The Triglyceride/HDL Ratio as a Non-Invasive Marker for Early-Stage NAFLD: A Retrospective Cross-Sectional Study of 2588 Patients
Abstract
1. Introduction
2. Patients and Methods
2.1. The Study Participants
2.2. The Laboratory and Radiological Analysis
2.3. The Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NAFLD | Non-alcoholic fatty liver disease |
| TG | Triglyceride |
| HDL | High-density lipoprotein |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| NASH | Non-alcoholic steatohepatitis |
| US | Ultrasound |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| APRI | AST-to-platelet ratio index |
| FIB-4 | Fibrosis-4 |
| EASL | European Association for the Study of the Liver |
| ROC | Receiver operating characteristic |
| WBC | White blood cell |
| ALP | Alkaline phosphatase |
| LDH | Lactate dehydrogenase |
| CRP | C-reactive protein |
| PLT | Platelet |
References
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, F.; Han, Y.; Zheng, L.; Bao, Z.; Liu, L.; Li, W. Association between chitinase-3-like protein 1 and metabolic-associated fatty liver disease in patients with type 2 diabetes mellitus. Ir. J. Med Sci. 2024, 193, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.J. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin. Liver Dis. 2004, 8, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Reccia, I.; Kumar, J.; Akladios, C.; Virdis, F.; Pai, M.; Habib, N.; Spalding, D. Non-alcoholic fatty liver disease: A sign of systemic disease. Metabolism 2017, 72, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Iuculano, F.; Pallini, G.; Fargion, S.; Fracanzani, A.L. Nutrients, Genetic Factors, and Their Interaction in Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 8761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Schwenzer, N.F.; Springer, F.; Schraml, C.; Stefan, N.; Machann, J.; Schick, F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J. Hepatol. 2009, 51, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, Y.; Zhong, L.; Hu, C.; Sheng, G. Association between the alanine aminotransferase/aspartate aminotransferase ratio and new-onset non-alcoholic fatty liver disease in a nonobese Chinese population: A population-based longitudinal study. Lipids Health Dis. 2020, 19, 245. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Bondesan, A.; Rondinelli, E.; Cella, S.G.; Sartorio, A. The Role of Aspartate Transaminase to Platelet Ratio Index (APRI) for the Prediction of Non-Alcoholic Fatty Liver Disease (NAFLD) in Severely Obese Children and Adolescents. Metabolites 2022, 12, 155, Erratum in Metabolites 2022, 12, 555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, Y.; Zhong, L.; Hu, C.; Zhong, M.; Peng, N.; Sheng, G. LDL/HDL cholesterol ratio is associated with new-onset NAFLD in Chinese non-obese people with normal lipids: A 5-year longitudinal cohort study. Lipids Health Dis. 2021, 20, 28. [Google Scholar] [CrossRef]
- Xuan, Y.; Hu, W.; Wang, Y.; Li, J.; Yang, L.; Yu, S.; Zhou, D. Association between RC/HDL-C ratio and risk of non-alcoholic fatty liver disease in the United States. Front. Med. 2024, 11, 1427138. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Tabacu, L.; Swami, S.; Ledbetter, M.; Siddiqui, M.S.; Smirnova, E.; Yáñez-Sepúlveda, R. Socioeconomic status and health disparities drive differences in accelerometer-derived physical activity in fatty liver disease and significant fibrosis. PLoS ONE 2024, 19, e0301774. [Google Scholar] [CrossRef] [PubMed]
- Summart, U.; Thinkhamrop, B.; Chamadol, N.; Khuntikeo, N.; Songthamwat, M.; Kim, C.S. Gender differences in the prevalence of nonalcoholic fatty liver disease in the Northeast of Thailand: A population-based cross-sectional study. F1000Research 2017, 6, 1630. [Google Scholar] [CrossRef] [PubMed]
- Nafld, J.; Eguchi, Y.; Hyogo, H.; Ono, M.; Mizuta, T.; Ono, N.; Fujimoto, K.; Chayama, K.; Saibara, T. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: A multicenter large retrospective study. J. Gastroenterol. 2012, 47, 586–595. [Google Scholar] [CrossRef]
- Kim, K.-S.; Hong, S.; Han, K.; Park, C.-Y. Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: Nationwide population based study. BMJ 2024, 384, e076388. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef]
- Amernia, B.; Moosavy, S.H.; Banookh, F.; Zoghi, G. FIB-4, APRI, and AST/ALT ratio compared to FibroScan for the assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease in Bandar Abbas, Iran. BMC Gastroenterol. 2021, 21, 453. [Google Scholar] [CrossRef]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018, 24, 3361–3373. [Google Scholar] [CrossRef]
- Long, M.T.; Pedley, A.; Colantonio, L.D.; Massaro, J.M.; Hoffmann, U.; Muntner, P.; Fox, C.S. Development and Validation of the Framingham Steatosis Index to Identify Persons With Hepatic Steatosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1172–1180.e2. [Google Scholar] [CrossRef]
- Lin, M.-S.; Lin, H.-S.; Chang, M.-L.; Tsai, M.-H.; Hsieh, Y.-Y.; Lin, Y.-S.; Tsai, M.-S.; Yang, C.-L.; Chen, M.-Y. Alanine aminotransferase to aspartate aminotransferase ratio and hepatitis B virus on metabolic syndrome: A community-based study. Front. Endocrinol. 2022, 13, 922312. [Google Scholar] [CrossRef] [PubMed]
- Thong, V.D.; Quynh, B.T.H. Correlation of Serum Transaminase Levels with Liver Fibrosis Assessed by Transient Elastography in Vietnamese Patients with Nonalcoholic Fatty Liver Disease. Int. J. Gen. Med. 2021, 14, 1349–1355. [Google Scholar] [CrossRef]
- Jamialahmadi, T.; Bo, S.; Abbasifard, M.; Sathyapalan, T.; Jangjoo, A.; Moallem, S.A.; Almahmeed, W.; Ashari, S.; Johnston, T.P.; Sahebkar, A. Association of C-reactive protein with histological, elastographic, and sonographic indices of non-alcoholic fatty liver disease in individuals with severe obesity. J. Health Popul. Nutr. 2023, 42, 30. [Google Scholar] [CrossRef]
- Hoekstra, M.; Van Eck, M. High-density lipoproteins and non-alcoholic fatty liver disease. Atheroscler. Plus 2023, 53, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Kawanabe, Y.; Shinozaki, F.; Sato, S.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Sueishi, M. Triglyceride is strongly associated with nonalcoholic fatty liver disease among markers of hyperlipidemia and diabetes. Biomed. Rep. 2014, 2, 633–636. [Google Scholar] [CrossRef]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhan, F.; Peng, T.; Xia, Z.; Li, J. Association between the Triglyceride–Glucose Index and Non-Alcoholic Fatty Liver Disease in patients with Atrial Fibrillation. Eur. J. Med Res. 2023, 28, 355. [Google Scholar] [CrossRef]
- Wu, K.-T.; Kuo, P.-L.; Su, S.-B.; Chen, Y.-Y.; Yeh, M.-L.; Huang, C.-I.; Yang, J.-F.; Lin, C.-I.; Hsieh, M.-H.; Hsieh, M.-Y.; et al. Nonalcoholic fatty liver disease severity is associated with the ratios of total cholesterol and triglycerides to high-density lipoprotein cholesterol. J. Clin. Lipidol. 2016, 10, 420–425.e1. [Google Scholar] [CrossRef]
- Fukuda, Y.; Hashimoto, Y.; Hamaguchi, M.; Fukuda, T.; Nakamura, N.; Ohbora, A.; Kato, T.; Kojima, T.; Fukui, M. Triglycerides to high-density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; a population-based cohort study. Liver Int. 2016, 36, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qin, H.; Qiu, S.; Chen, G.; Chen, Y. Correlation of triglyceride to high-density lipoprotein cholesterol ratio with nonalcoholic fatty liver disease among the non-obese Chinese population with normal blood lipid levels: A retrospective cohort research. Lipids Health Dis. 2019, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-G.; Tian, N.; Xie, W.-N. Total cholesterol to high-density lipoprotein ratio and nonalcoholic fatty liver disease in a population with chronic hepatitis B. World J. Hepatol. 2022, 14, 791–801. [Google Scholar] [CrossRef]
- Ozkan, S. Incidence of nonalcoholic fatty liver disease in type 2 diabetic patients. Yeditepe Med. J. 2016, 12, 960–967. [Google Scholar] [CrossRef]
- Sayar, M.S.; Bulut, D.; Acar, A. Evaluation of hepatosteatosis in patients with chronic hepatitis B virus infection. Arab. J. Gastroenterol. 2023, 24, 11–15. [Google Scholar] [CrossRef]
- Yin, J.-Y.; Yang, T.-Y.; Yang, B.-Q.; Hou, C.-X.; Li, J.-N.; Li, Y.; Wang, Q. FibroScan-aspartate transaminase: A superior non-invasive model for diagnosing high-risk metabolic dysfunction-associated steatohepatitis. World J. Gastroenterol. 2024, 30, 2440–2453. [Google Scholar] [CrossRef]
- Rigor, J.; Diegues, A.; Presa, J.; Barata, P.; Martins-Mendes, D. Noninvasive fibrosis tools in NAFLD: Validation of APRI, BARD, FIB-4, NAFLD fibrosis score, and Hepamet fibrosis score in a Portuguese population. Postgrad. Med. 2022, 134, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Nanji, A.A.; French, S.W.; Freeman, J.B. Serum alanine aminotransferase to aspartate aminotransferase ratio and degree of fatty liver in morbidly obese patients. Enzyme 1986, 36, 266–269. [Google Scholar] [CrossRef]

| Characteristics and Findings | All Patients (n: 2588, Mean ± Std) | Patients Without NAFLD (n: 1270, Mean ± Std) | Patients with NAFLD (n: 1318, Mean ± Std) | p-Value |
|---|---|---|---|---|
| Age (years) | 45.31 ± 12.50 | 41.42 ± 13.33 | 49.07 ± 10.34 | <0.001 |
| Gender (F/M) | 1631/957 | 811/459 | 820/498 | 0.249 |
| WBCs (109/L) | 7.35 ± 1.93 | 7.11 ± 1.91 | 7.58 ± 1.92 | <0.001 |
| Neutrophil (109/L) | 4.32 ± 1.50 | 4.23 ± 1.56 | 4.40 ± 1.45 | 0.005 |
| Hemoglobin (g/L) | 13.58 ± 1.78 | 13.4 ± 1.85 | 13.76 ± 1.70 | <0.001 |
| Platelets (109/L) | 258.81 ± 70.92 | 257.26 ± 76.02 | 260.31 ± 65.62 | 0.275 |
| Glucose (mg/dL) | 109.34 ± 41.40 | 100.63 ± 31.48 | 117.73 ± 47.61 | <0.001 |
| HbA1c (%) | 6.25 ± 1.48 | 5.88 ± 1.30 | 6.52 ± 1.54 | <0.001 |
| AST (U/L) | 24.80 ± 38.88 | 22.67 ± 30.62 | 26.84 ± 45.33 | <0.001 |
| ALT (U/L) | 29.91 ± 49.99 | 24.02 ± 36.87 | 35.60 ± 59.45 | <0.001 |
| ALP (IU/L) | 84.37 ± 36.09 | 82.38 ± 42.0 | 86.45 ± 28.49 | 0.013 |
| LDH (U/L) | 182.75 ± 47.96 | 178.05 ± 46.45 | 187.77 ± 49.06 | <0.001 |
| Creatinine (mg/dL) | 0.75 ± 0.16 | 0.74 ± 0.15 | 0.76 ± 0.16 | <0.001 |
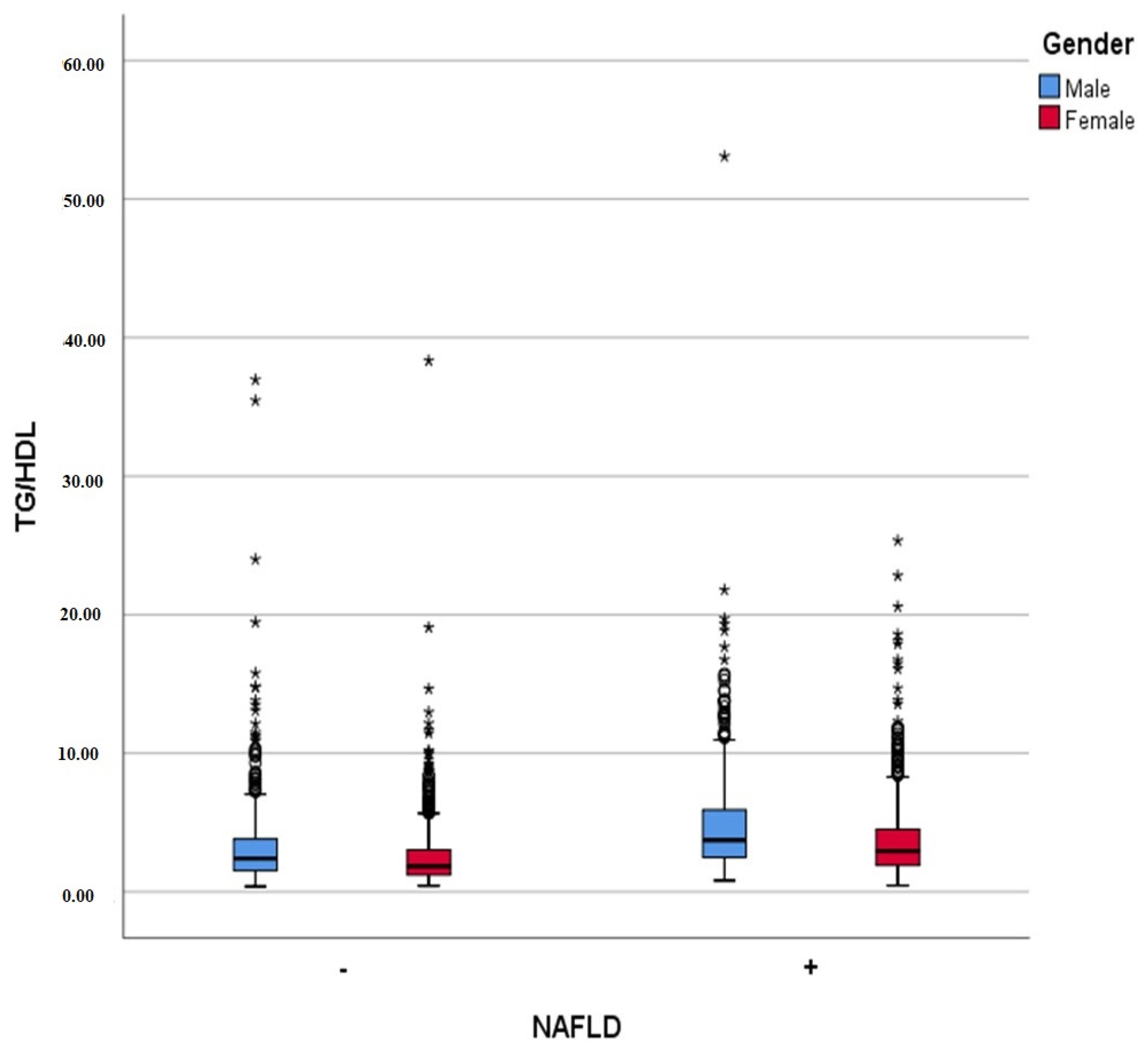
| Triglyceride (mg/dL) | 147.42 ± 93.89 | 122.67 ± 80.57 | 171.27 ± 99.50 | <0.001 |
| HDL-cholesterol (mg/dL) | 49.28 ± 12.82 | 51.26 ± 13.54 | 47.37 ± 11.78 | <0.001 |
| Total cholesterol (mg/dL) | 189.23 ± 41.0 | 181.16 ± 40.62 | 197.02 ± 39.88 | <0.001 |
| Uric acid (mg/dL) | 4.70 ± 1.31 | 4.34 ± 1.22 | 5.06 ± 1.30 | <0.001 |
| Albumin (g/L) | 45.63 ± 3.0 | 45.41 ± 3.28 | 45.83 ± 2.70 | 0.002 |
| CRP (mg/L) | 4.91 ± 9.32 | 4.31 ± 9.85 | 5.49 ± 8.75 | 0.007 |
| Patients Without NAFLD (n: 1270) | Patients with NAFLD (n: 1318) | p-Value | |
|---|---|---|---|
| TG/HDL ratio | 2.79 ± 2.83 | 4.08 ± 3.30 | <0.001 |
| ALT/AST ratio | 0.99 ± 0.39 | 1.23 ± 0.49 | <0.001 |
| APRI | 0.32 ± 0.64 | 0.35 ± 0.62 | <0.001 |
| FIB-4 score | 0.85 ± 0.80 | 0.93 ± 0.50 | <0.001 |
| TG/Glucose ratio | 1.24 ± 0.74 | 1.54 ± 0.88 | <0.001 |
| B | S.E. | Odds Ratio | p | |
|---|---|---|---|---|
| (Constant) | −2.518 | 0.188 | 0.081 | |
| FIB-4 | 0.734 | 0.116 | 2.082 | <0.001 |
| APRI | −61.141 | 14.798 | 0 | <0.001 |
| Tg/HDL ratio | 0.112 | 0.03 | 1.118 | <0.001 |
| Tg/glucose ratio | 0.101 | 0.095 | 1.107 | 0.288 |
| ALT/AST ratio | 1.452 | 0.122 | 4.27 | <0.001 |
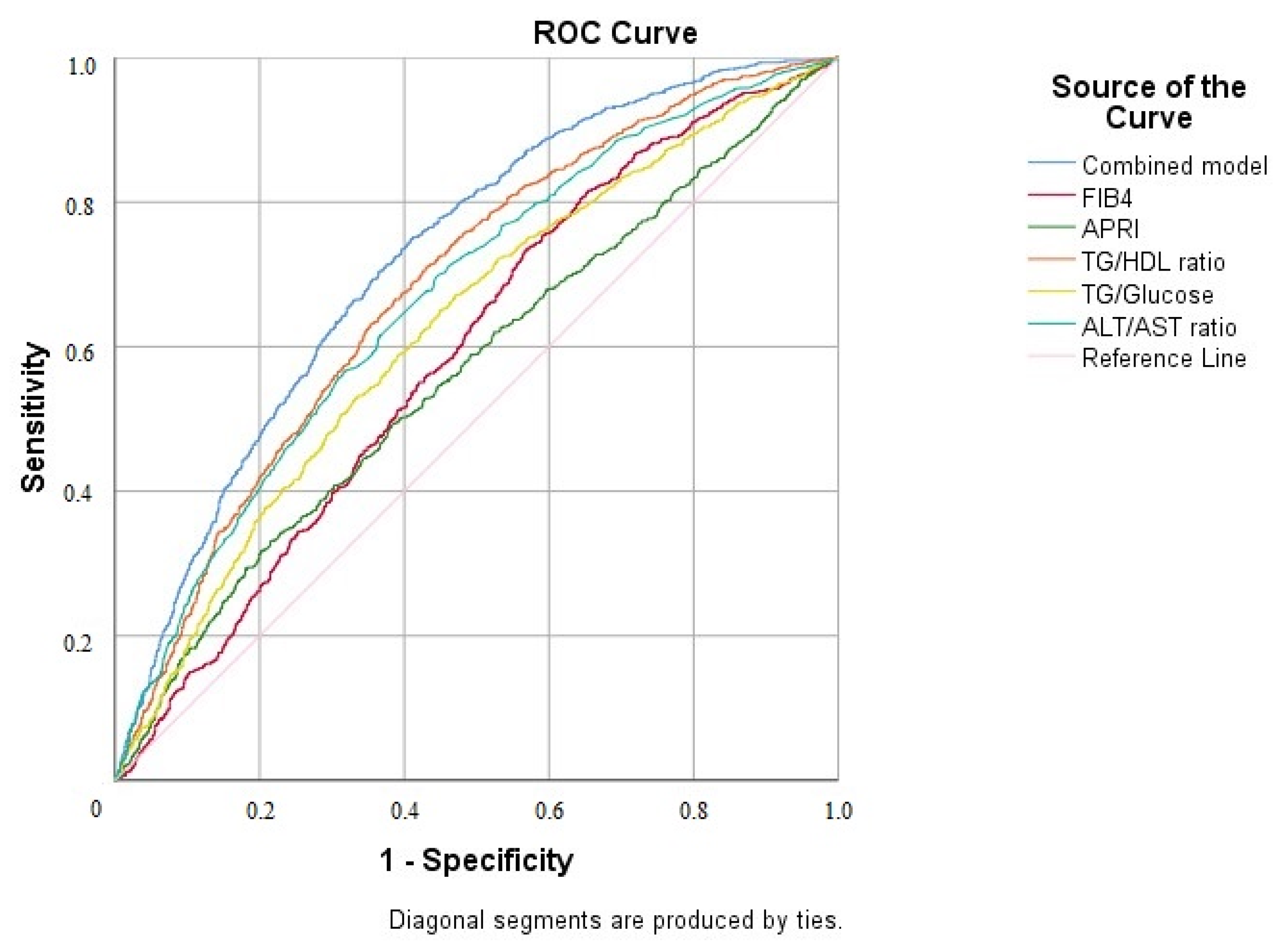
| AUROC for NAFLD (%95 CI) | p-Value | |
|---|---|---|
| TG/HDL ratio | 0.682 (0.662–0.703) | <0.001 |
| Combined model | 0.723 (0.704–0.743) | <0.001 |
| ALT/AST ratio | 0.668 (0.647–0.689) | <0.001 |
| APRI | 0.565 (0.543–0.587) | <0.001 |
| FIB-4 | 0.591 (0.569–0.613) | <0.001 |
| TG/glucose ratio | 0.626 (0.604–0.647) | <0.001 |
| TG/HDL Ratio | N of Patients Without NAFLD | N of Patients with NAFLD | Total | p-Value |
|---|---|---|---|---|
| <1.86 | 578 | 254 | 832 | <0.001 |
| ≥1.86 | 692 | 1064 | 1756 | |
| Total | 1270 | 1318 | 2588 |
| Parameter | r (95% CI) | p |
|---|---|---|
| Age | 0.139 (0.066–0.210) | <0.001 |
| WBCs | 0.183 (0.114–0.246) | <0.001 |
| Hgb | 0.213 (0.140–0.287) | <0.001 |
| PLTs | −0.008 (−0.083–0.062) | 0.818 |
| Creatinine | 0.174 (0.102–0.237) | <0.001 |
| AST | 0.146 (0.077–0.212) | <0.001 |
| ALT | 0.229 (0.158–0.296) | <0.001 |
| Uric acid | 0.272 (0.208–0.337) | <0.001 |
| Glucose | 0.244 (0.179–0.309) | <0.001 |
| HbA1c | 0.242 (0.168–0.311) | <0.001 |
| LDH | 0.021 (−0.044–0.090) | 0.554 |
| APRI | 0.109 (0.038–0.178) | 0.002 |
| FIB-4 | 0.061 (−0.010–0.134) | 0.084 |
| ALT/AST ratio | 0.249 (0.183–0.315) | <0.001 |
| TG/glucose ratio | 0.795 (0.763–0.825) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoca, E.; Cangir, B.; Ahbab, S.; Şahin, S.İ.; Çiftçi Öztürk, E.; Urvasızoğlu, A.Ö.; Kalaycı, N.; Engin, İ.; Ataoğlu, H.E. The Triglyceride/HDL Ratio as a Non-Invasive Marker for Early-Stage NAFLD: A Retrospective Cross-Sectional Study of 2588 Patients. Diagnostics 2025, 15, 2045. https://doi.org/10.3390/diagnostics15162045
Hoca E, Cangir B, Ahbab S, Şahin Sİ, Çiftçi Öztürk E, Urvasızoğlu AÖ, Kalaycı N, Engin İ, Ataoğlu HE. The Triglyceride/HDL Ratio as a Non-Invasive Marker for Early-Stage NAFLD: A Retrospective Cross-Sectional Study of 2588 Patients. Diagnostics. 2025; 15(16):2045. https://doi.org/10.3390/diagnostics15162045
Chicago/Turabian StyleHoca, Emre, Bilal Cangir, Süleyman Ahbab, Seher İrem Şahin, Ece Çiftçi Öztürk, Ayşe Öznur Urvasızoğlu, Nilsu Kalaycı, İsmail Engin, and Hayriye Esra Ataoğlu. 2025. "The Triglyceride/HDL Ratio as a Non-Invasive Marker for Early-Stage NAFLD: A Retrospective Cross-Sectional Study of 2588 Patients" Diagnostics 15, no. 16: 2045. https://doi.org/10.3390/diagnostics15162045
APA StyleHoca, E., Cangir, B., Ahbab, S., Şahin, S. İ., Çiftçi Öztürk, E., Urvasızoğlu, A. Ö., Kalaycı, N., Engin, İ., & Ataoğlu, H. E. (2025). The Triglyceride/HDL Ratio as a Non-Invasive Marker for Early-Stage NAFLD: A Retrospective Cross-Sectional Study of 2588 Patients. Diagnostics, 15(16), 2045. https://doi.org/10.3390/diagnostics15162045





