Incidental Discovery of a Right Atrial Diverticulum in an Adult Patient
Abstract
1. Introduction
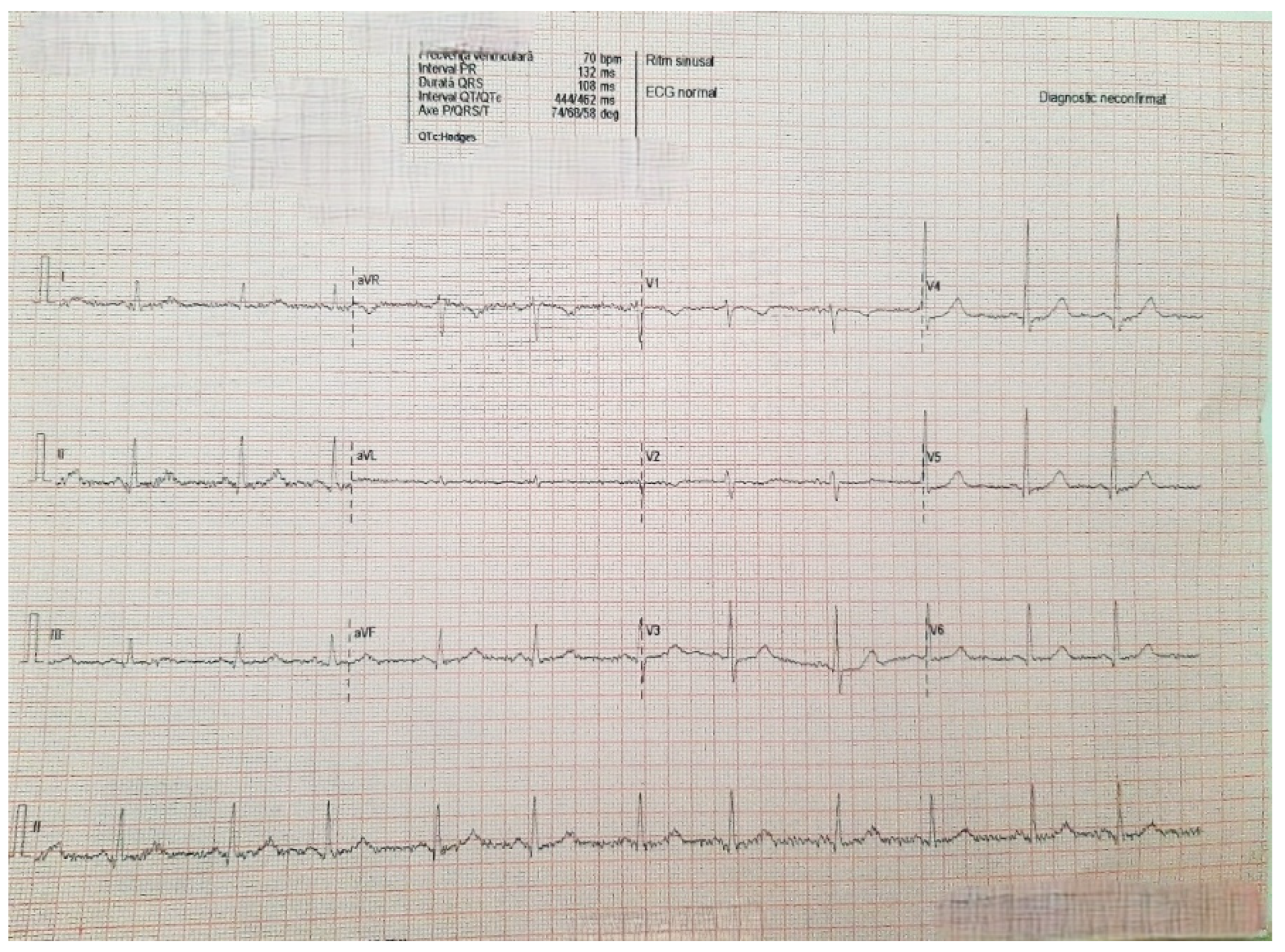
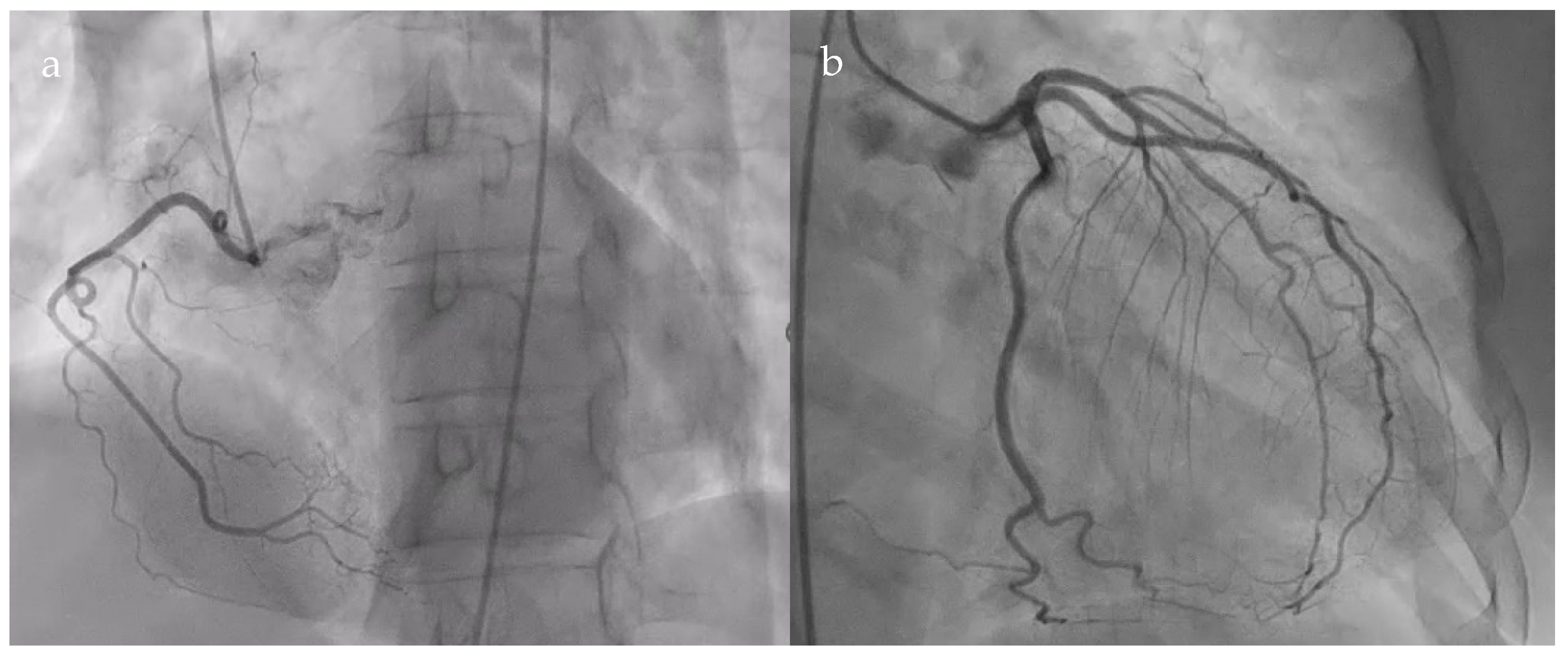
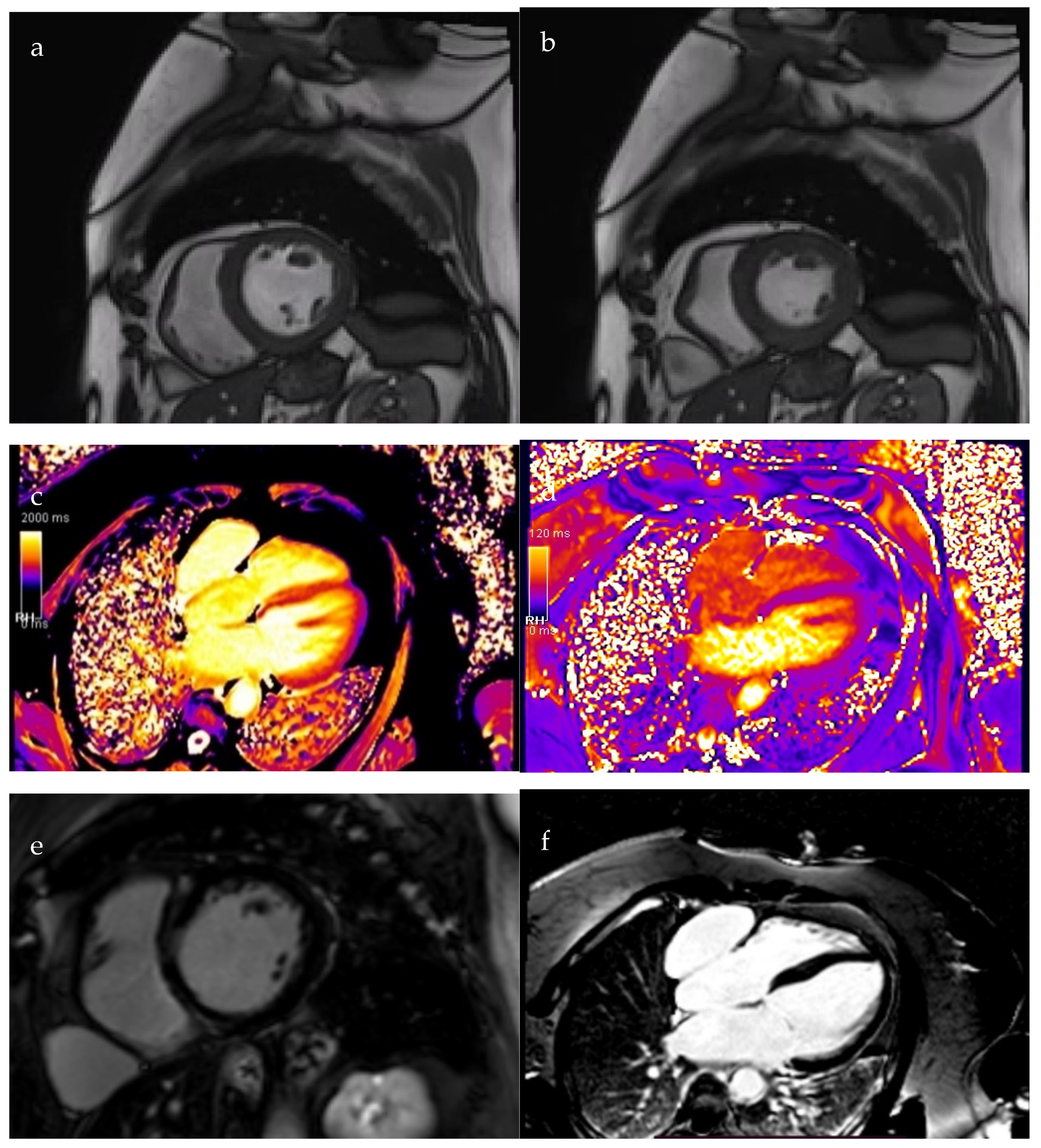
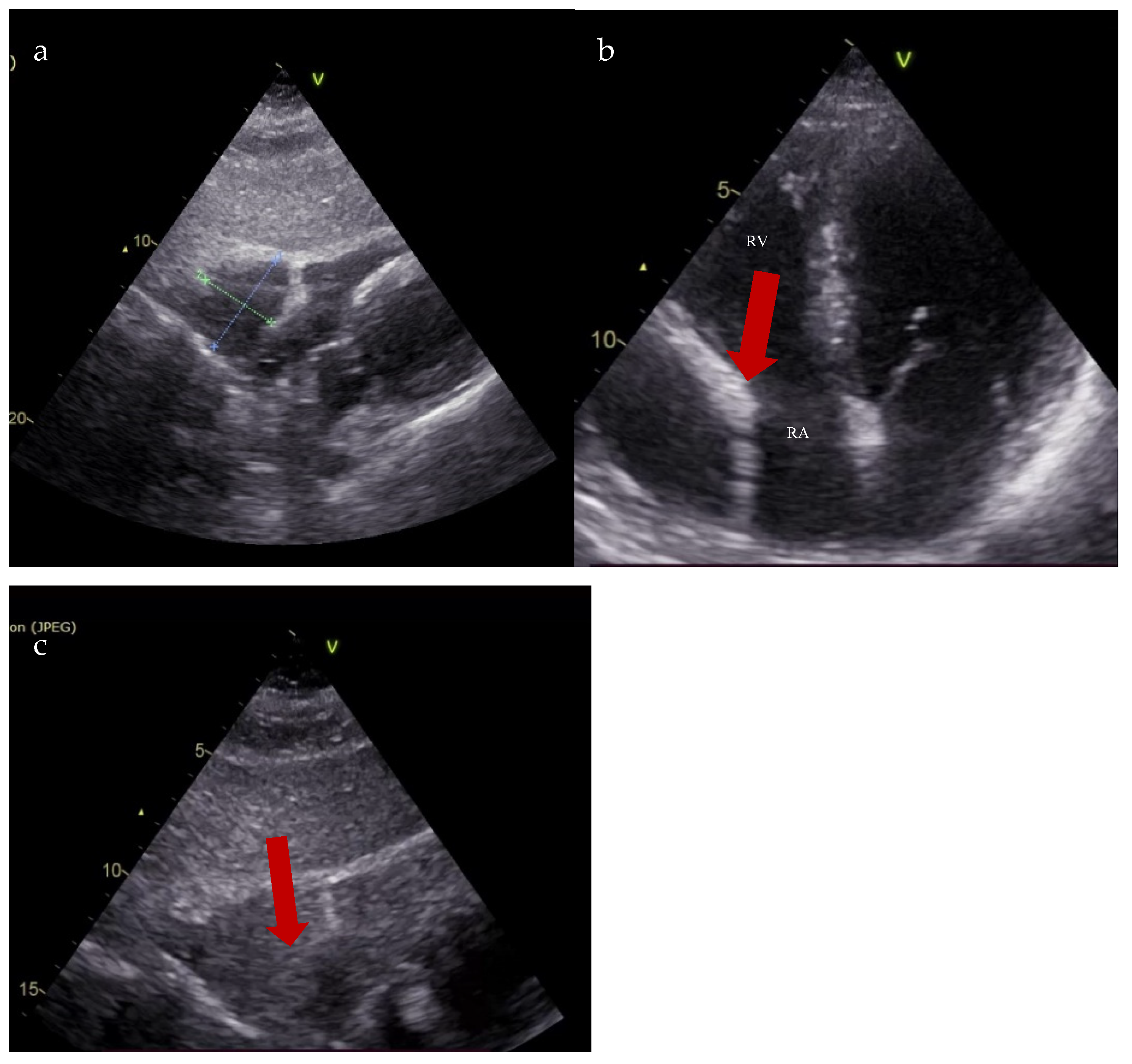
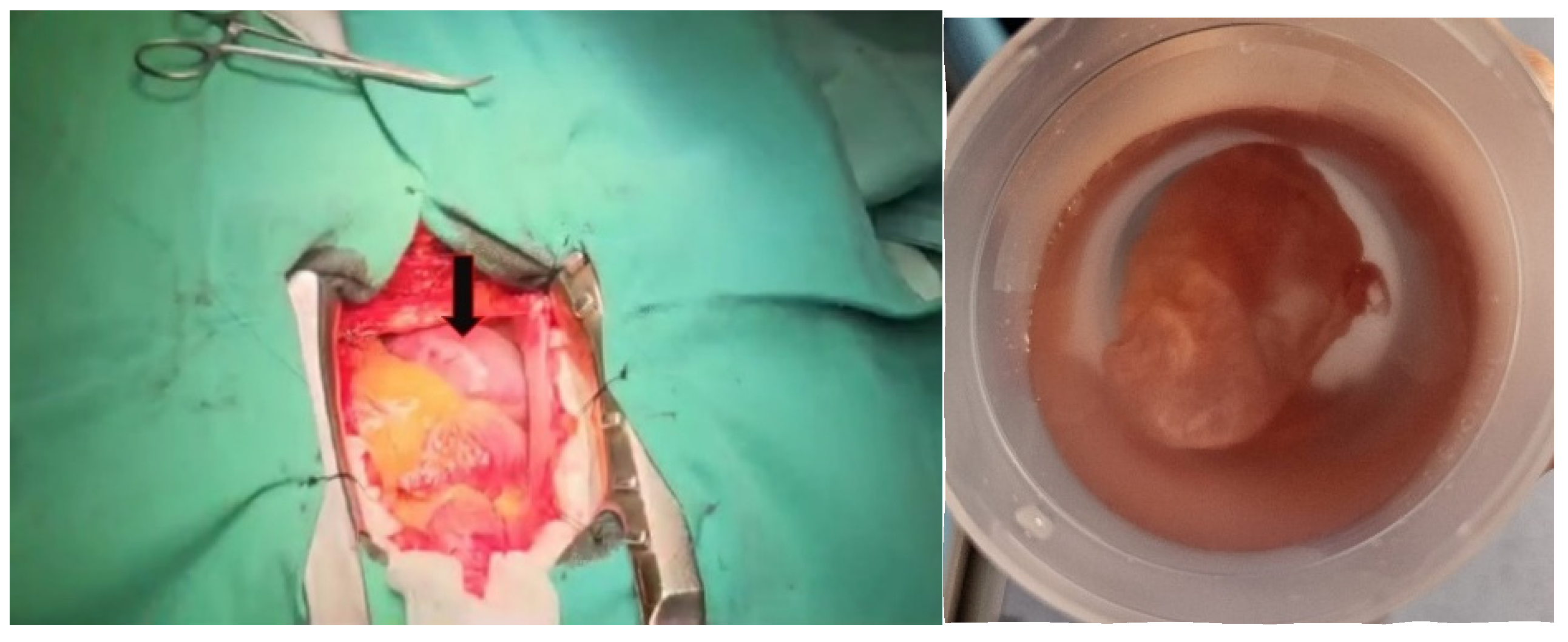
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID 19 | Coronavirus disease 2019 |
| LGE | late post-contrast sequences |
| MRI | magnetic resonance imaging |
References
- Binder, T.M.; Rosenhek, R.; Frank, H.; Gwechenberger, M.; Baumgartner, H. Congenital malformations of the right atrium and the coronary sinus: An analysis based on 103 cases reported in the literature and two additional cases. Chest 2000, 117, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.P. Surgery of the Heart; Lea and Febiger: Philadelphia, PA, USA, 1955. [Google Scholar]
- Kadlubik, S.D.; Vazquez, J.F.; Aguilar, J.P.; Cuéllar, M.L. Right atrial dilatation and primary hypertrophic cardiomyopathy: Report of a case and review of the literature. Arch. Inst. Cardiol. Mex. 1972, 42, 788–795. [Google Scholar]
- Fan, Y.; Jiang, H.; Wei, Y.; Zhang, B.; Ge, J. A Case of Giant Right Atrium Diverticulum with Atrial Septal Defect. Heart Surg. Forum 2021, 24, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.; Eicher, J.C.; Louis, P. Congenital diverticulum of the right atrium situated on the floor of the coronary sinus. Br. Heart J. 1988, 59, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Hu, J.; Zhang, Y.; Geng, Z.; Zhang, B. A rare case of an unexpected trigger of paroxysmal atrial fibrillation in the right atrial appendage diverticulum. BMC Cardiovasc. Disord. 2024, 24, 130. [Google Scholar] [CrossRef]
- Di Segni, E.; Siegal, A.; Katzenstein, M. Congenital diverticulum of the heart arising from the coronary sinus. Br. Heart J. 1986, 56, 380–384. [Google Scholar] [CrossRef]
- Tomar, M.; Radhakrishnan, S.; Iyer, K.S.; Shrivastava, S. Giant congenital diverticulum of the right atrium. Indian Heart J. 2008, 60, 359–362. [Google Scholar]
- Bezuska, L.; Bu’Lock, F.A.; Anderson, R.H.; Speggiorin, S.; Corno, A.F. Giant Right Atrial Aneurysm: Antenatal Diagnosis and Surgical Treatment. World J. Pediatr. Congenit. Heart Surg. 2018, 9, 459–462. [Google Scholar] [CrossRef]
- Angoff, R.K.; Waks, J.W.; Gavin, M.C.; Stabenau, H.F.; Strom, J.B. A Focus on the Right Atrium: Right Atrial Diverticulum and Atrial Flutter. JACC Case Rep. 2023, 11, 101788. [Google Scholar] [CrossRef]
- Kong, L.C.; Shuang, T.; Zhao, L.; Pu, J.; Wang, X.H. Catheter Ablation of a Right Atrial Free Wall Diverticulum-Related Accessory Pathway Masquerading as a Septal One. Int. Heart J. 2023, 64, 81–84. [Google Scholar] [CrossRef]
- Shakerian, B.; Mandegar, M.H. Right Atrial Diverticulum in an Adult Woman with Left Bundle Branch Block. Sultan Qaboos Univ. Med. J. 2020, 20, e394–e396. [Google Scholar] [CrossRef]
- Morishita, Y.; Kawashima, S.; Shimokawa, S.; Taira, A.; Kawagoe, H.; Nakamura, K. Multiple diverticula of the right atrium. Am. Heart J. 1990, 120, 1225–1227. [Google Scholar] [CrossRef]
- Agematsu, K.; Okamura, T.; Ishihara, K.; Kurosawa, H. Remarkable giant right atrial diverticulum in asymptomatic patient. Interact. Cardiovasc. Thorac. Surg. 2009, 8, 705–707. [Google Scholar] [CrossRef]
- Gray, H. Anatomy of the Human Body; Warren, H.L., Ed.; Lea & Febiger: New York, NY, USA, 1918. [Google Scholar]
- Yuan, G.; Yan, B.P.; Hu, J.; Wang, S.; Sun, J.P. Compressive giant right atrial diverticulum. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1065. [Google Scholar] [CrossRef]
- Chen, X.; Guo, X.; Ni, Y.; Yu, J.; Cha, Y.; Yang, Y. Giant right atrial diverticulum in an adult. J. Card. Surg. 2010, 25, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Shojima, T.; Tahara, N.; Morita, K.; Nakayoshi, T.; Tahara, A.; Bekki, M.; Maeda-Ogata, S.; Sugiyama, Y.; Igata, S.; et al. Life-threatening huge right atrial diverticulum. Eur. Heart J. Case Rep. 2020, 4, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, X.; Xu, J.; Wu, X.; Peng, S.; Zhang, Q.; Zhou, G.; Wei, Y.; Liu, S.; Chen, S. Right Atrial Diverticulum: An Unexpected Trigger of Paroxysmal Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiao, Y.; Hu, D.; Chen, M.; Li, X. Atrial tachycardia originating from a right atrial free wall diverticulum: Case report. Eur. Heart J. Case Rep. 2024, 8, ytae497. [Google Scholar] [CrossRef]
- Borgohain, S.; Malik, L.; Prasad, S.; Gupta, A.; Grover, V.; Gupta, V.K. Case of single right atrial diverticulum and review of etiology and management. Asian Cardiovasc. Thorac. Ann. 2013, 21, 592–595. [Google Scholar] [CrossRef]
- Morrow, A.G.; Behrendt, D.M. Congenital aneurysm (diverticulum) of the right atrium. Clinical manifestations and results of operative treatment. Circulation 1968, 38, 124–128. [Google Scholar] [CrossRef]
- Sepulveda, S.; Rosental, C.; Choe, H.J.; Cornelis, J.; Salgado, G. Congenital giant right atrial aneurysm. Int. J. Cardiovasc. Imaging 2024, 40, 687–691. [Google Scholar] [CrossRef]
- Forderer, N.; Akintürk, H.; Jux, C. Idiopathic enlargement of the right atrium masking left atrial aneurysm in a neonate. Cardiol. Young 2023, 33, 2446–2448. [Google Scholar] [CrossRef]
- Aggarwal, N.; Joshi, R.; Joshi, R.K.; Agarwal, M. Right Atrial Diverticulosis and Early-onset Arrhythmia: Rare Cause of Incessant Neonatal Arrhythmia. Indian Pediatr. 2017, 54, 503–504. [Google Scholar] [CrossRef]

| Blood count and inflammation markers | Hemoglobin 15 g/dL Hematocrit 45.9% Red blood cells 5,460,000/mm3 Platelets 251,000/mm3 White blood cells 6180/mm3 Protein C reactive 0.3 mg/dL Fibrinogen 374 mg/dL |
| Liver function | Aspartate aminotransferase 23 U/L Alanine aminotransferase 27 U/L Gamma-glutamyl transferase 18 U/L |
| Renal function | Urea 54 mg/dL Creatinine 0.6 mg/dL GFRe 101 mL/min/1.73 m2 Na+ 143 mmol/L K+ 4.8 mmol/L |
| Cardiac biomarkers | NT-proBNP 2869 pg/mL Creatine kinase MB izoenzyme 20 U/L High-sensitivity cardiac troponine test negative |
| Cardiovascular risk profile | Total cholesterol 219 mg/dL LDL-cholesterol 170 mg/dL HDL-cholesterol 44 mg/dL Non-HDL cholesterol 175 mg/dL Triglyceride 118 mg/dL Blood glucose 89 mg/dL Uric acid 4.8 mg/dL |
| LVDd | 49 mm | RVD basal | 25 mm |
| IVST | 8 mm | TAPSE | 20 mm |
| PWT | 8 mm | RV-RA gradient | 42 mmHg |
| E/A | 1.2 | PAP | 47 mmHg |
| mean E/E’ | 15 | ||
| LVEF | 20% | ||
| LA area | 22 cm/m2 | RA area | 20 cm/m2 |
| LA volume | 33 mL/m2 | RA volume | 25 mL/m2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onofrei, V.; Rusu, I.; Manole, O.-M. Incidental Discovery of a Right Atrial Diverticulum in an Adult Patient. Diagnostics 2025, 15, 2058. https://doi.org/10.3390/diagnostics15162058
Onofrei V, Rusu I, Manole O-M. Incidental Discovery of a Right Atrial Diverticulum in an Adult Patient. Diagnostics. 2025; 15(16):2058. https://doi.org/10.3390/diagnostics15162058
Chicago/Turabian StyleOnofrei, Viviana, Iuliana Rusu, and Oana-Mădălina Manole. 2025. "Incidental Discovery of a Right Atrial Diverticulum in an Adult Patient" Diagnostics 15, no. 16: 2058. https://doi.org/10.3390/diagnostics15162058
APA StyleOnofrei, V., Rusu, I., & Manole, O.-M. (2025). Incidental Discovery of a Right Atrial Diverticulum in an Adult Patient. Diagnostics, 15(16), 2058. https://doi.org/10.3390/diagnostics15162058





