, delta chloride levels emerged as a key predictive factor. These findings not only validate the potential of ML in refining clinical diagnostics but also pave the way for improved patient management and outcomes in neurocritical care, suggesting a promising avenue for future research in this domain.
Author Contributions
Conceptualization, K.S.K. and J.-A.R.; Methodology, K.S.K., T.J.Y. and J.-A.R.; Formal analysis, J.A. and J.-A.R.; Investigation, J.-A.R.; Resources, J.A. and J.-A.R.; Data curation, K.S.K. and T.J.Y.; Writing—original draft, K.S.K., J.A. and J.-A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Regarding data availability, our data are available on the Harvard Dataverse Network (http://dx.doi.org/10.7910/DVN/PWBWI6).
Acknowledgments
We would like to thank the nursing director of the neurosurgical ICU, Suk Kyung Choo, for providing excellent advice and fruitful discussions. We would also like to thank all the nurses of the neurosurgical ICU at Samsung Medical Center for their support in the completion of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Christiansen, C.L.; Cleary, P.D.; Munro, C.; Lazarus, J.M. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch. Intern. Med. 1995, 155, 1505–1511. [Google Scholar] [CrossRef]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef]
- Buttner, S.; Stadler, A.; Mayer, C.; Patyna, S.; Betz, C.; Senft, C.; Geiger, H.; Jung, O.; Finkelmeier, F. Incidence, Risk Factors, and Outcome of Acute Kidney Injury in Neurocritical Care. J. Intensive Care Med. 2020, 35, 338–346. [Google Scholar] [CrossRef]
- Husain-Syed, F.; Takeuchi, T.; Neyra, J.A.; Ramírez-Guerrero, G.; Rosner, M.H.; Ronco, C.; Tolwani, A.J. Acute kidney injury in neurocritical care. Crit. Care 2023, 27, 341. [Google Scholar] [CrossRef]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef]
- Ramirez-Guerrero, G.; Baghetti-Hernandez, R.; Ronco, C. Acute Kidney Injury at the Neurocritical Care Unit. Neurocrit. Care 2022, 36, 640–649. [Google Scholar] [CrossRef]
- Malek, M. Brain consequences of acute kidney injury: Focusing on the hippocampus. Kidney Res. Clin. Pract. 2018, 37, 315–322. [Google Scholar] [CrossRef]
- Pickkers, P.; Ostermann, M.; Joannidis, M.; Zarbock, A.; Hoste, E.; Bellomo, R.; Prowle, J.; Darmon, M.; Bonventre, J.V.; Forni, L.; et al. The intensive care medicine agenda on acute kidney injury. Intensive Care Med. 2017, 43, 1198–1209. [Google Scholar] [CrossRef]
- Tomašev, N.; Glorot, X.; Rae, J.W.; Zielinski, M.; Askham, H.; Saraiva, A.; Mottram, A.; Meyer, C.; Ravuri, S.; Protsyuk, I.; et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 2019, 572, 116–119. [Google Scholar] [CrossRef]
- Sadan, O.; Singbartl, K.; Kandiah, P.A.; Martin, K.S.; Samuels, O.B. Hyperchloremia Is Associated with Acute Kidney Injury in Patients with Subarachnoid Hemorrhage. Crit. Care Med. 2017, 45, 1382–1388. [Google Scholar] [CrossRef]
- Yunos, N.M.; Bellomo, R.; Hegarty, C.; Story, D.; Ho, L.; Bailey, M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012, 308, 1566–1572. [Google Scholar] [CrossRef]
- Peng, C.; Yang, F.; Li, L.; Peng, L.; Yu, J.; Wang, P.; Jin, Z. A Machine Learning Approach for the Prediction of Severe Acute Kidney Injury Following Traumatic Brain Injury. Neurocrit. Care 2023, 38, 335–344. [Google Scholar] [CrossRef]
- Al-Mufti, F.; Kim, M.; Dodson, V.; Sursal, T.; Bowers, C.; Cole, C.; Scurlock, C.; Becker, C.; Gandhi, C.; Mayer, S.A. Machine Learning and Artificial Intelligence in Neurocritical Care: A Specialty-Wide Disruptive Transformation or a Strategy for Success. Curr. Neurol. Neurosci. Rep. 2019, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.J.; Wang, S.V.; Vaduganathan, M.; Evers, T.; Schneeweiss, S. Comparison of Machine Learning Methods with Traditional Models for Use of Administrative Claims with Electronic Medical Records to Predict Heart Failure Outcomes. JAMA Netw. Open 2020, 3, e1918962. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.L.; Kohane, I.S. Big Data and Machine Learning in Health Care. JAMA 2018, 319, 1317–1318. [Google Scholar] [CrossRef]
- Shah, N.H.; Milstein, A.; Bagley, S. Making Machine Learning Models Clinically Useful. JAMA 2019, 322, 1351–1352. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, J.D.; Liang, L.; Schulte, P.J.; Yancy, C.W.; Heidenreich, P.A.; Hernandez, A.F.; Bhatt, D.L.; Fonarow, G.C.; Laskey, W.K. Prediction of 30-Day All-Cause Readmissions in Patients Hospitalized for Heart Failure: Comparison of Machine Learning and Other Statistical Approaches. JAMA Cardiol. 2017, 2, 204–209. [Google Scholar] [CrossRef]
- Khera, R.; Haimovich, J.; Hurley, N.C.; McNamara, R.; Spertus, J.A.; Desai, N.; Rumsfeld, J.S.; Masoudi, F.A.; Huang, C.; Normand, S.L.; et al. Use of Machine Learning Models to Predict Death After Acute Myocardial Infarction. JAMA Cardiol. 2021, 6, 633–641. [Google Scholar] [CrossRef]
- Adamson, A.S.; Smith, A. Machine Learning and Health Care Disparities in Dermatology. JAMA Dermatol. 2018, 154, 1247–1248. [Google Scholar] [CrossRef]
- Goldstein, B.A.; Navar, A.M.; Carter, R.E. Moving beyond regression techniques in cardiovascular risk prediction: Applying machine learning to address analytic challenges. Eur. Heart J. 2017, 38, 1805–1814. [Google Scholar] [CrossRef]
- Koyner, J.L.; Carey, K.A.; Edelson, D.P.; Churpek, M.M. The Development of a Machine Learning Inpatient Acute Kidney Injury Prediction Model. Crit. Care Med. 2018, 46, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Kate, R.J.; Perez, R.M.; Mazumdar, D.; Pasupathy, K.S.; Nilakantan, V. Prediction and detection models for acute kidney injury in hospitalized older adults. BMC Med. Inform. Decis. Mak. 2016, 16, 39. [Google Scholar] [CrossRef]
- Rashidi, P.; Bihorac, A. Artificial intelligence approaches to improve kidney care. Nat. Rev. Nephrol. 2020, 16, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Kumar, A.B.; Shi, Y.; Shotwell, M.S.; Richards, J.; Ehrenfeld, J.M. Hypernatremia is a significant risk factor for acute kidney injury after subarachnoid hemorrhage: A retrospective analysis. Neurocrit. Care 2015, 22, 184–191. [Google Scholar] [CrossRef]
- Riha, H.M.; Erdman, M.J.; Vandigo, J.E.; Kimmons, L.A.; Goyal, N.; Davidson, K.E.; Pandhi, A.; Jones, G.M. Impact of Moderate Hyperchloremia on Clinical Outcomes in Intracerebral Hemorrhage Patients Treated with Continuous Infusion Hypertonic Saline: A Pilot Study. Crit. Care Med. 2017, 45, e947–e953. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Jeon, Y.-T.; Sohn, H.; Chung, S.H.; Do, S.-H. Association of Perioperative Hyperchloremia and Hyperchloremic Metabolic Acidosis with Acute Kidney Injury After Craniotomy for Intracranial Hemorrhage. World Neurosurg. 2019, 125, e1226–e1240. [Google Scholar] [CrossRef]
- Sigmon, J.; May, C.C.; Bryant, A.; Humanez, J.; Singh, V. Assessment of Acute Kidney Injury in Neurologically Injured Patients Receiving Hypertonic Sodium Chloride: Does Chloride Load Matter? Ann. Pharmacother. 2020, 54, 541–546. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; Acute Kidney Injury Network. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Fink, M.E. Osmotherapy for intracranial hypertension: Mannitol versus hypertonic saline. Continuum 2012, 18, 640–654. [Google Scholar] [CrossRef]
- Lee, K.; Jeon, J.; Kim, J.M.; Kim, G.; Kim, K.; Jang, H.R.; Lee, J.E.; Joh, J.W.; Lee, S.K.; Huh, W. Perioperative risk factors of progressive chronic kidney disease following liver transplantation: Analyses of a 10-year follow-up single-center cohort. Ann. Surg. Treat. Res. 2020, 99, 52–62. [Google Scholar] [CrossRef]
- Batista, G.E.A.P.A.; Monard, M.C. A Study of K-Nearest Neighbour as an Imputation Method. Computing Systems—Design, Management and Applications, HIS 2002, December 1–4, 2002, Santiago, Chile. Available online: https://www.researchgate.net/profile/Maria-Carolina-Monard/publication/2475229_A_Study_of_K-Nearest_Neighbour_as_an_Imputation_Method/links/0deec51ae1802c9861000000/A-Study-of-K-Nearest-Neighbour-as-an-Imputation-Method.pdf (accessed on 1 February 2024).
- Jonsson, P.; Wohlin, C. An evaluation of k-nearest neighbour imputation using likert data. In Proceedings of the 10th International Symposium on Software Metrics, 2004, Proceedings, Chicago, IL, USA, 11–17 September 2004; Available online: https://ieeexplore.ieee.org/document/1357895 (accessed on 1 February 2024).
- Pan, R.; Yang, T.; Cao, J.; Lu, K.; Zhang, Z. Missing data imputation by K nearest neighbours based on grey relational structure and mutual information. Appl. Intell. 2015, 43, 614–632. [Google Scholar] [CrossRef]
- Barlow, B.; Thompson Bastin, M.L.; Shadler, A.; Cook, A.M. Association of chloride-rich fluids and medication diluents on the incidence of hyperchloremia and clinical consequences in aneurysmal subarachnoid hemorrhage. J. Neurocrit. Care 2022, 15, 113–121. [Google Scholar] [CrossRef]
- Goecks, J.; Jalili, V.; Heiser, L.M.; Gray, J.W. How Machine Learning Will Transform Biomedicine. Cell 2020, 181, 92–101. [Google Scholar] [CrossRef]
- Shaw, A.D.; Raghunathan, K.; Peyerl, F.W.; Munson, S.H.; Paluszkiewicz, S.M.; Schermer, C.R. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. 2014, 40, 1897–1905. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).