Assessment of the Fascial System Thickness in Patients with and Without Low Back Pain: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Questions
2.2. Keywords
2.3. Article Inclusion Criteria
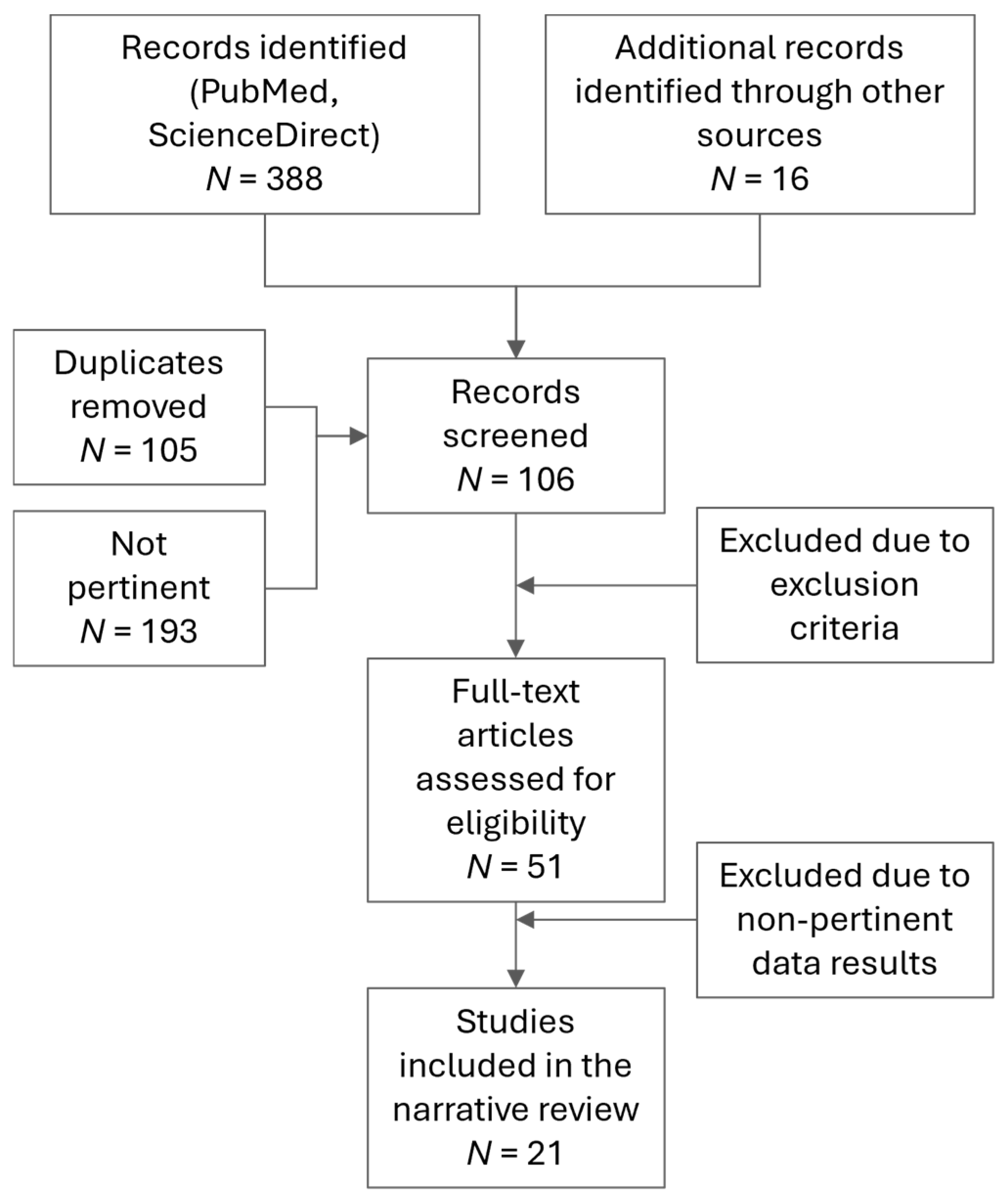
3. Results
| Structure | Reference | Study Population (Number) | Sex | BMI (kg/m2) | Age (Mean ± s.d.) | Spinal Level | Method |
|---|---|---|---|---|---|---|---|
| SF | [26] | 10 | M 100% | 23.06 ± 2.6 | 30.6 ± 4.99 (non-LBP) | Posterior chest | US |
| [27] | 6 | M 50%, F 50% | - | 73–85 (not specified) | - | Ex vivo | |
| DF | [28] | 18 | F 100% | 23 ± 4 | 22 ± 1 (non-LBP) | L2 | US |
| 17 | 27 ± 4 | 69 ± 4 (non-LBP) | |||||
| [4] | 107 (47 non-LBP, 60 LBP) | M (43% non-LBP, 42% LBP) F (57% non-LBP, 58% LBP) | 25.9 ± 0.7 (non-LBP) 25.7 ± 0.6 (LBP) | 39.3 ± 14.1 (non-LBP) 38.3 ± 13.3 (LBP | L2–L3 | US | |
| [5] | 121 (50 non-LBP, 71 LBP) | M (24 non-LBP, 38 LBP) F (26 non-LBP, 33 LBP) | 26.1 ± 0.6 (non-LBP) 26 ± 0.5 (LBP) | 41.8 ± 2.3 (non-LBP) 44.6 ± 1.8 (LBP) | L2–L3 | US | |
| [29] | 48 | M 50%, F 50% | 24.5 | 37.4 ± 13.3 (LBP) | L2–L3 | US | |
| [30] | 22 | - | Lower than 28.5 (exclusion criteria) | 25–65 (LBP) | L2–L3 | US | |
| [31] | 66 | M 100% | 23 ± 1.5 | 22.6 ± 3.7 (non-LBP) | L2–L3 | US | |
| [32] | 50 | M 58%, F 42% | 23.91 ± 3.58 | 36 (non-LBP) | L3 | US | |
| [33] | 92 | M 49% F 51% | 24.03 ± 6.1 (non-LBP) 23.37 ± 5.22 (LBP) | 27.09 ± 12.38 (non-LBP) 28.96 ± 10.54 (LBP) | L3 | US | |
| [20] | 14 | M 100% | Mass 78.9 ± 8.02 kg Height 181 ± 9.71 cm | 23.7 ± 7.31 (non-LBP) | L3–L4 | US | |
| [34] | 54 | M (12 non-LBP, 10 LBP) F (19 non-LBP, 13 LBP) | 22.30 ± 2.00 (non-LBP) 22.11 ± 2.84 (LBP) | 22.94 ± 5.23 (non-LBP) 25.13 ± 10.04 (LBP) | L4 | US | |
| [35] | 30 | M 100% | 24.03 ± 2.14 | 24 ± 5 (non-LBP) 28 ± 10 (LBP) | L4 | US | |
| [36] | 63 | M (15 non-LBP, 15 LBP) F (15 non-LBP, 18 LBP) | 24.4 ± 3.2 M (non-LBP) 23.3 ± 3.6 (F non-LBP) 25.8 ± 3.9 M (LBP) 26.8 ± 3.0 (F LBP) | Non-LBP: 39.3 ± 14.3 (M) 39.8 ± 14.1 (F) LBP: 44.5 ± 13.9 (M) 47.8 ± 11.9 (F) | L4–L5 | US | |
| [37] | 65 | M (15 non-LBP, 16 LBP) F (15 non-LBP, 19 LBP) | 24.4 ± 3.2 M (non-LBP) 23.3 ± 3.6 (F non-LBP) 25.6 ± 3.8 M (LBP) 26.8 ± 3.0 (F LBP) | Non-LBP: 39.3 ± 14.3 (M) 39.8 ± 13.7 (F) LBP: 44.1 ± 13.5 (M) 47.8 ± 11.9 (F) | L4–L5 | US | |
| [38] | 63 | M (15 non-LBP, 15 LBP) F (15 non-LBP, 18 LBP) | 24.4 ± 3.2 M (non-LBP) 23.3 ± 3.6 (F non-LBP) 25.8 ± 3.9 M (LBP) 26.8 ± 3.0 (F LBP) | Non-LBP: 39.3 ± 14.3 (M) 39.8 ± 14.1 (F) LBP: 44.5 ± 13.9 (M) 47.8 ± 11.9 (F) | L4–L5 | US | |
| [11] | 20 | - | - | 50–75 (not specified) | At different level | Ex vivo | |
| [26] | Reported above | ||||||
| [39] | 29 | F 100% | 20.4 and 22.4 according to different testing groups | 22.5 (18–29) (not specified) | n/a | US | |
| [17] | 10 | M 40% F 60% | - | 77 ± 10 (non-LBP) | n/a | Ex vivo | |
| [40] | 40 | M 45% F 55% | - | 59–84 (non-LBP) | n/a | Ex vivo | |
| Subcutaneous tissue | [4] | Reported above | |||||
| [30] | Reported above | ||||||
| [32] | Reported above | ||||||
| [37] | Reported above | ||||||
| [36] | Reported above | ||||||
| [41] | 165 | M 47% F 53% | - | 39.1 (LBP) | L3–S1 | MRI | |
| [23] | 280 | M (50 non-LBP, 52 LBP) F (50 non-LBP, 111 LBP) | 24.5 ± 2.7 (non-LBP) 26.7 ± 4.3 (LBP) | 32.5 ± 8.1 (non-LBP) 35.8 ± 6.8 (LBP) | L1–S1 | MRI | |
| [26] | Reported above | ||||||
3.1. Deep Fascia Thickness
| Reference | Thickness (mm) | |
|---|---|---|
| Non-LBP | LBP | |
| [28] | 1.33 | - |
| 2.35 | ||
| [4] | - | +25% |
| [5] * | 3.70 ± 0.40 (M) 4.10 ± 0.30 (F) | 4.90 ± 0.30 (M) 4.10 ± 0.30 (F) |
| [29] | - | Before treatment (divided into two populations) 2.00 ± 0.64 and 1.80 ± 0.45 (right) 1.90 ± 0.50 and 1.78 ± 0.42 (left) |
| [30] | - | 0.36 (left) 0.34 (right) |
| [31] | 2.56 ± 0.69 (left) 2.69 ± 0.67 (right) | - |
| [32] | 2.8 (median value and referred to by authors as SF) | - |
| [33] | 1.75 ± 0.85 | 2.11 ± 0.65 |
| [20] | <3.00 | - |
| [34] | 1.80 ± 0.60 | 1.80 ± 0.60 |
| [35] | 1.60 ± 0.40 (right) 1.50 ± 0.40 (left) | 1.70 ± 0.40 (right) 1.50 ± 0.40 (left) |
| [36] | 3.30 ± 0.90 (right) 3.50 ± 1.10 (left) | 3.90 ± 1.30 (right) 4.00 ± 1.40 (left) |
| [37] | 3.90 (M) 3.20 (F) | 4.10 (M) 3.50 (F) |
| [38] | 3.70 ± 1.10 | |
| [11] * | 1.93 (thoracic right) 1.82 (thoracic left) 2.44 (lumbar right) 2.42 (lumbar left) 4.29 (sacral right) 4.34 (sacral left) | |
| [26] | 2.00 | - |
| [39] | Users and non-users of hormonal contraceptives: 4.20 and 5.60 (1st measurement) 4.00 and 5.40 (2nd measurement) | |
| [17] | 0.96 ± 0.15 (posterior layer) 0.41 ± 0.05 (middle layer) | - |
| [40] | 3.00 ± 0.50 | - |
3.2. Superficial Fascia Thickness
| Reference | Thickness (mm) | |
|---|---|---|
| Non-LBP | LBP | |
| [26] | 0.600 | - |
| [27] * | 0.145 ± 0.015 (M) 0.165 ± 0.009 (F) | |
3.3. Subcutaneous Tissue Thickness
| Reference | Thickness (mm) | |
|---|---|---|
| Non-LBP | LBP | |
| [4] * | 4.8 ± 0.6 | 5.3 ± 0.5 |
| [30] | - | 8.32 (left) |
| [32] | 3.0 (median) | - |
| [37] | 5.0 ± 1.9 (M) 7.4 ± 3.3 (F) | 5.3 ± 2.9 (M) 11.1 ± 4.7 (F) |
| [36] | 6.4 ± 3.0 (right) 6.0 ± 2.8 (left) 5.0 ± 1.9 (M) 7.4 ± 3.3 (F) | 8.6 ± 4.8 (right) 8.4 ± 4.7 (left) 5.3 ± 2.9 (M) 11.1 ± 4.7 (F) |
| [41] | - | 25.4 (L3–L4) 29.1 (L4–L5) 32.2 (L5–S1) |
| [23] | 14.2 ± 8.7 (L1–L2) 14.8 ± 8.8 (L2–L3) 18.7 ± 10.4 (L3–L4) 25.6 ± 12.6 (L4–L5) 30.2 ± 13.3 (L5–S1) | |
| 10.8 ± 4.5 | 22.4 ± 9.7 (F) 17.9 ± 9.7 (M) | |
| [26] | <4.0 | - |
4. Discussion
4.1. Answer to Research Question 1: Typical Thickness Values and Variability for SF, DF, and Subcutaneous Tissue in the Thoracolumbar Region in Asymptomatic Individuals
4.2. Answer to Research Question 2: Thickness Variability in Relation to Factors Such as Clinical Conditions (i.e., Non-LBP vs. LBP Individuals), Age, Sex, and Surrounding Structures
4.3. Sex and Age Differences
4.4. Clinical Relevance and Functional Implications
4.5. Limitations and Future Developments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LBP | Low back pain |
| BMI | Body mass index |
| DF | Deep fascia |
| TLF | Thoracolumbar fascia |
| SF | Superficial fascia |
| MRI | Magnetic resonance imaging |
| US | Ultrasonography |
References
- Bonaldi, L.; Berardo, A.; Fontanella, C.G. The Mechanical Behavior of the Fascial System. In Fascia, Function, and Medical Applications; CRC Press: Boca Raton, FL, USA, 2024; pp. 107–116. [Google Scholar]
- Stecco, C.; Hammer, W.; Vleeming, A.; De Caro, R. Functional Atlas of the Human Fascial System; Livingstone, C., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9780702044304. [Google Scholar]
- Langevin, H.M.; Sherman, K.J. Pathophysiological Model for Chronic Low Back Pain Integrating Connective Tissue and Nervous System Mechanisms. Med. Hypotheses 2007, 68, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Stevens-Tuttle, D.; Fox, J.R.; Badger, G.J.; Bouffard, N.A.; Krag, M.H.; Wu, J.; Henry, S.M. Ultrasound Evidence of Altered Lumbar Connective Tissue Structure in Human Subjects with Chronic Low Back Pain. BMC Musculoskelet. Disord. 2009, 10, 151. [Google Scholar] [CrossRef]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan- Naumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.N.; Triano, J.J.; Henry, S.M. Reduced Thoracolumbar Fascia Shear Strain in Human Chronic Low Back Pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef]
- Ferreira, M.L.; de Luca, K.; Haile, L.M.; Steinmetz, J.D.; Culbreth, G.T.; Cross, M.; Kopec, J.A.; Ferreira, P.H.; Blyth, F.M.; Buchbinder, R.; et al. Global, Regional, and National Burden of Low Back Pain, 1990–2020, Its Attributable Risk Factors, and Projections to 2050: A Systematic Analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef]
- Buchbinder, R.; Underwood, M.; Hartvigsen, J.; Maher, C.G. The Lancet Series Call to Action to Reduce Low Value Care for Low Back Pain: An Update. Pain 2020, 161, S57–S64. [Google Scholar] [CrossRef]
- Vining, R.; Onifer, S.M.; Twist, E.; Ziegler, A.M.; Corber, L.; Long, C.R. Thoracolumbar Fascia Mobility and Chronic Low Back Pain: Phase 2 of a Pilot and Feasibility Study Including Multimodal Chiropractic Care. Chiropr. Man. Ther. 2022, 30, 46. [Google Scholar] [CrossRef]
- Simons, K.; Rapp, A.; Yilmaz, E.; Halalmeh, D.R.; Moisi, M.D. Innervation of the Thoracolumbar Fascia and Its Relationship to Lower Back Pain. Spine Sch. 2018, 2, 19–21. [Google Scholar] [CrossRef]
- Platzer, W. Color Atlas of Human Anatomy—Locomotor System, 6th ed.; Thieme Publishing Group: Stuttgart, Germany, 2014; Volume 1. [Google Scholar]
- Marpalli, S.; Mohandas Rao, K.G.; Venkatesan, P.; George, B.M. The Morphological and Microscopical Characteristics of Posterior Layer of Human Thoracolumbar Fascia; A Potential Source of Low Back Pain. Morphologie 2021, 105, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Schleip, R.; Klingler, W.; Stecco, C. The Lumbodorsal Fascia as a Potential Source of Low Back Pain: A Narrative Review. BioMed Res. Int. 2017, 2017, 5349620. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, F.; Gaudreault, N.; Venne, G. The Deep Fascia and Its Role in Chronic Pain and Pathological Conditions: A Review. Clin. Anat. 2022, 35, 649–659. [Google Scholar] [CrossRef]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The Thoracolumbar Fascia: Anatomy, Function and Clinical Considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef]
- Gatton, M.L.; Pearcy, M.J.; Pettet, G.J.; Evans, J.H. A Three-Dimensional Mathematical Model of the Thoracolumbar Fascia and an Estimate of Its Biomechanical Effect. J. Biomech. 2010, 43, 2792–2797. [Google Scholar] [CrossRef]
- Schilder, A.; Hoheisel, U.; Magerl, W.; Benrath, J.; Klein, T.; Treede, R.D. Sensory Findings after Stimulation of the Thoracolumbar Fascia with Hypertonic Saline Suggest Its Contribution to Low Back Pain. Pain 2014, 155, 222–231. [Google Scholar] [CrossRef]
- Creze, M.; Soubeyrand, M.; Nyangoh Timoh, K.; Gagey, O. Organization of the Fascia and Aponeurosis in the Lumbar Paraspinal Compartment. Surg. Radiol. Anat. 2018, 40, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Wilke, J.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Thoracolumbar Fascia Deformation during Deadlifting and Trunk Extension in Individuals with and without Back Pain. Front. Med. 2023, 10, 1177146. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study. Life 2022, 12, 1735. [Google Scholar] [CrossRef]
- Kellis, E.; Kekelekis, A.; Drakonaki, E.E. Is Thoracolumbar Fascia Shear-Wave Modulus Affected by Active and Passive Knee Flexion? J. Anat. 2023, 244, 438–447. [Google Scholar] [CrossRef] [PubMed]
- D’Hooge, R.; Cagnie, B.; Crombez, G.; Vanderstraeten, G.; Dolphens, M.; Danneels, L. Increased Intramuscular Fatty Infiltration without Differences in Lumbar Muscle Cross-Sectional Area during Remission of Unilateral Recurrent Low Back Pain. Man. Ther. 2012, 17, 584–588. [Google Scholar] [CrossRef]
- Heuch, I.; Hagen, K.; Heuch, I.; Nygaard, Ø.; Zwart, J.-A. The Impact of Body Mass Index on the Prevalence of Low Back Pain The HUNT Study. Spine 2010, 35, 764–768. [Google Scholar] [CrossRef]
- Özcan-Ekşi, E.E.; Kara, M.; Berikol, G.; Orhun, Ö.; Turgut, V.U.; Ekşi, M.Ş. A New Radiological Index for the Assessment of Higher Body Fat Status and Lumbar Spine Degeneration. Skelet. Radiol. 2022, 51, 1261–1271. [Google Scholar] [CrossRef]
- Brooks, C.; Siegler, J.C.; Marshall, P.W.M. Relative Abdominal Adiposity Is Associated with Chronic Low Back Pain: A Preliminary Explorative Study. BMC Public Health 2016, 16, 700. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hammoudeh, D.S.N.; Dohi, T.; Cho, H.; Ogawa, R. In Vivo Analysis of the Superficial and Deep Fascia. Plast. Reconstr. Surg. 2022, 150, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hijleh, M.F.; Roshier, A.L.; Al-Shboul, Q.; Dharap, A.S.; Harris, P.F. The Membranous Layer of Superficial Fascia: Evidence for Its Widespread Distribution in the Body. Surg. Radiol. Anat. 2006, 28, 606–619. [Google Scholar] [CrossRef]
- Wilke, J.; Macchi, V.; De Caro, R.; Stecco, C. Fascia Thickness, Aging and Flexibility: Is There an Association? J. Anat. 2019, 234, 43–49. [Google Scholar] [CrossRef]
- Devantéry, K.; Morin, M.; Grimard, J.; Gaudreault, N. Effects of a Myofascial Technique on the Stiffness and Thickness of the Thoracolumbar Fascia and Lumbar Erector Spinae Muscles in Adults with Chronic Low Back Pain: A Randomized before-and-after Experimental Study. Bioengineering 2023, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Sanjana, F.; Chaudhry, H.; Findley, T. Effect of MELT Method on Thoracolumbar Connective Tissue: The Full Study. J. Bodyw. Mov. Ther. 2017, 21, 179–185. [Google Scholar] [CrossRef]
- Yang, C.; Huang, X.; Li, Y.; Sucharit, W.; Sirasaporn, P.; Eungpinichpong, W. Acute Effects of Percussive Massage Therapy on Thoracolumbar Fascia Thickness and Ultrasound Echo Intensity in Healthy Male Individuals: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 1073. [Google Scholar] [CrossRef]
- Calvo-Lobo, C.; Díez-Vega, I.; García-Mateos, M.; Molina-Martín, J.J.; Díaz-Ureña, G.; Rodríguez-Sanz, D. Relationship of the Skin and Subcutaneous Tissue Thickness in the Tensiomyography Response: A Novel Ultrasound Observational Study. Rev. Assoc. Med. Bras. 2018, 64, 549–553. [Google Scholar] [CrossRef]
- Pirri, C.; Pirri, N.; Guidolin, D.; Macchi, V.; Porzionato, A.; De Caro, R.; Stecco, C. Ultrasound Imaging of Thoracolumbar Fascia Thickness: Chronic Non-Specific Lower Back Pain versus Healthy Subjects; A Sign of a “Frozen Back”? Diagnostics 2023, 13, 1436. [Google Scholar] [CrossRef]
- Perez, A.M.; Fernández-Carnero, S.; Sicilia-Gomez-de-Parada, C.; Cuenca-Zaldívar, N.; Naranjo-Cinto, F.; Pecos-Martín, D.; Gallego-Izquierdo, T.; Nuñez-Nagy, S. Diaphragmatic Activation Correlated with Lumbar Multifidus Muscles and Thoracolumbar Fascia by B-Mode and M-Mode Ultrasonography in Subjects with and without Non-Specific Low Back Pain: A Pilot Study. Medicina 2023, 59, 315. [Google Scholar] [CrossRef]
- Almazán-Polo, J.; López-López, D.; Romero-Morales, C.; Rodríguez-Sanz, D.; Becerro-De-bengoa-vallejo, R.; Losa-Iglesias, M.E.; Bravo-Aguilar, M.; Calvo-Lobo, C. Quantitative Ultrasound Imaging Differences in Multifidus and Thoracolumbar Fasciae between Athletes with and without Chronic Lumbopelvic Pain: A Case-Control Study. J. Clin. Med. 2020, 9, 2647. [Google Scholar] [CrossRef] [PubMed]
- Larivière, C.; Gagnon, D.H.; Preuss, R. Structural Remodeling of the Lumbar Multifidus, Thoracolumbar Fascia and Lateral Abdominal Wall Perimuscular Connective Tissues: Medium-Term Test-Retest Reliability of Ultrasound Measures. J. Bodyw. Mov. Ther. 2021, 27, 265–273. [Google Scholar] [CrossRef]
- Larivière, C.; Preuss, R.; Gagnon, D.H.; Mecheri, H.; Henry, S.M. Structural Remodelling of the Lumbar Multifidus, Thoracolumbar Fascia and Lateral Abdominal Wall Perimuscular Connective Tissues: A Cross-Sectional and Comparative Ultrasound Study. J. Bodyw. Mov. Ther. 2020, 24, 293–302. [Google Scholar] [CrossRef]
- Larivière, C.; Henry, S.M.; Preuss, R. Structural Remodeling of the Lumbar Multifidus, Thoracolumbar Fascia and Lateral Abdominal Wall Perimuscular Connective Tissues: A Search for Its Potential Determinants. J. Anat. 2021, 238, 536–550. [Google Scholar] [CrossRef]
- Vita, M.; Sedlackova, Z.; Herman, M.; Furst, T.; Smekal, D.; Cech, Z. Influence of Female Hormones on Fascia Elasticity: An Elastography Study. Clin. Anat. 2019, 32, 941–947. [Google Scholar] [CrossRef]
- Loukas, M.; Shoja, M.M.; Thurston, T.; Jones, V.L.; Linganna, S.; Tubbs, R.S. Anatomy and Biomechanics of the Vertebral Aponeurosis Part of the Posterior Layer of the Thoracolumbar Fascia. Surg. Radiol. Anat. 2008, 30, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Bhadresha, A.; Lawrence, O.J.; McCarthy, M.J.H. A Comparison of Magnetic Resonance Imaging Muscle Fat Content in the Lumbar Paraspinal Muscles with Patient-Reported Outcome Measures in Patients with Lumbar Degenerative Disk Disease and Focal Disk Prolapse. Glob. Spine J. 2016, 6, 401–410. [Google Scholar] [CrossRef]
- Stecco, A.; Bonaldi, L.; Fontanella, C.G.; Stecco, C.; Pirri, C. The Effect of Mechanical Stress on Hyaluronan Fragments’ Inflammatory Cascade: Clinical Implications. Life 2023, 13, 2277. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P.; Soundararajan, K.; Kishen, T.J.; Janardhan, S.; Kumar CR, S. Comparison of Yoga and Dynamic Neuromuscular Stabilization Exercise in Chronic Low Back Pain on Magnetic Resonance Imaging of Lumbar Multifidus—Protocol for a Randomized Controlled Trial. Contemp. Clin. Trials Commun. 2022, 28, 100937. [Google Scholar] [CrossRef]
- Berardo, A.; Bonaldi, L.; Stecco, C.; Fontanella, C.G. Biomechanical Properties of the Human Superficial Fascia: Site-Specific Variability and Anisotropy of Abdominal and Thoracic Regions. J. Mech. Behav. Biomed. Mater. 2024, 157, 106637. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, H.; Miyatani, M.; Azuma, K.; Kuno, S.; Fukunaga, T. Influences of Age and Sex on Abdominal Muscle and Subcutaneous Fat Thickness. Eur. J. Appl. Physiol. 2004, 91, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Rummens, S.; Bosch, S.; Dierckx, S.; Vanmechelen, A.; Peeters, R.; Brumagne, S.; Desloovere, K.; Peers, K. Reliability and Agreement of Lumbar Multifidus Volume and Fat Fraction Quantification Using Magnetic Resonance Imaging. Musculoskelet. Sci. Pract. 2022, 59, 102532. [Google Scholar] [CrossRef]
- Crook, J.; Masi, S.; Naghdi, N.; Roussac, A.; Rye, M.; Rosenstein, B.; Rivaz, H.; Boily, M.; Weber, M.H.; Fortin, M. Comparison of Multifidus Muscle Intramuscular Fat by Ultrasound Echo Intensity and Fat-Water Based MR Images in Individuals with Chronic Low Back Pain. Musculoskelet. Sci. Pract. 2023, 63, 102717. [Google Scholar] [CrossRef]
- Ogon, I.; Takebayashi, T.; Takashima, H.; Morita, T.; Yoshimoto, M.; Terashima, Y.; Yamashita, T. Quantitative Analysis Concerning Atrophy and Fat Infiltration of the Multifidus Muscle with Magnetic Resonance Spectroscopy in Chronic Low Back Pain. Spine Surg. Relat. Res. 2019, 3, 163–170. [Google Scholar] [CrossRef]
- Goubert, D.; De Pauw, R.; Meeus, M.; Willems, T.; Cagnie, B.; Schouppe, S.; Van Oosterwijck, J.; Dhondt, E.; Danneels, L. Lumbar Muscle Structure and Function in Chronic versus Recurrent Low Back Pain: A Cross-Sectional Study. Spine J. 2017, 17, 1285–1296. [Google Scholar] [CrossRef]
- Bonaldi, L.; Fontanella, C.G.; Stecco, C.; Berardo, A. Design, Implementation and Effectiveness of Human Fascia Lata Biomechanics for Tissue Engineering. J. Biomech. 2024, 176, 112369. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonaldi, L.; Berardo, A.; Stecco, A.; Stecco, C.; Fontanella, C.G. Assessment of the Fascial System Thickness in Patients with and Without Low Back Pain: A Narrative Review. Diagnostics 2025, 15, 2059. https://doi.org/10.3390/diagnostics15162059
Bonaldi L, Berardo A, Stecco A, Stecco C, Fontanella CG. Assessment of the Fascial System Thickness in Patients with and Without Low Back Pain: A Narrative Review. Diagnostics. 2025; 15(16):2059. https://doi.org/10.3390/diagnostics15162059
Chicago/Turabian StyleBonaldi, Lorenza, Alice Berardo, Antonio Stecco, Carla Stecco, and Chiara Giulia Fontanella. 2025. "Assessment of the Fascial System Thickness in Patients with and Without Low Back Pain: A Narrative Review" Diagnostics 15, no. 16: 2059. https://doi.org/10.3390/diagnostics15162059
APA StyleBonaldi, L., Berardo, A., Stecco, A., Stecco, C., & Fontanella, C. G. (2025). Assessment of the Fascial System Thickness in Patients with and Without Low Back Pain: A Narrative Review. Diagnostics, 15(16), 2059. https://doi.org/10.3390/diagnostics15162059










