A Partial Hydatidiform Mole in an Ovarian Ectopic Pregnancy: An Exceptional Occurrence
Abstract
1. Introduction
2. Case Presentation
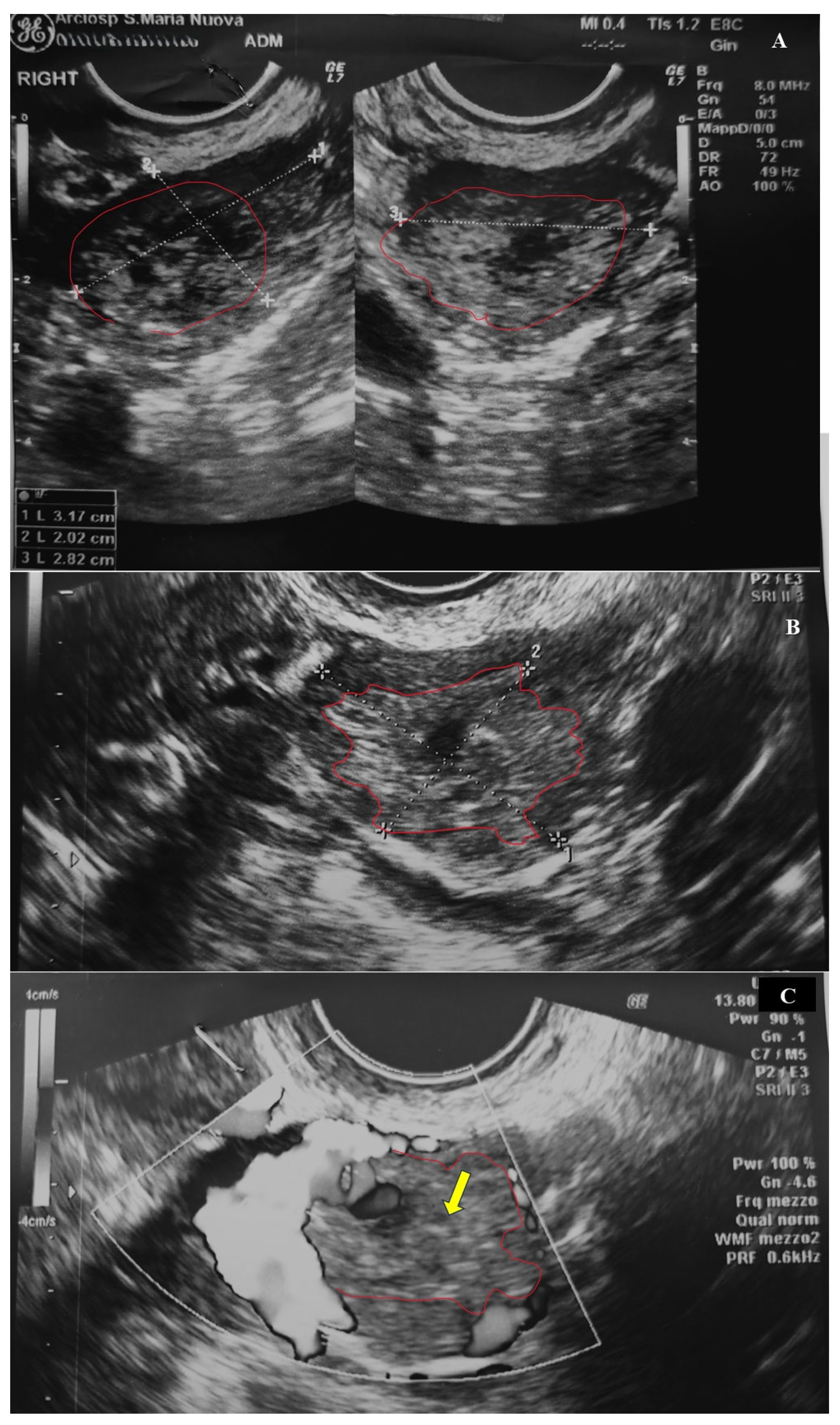
2.1. Clinical History
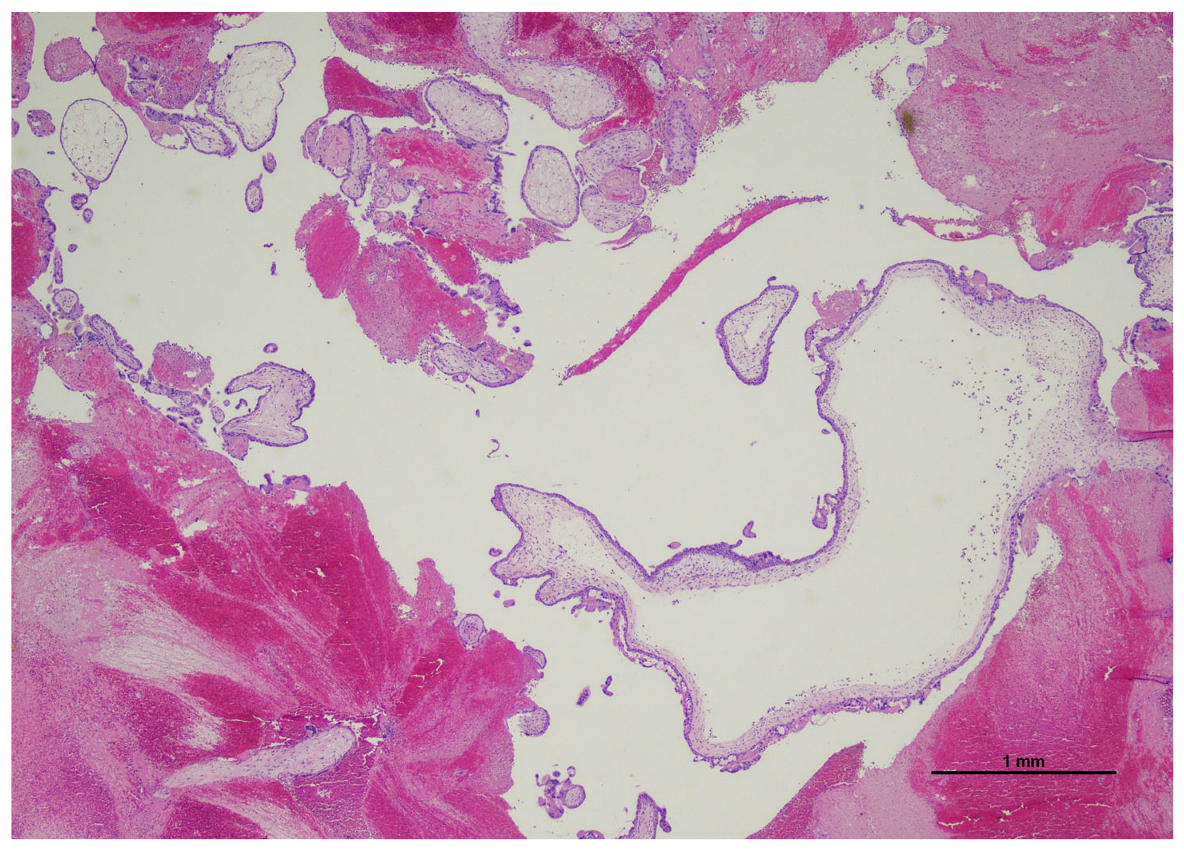
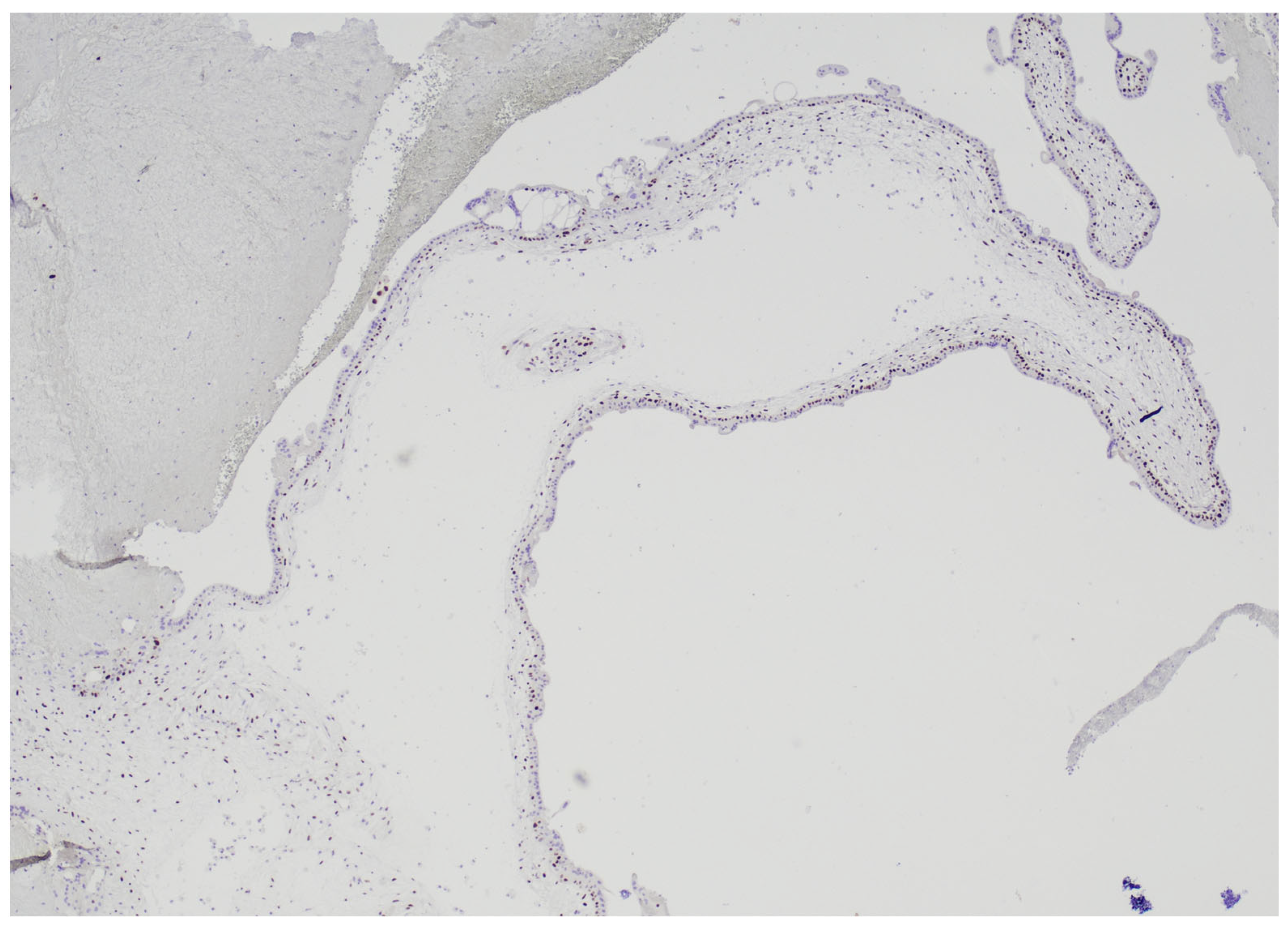
2.2. Histological Examination
2.3. HER2 FISH Analysis
2.4. STR Analysis
2.5. Patient’s Follow-Up
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tonick, S.; Conageski, C. Ectopic Pregnancy. Obstet. Gynecol. Clin. N. Am. 2022, 49, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Solangon, S.A.; Naftalin, J.; Jurkovic, D. Ovarian Ectopic Pregnancy: Clinical Characteristics, Ultrasound Diagnosis and Management. Ultrasound Obstet. Gynecol. 2024, 63, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, L.; Wood, F.; Hallchurch, J.; Douce, G.; Pinto, S. Ruptured Ovarian Ectopic Pregnancy Presenting with an Acute Abdomen. BMJ Case Rep. 2022, 15, e252499. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Als-Nielsen, A. Cas de Mole Hydatideuse Ovarienne. Acta Obstet. Gynecol. Scand. 1925, 3, 325–336. [Google Scholar] [CrossRef]
- Barr, F.G.; Oktay, A. Primary Ovarian Hydatidiform Mole: A Case Report. Am. J. Obstet. Gynecol. 1960, 79, 1088–1090. [Google Scholar] [CrossRef]
- Yenen, E.; Inanc, F.A.; Babuna, C. Primary Ovarian Hydatidiform Mole. Report of a Case. Obstet. Gynecol. 1965, 26, 721–724. [Google Scholar]
- Adli, A.G. Primary Ovarian Hydatidiform Mole. Int. Surg. 1978, 63, 103. [Google Scholar]
- Jock, D.E.; Schwartz, P.E.; Portnoy, L. Primary Ovarian Hydatidiform Mole: Addition of a Sixth Case to the Literature. Obstet. Gynecol. 1981, 58, 657–660. [Google Scholar]
- D’Aguillo, A.F.; Goldberg, M.I.; Kamalamma, M.; Yuliano, S.E.; Scully, J.T. Primary Ovarian Hydatidiform Mole. Hum. Pathol. 1982, 13, 279–281. [Google Scholar] [CrossRef]
- Stanhope, C.R.; Stuart, G.C.; Curtis, K.L. Primary Ovarian Hydatidiform Mole: Review of the Literature and Report of a Case. Am. J. Obstet. Gynecol. 1983, 145, 886–888. [Google Scholar] [CrossRef]
- Switzer, J.M.; Weckstein, M.L.; Campbell, L.F.; Curtis, K.L.; Powaser, J.T. Ovarian Hydatidiform Mole. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 1984, 3, 471–473. [Google Scholar] [CrossRef]
- Church, E.; Hanna, L.; New, F.; Uku, A.; Awad, H.; Watson, A.J.S. Ovarian Molar Pregnancy. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2008, 28, 660–661. [Google Scholar] [CrossRef]
- Leung, F.; Terzibachian, J.-J.; Chung Fat, B.; Lassabe, C.; Knoepffler, F.; Maillet, R.; Riethmuller, D. Môle Hydatiforme Hétérotopique Ovarienne. À Propos d’un Cas. Gynécologie Obs. Fertil. 2009, 37, 749–751. [Google Scholar] [CrossRef]
- Leung, F.; Terzibachian, J.-J.; Lassabe, C. One Mole May Hide Another: The Case of an Ovarian Hydatidiform Mole Coexistent with Intrauterine Hydatidiform Mole. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 148, 98–99. [Google Scholar] [CrossRef]
- Joneborg, U.; Papadogiannakis, N.; Lindell, G.; Marions, L. Choriocarcinoma Following Ovarian Hydatidiform Mole: A Case Report. J. Reprod. Med. 2011, 56, 511–514. [Google Scholar]
- Sehn, J.K.; Kuroki, L.M.; Hopeman, M.M.; Longman, R.E.; McNicholas, C.P.; Huettner, P.C. Ovarian Complete Hydatidiform Mole: Case Study with Molecular Analysis and Review of the Literature. Hum. Pathol. 2013, 44, 2861–2864. [Google Scholar] [CrossRef] [PubMed]
- Aravapalli, Y.; Mane, A.; Kathrani, N.; Chauhan, R.S. Ruptured Large Ectopic Hydatidiform Mole: An Infrequent Presentation of Gestational Trophoblastic Disease. J. Ultrasound 2024, 27, 941–945. [Google Scholar] [CrossRef] [PubMed]
- LeGallo, R.D.; Stelow, E.B.; Ramirez, N.C.; Atkinsx, K.A. Diagnosis of Hydatidiform Moles Using P57 Immunohistochemistry and HER2 Fluorescent In Situ Hybridization. Am. J. Clin. Pathol. 2008, 129, 749–755. [Google Scholar] [CrossRef]
- Cavalari, C.A.A.; Mehrtash, H.; Brizuela, V.; Baguiya, A.; Adu-Bonsaffoh, K.; Cecatti, J.G.; Bahamondes, L.; Charles, C.M.; Govule, P.; Dossou, J.-P.; et al. Prevalence and Management of Ectopic and Molar Pregnancies in 17 Countries in Africa and Latin America and the Caribbean: A Secondary Analysis of the WHO Multi-Country Cross-Sectional Survey on Abortion. BMJ Open 2024, 14, e086723. [Google Scholar] [CrossRef]
- Pape, J.; Bajka, A.; Strutas, D.; Burkhardt, T.; Imesch, P.; Fink, D.; Samartzis, E.P.; Bajka, M. The Predictive Value of Decisive and Soft Ultrasound Criteria for Ectopic Pregnancy Identification in 321 Preoperative Cases. Ultraschall Med. Eur. J. Ultrasound 2023, 44, e47–e61. [Google Scholar] [CrossRef]
- Pape, J.; Bajka, A.; Seifert, B.; Asmis, L.; Imesch, P.; Metzler, J.; Burkhardt, T.; Condous, G.; Samartzis, E.P.; Bajka, M. Judging Urgency in 343 Ectopic Pregnancies Prior to Surgery—The Importance of Transvaginal Sonographic Diagnosis of Intraabdominal Free Blood. Ultraschall Med. 2023, 44, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Soper, J.T. Gestational Trophoblastic Disease: Current Evaluation and Management. Obstet. Gynecol. 2021, 137, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B. Pathology of Gestational Trophoblastic Disease (GTD). Hematol. Oncol. Clin. N. Am. 2024, 38, 1191–1217. [Google Scholar] [CrossRef]
- Sarmadi, S.; Izadi-Mood, N.; Abbasi, A.; Sanii, S. p57KIP2 Immunohistochemical Expression: A Useful Diagnostic Tool in Discrimination between Complete Hydatidiform Mole and Its Mimics. Arch. Gynecol. Obstet. 2011, 283, 743–748. [Google Scholar] [CrossRef]
- Braga, A.; Andrade, T.; de Souza, M.D.C.B.; Campos, V.; Freitas, F.; Maestá, I.; Sun, S.Y.; Pedrotti, L.G.; Bessel, M.; Junior, J.A.; et al. Presentation, Medical Complications and Development of Gestational Trophoblastic Neoplasia of Hydatidiform Mole after Intracytoplasmic Sperm Injection as Compared to Hydatidiform Mole after Spontaneous Conception—A Retrospective Cohort Study and Literature Review. Gynecol. Oncol. 2023, 170, 179–185. [Google Scholar] [CrossRef]
- Toufaily, M.H.; Roberts, D.J.; Westgate, M.-N.; Holmes, L.B. Triploidy: Variation of Phenotype. Am. J. Clin. Pathol. 2016, 145, 86–95. [Google Scholar] [CrossRef]
- Ross, J.A.; Unipan, A.; Clarke, J.; Magee, C.; Johns, J. Ultrasound Diagnosis of Molar Pregnancy. Ultrasound Leeds Engl. 2018, 26, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yang, X.; Yin, H. Application of Short Tandem Repeat (STR) Genotyping in Partial Hydatidiform Mole. Am. J. Transl. Res. 2023, 15, 3731–3738. [Google Scholar]

| Marker | Chromosome Location | Size Range | Allele Size | Allele |
|---|---|---|---|---|
| D8s1179 | 8 | 122–169 | 123-135-148 | 8-11-14 |
| D21s11 | 21q11.2-q21 | 184–240 | 206-210 | 29.2-30.2 |
| D7s820 | 7q11.21-22 | 255–292 | 263-266-279 | 8-9-12 |
| CSF1PO | 5q33.3-34 | 304–340 | 314-322-330 | 9-11-13 |
| D3s1358 | 3p | 111–140 | 120-128-132-136 | 14-16-17-18 |
| TH01 | 11p15.5 | 162–202 | 172-181-184 | 7-9-9.3 |
| D13s317 | 13q22-31 | 216–244 | 232-236-240 | 12-13-14 |
| D16s539 | 16q24-qter | 252–292 | 276-279-279 | 11-12-12 |
| D2s1338 | 2q35-37.1 | 306–359 | 314-318-326 | 17-18-20 |
| D19s433 | 19q12-13.1 | 101–135 | 116-120 | 13-14 |
| Vwa | 12p12-pter | 154–206 | 173-176-176 | 16-17-17 |
| TPOX | 2p23-2per | 221–250 | 229-229-236 | 8-8-10 |
| D18s51 | 18q21.3 | 261–344 | 292-292-300 | 14.2-14.2-21 |
| Amelogenin | X: p22.1-22.3 Y: p11.2 | 106–111 | 106 | X |
| D5s818 | 5q21.31 | 133–172 | 149-153-157 | 11-12-13 |
| FGA | 4q28 | 214–355 | 244 | 25 |
| Marker | Allele Size | Allele Height |
|---|---|---|
| X1 | 139-151 | 850-1659 |
| XY2 | 191-199 | 339-723 |
| X3 | 281-285-269 | 390-379-360 |
| X9 | 322-334-338 | 412-369-358 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonasoni, M.P.; Zuntini, R.; Shah, K.; De Marco, L.; Zanetti, E.; Pagliai, L.; Blasi, I.; Carossino, E.; Ferretti, A.; Mandato, V.D.; et al. A Partial Hydatidiform Mole in an Ovarian Ectopic Pregnancy: An Exceptional Occurrence. Diagnostics 2025, 15, 2024. https://doi.org/10.3390/diagnostics15162024
Bonasoni MP, Zuntini R, Shah K, De Marco L, Zanetti E, Pagliai L, Blasi I, Carossino E, Ferretti A, Mandato VD, et al. A Partial Hydatidiform Mole in an Ovarian Ectopic Pregnancy: An Exceptional Occurrence. Diagnostics. 2025; 15(16):2024. https://doi.org/10.3390/diagnostics15162024
Chicago/Turabian StyleBonasoni, Maria Paola, Roberta Zuntini, Khush Shah, Loredana De Marco, Eleonora Zanetti, Luca Pagliai, Immacolata Blasi, Emanuela Carossino, Alice Ferretti, Vincenzo Dario Mandato, and et al. 2025. "A Partial Hydatidiform Mole in an Ovarian Ectopic Pregnancy: An Exceptional Occurrence" Diagnostics 15, no. 16: 2024. https://doi.org/10.3390/diagnostics15162024
APA StyleBonasoni, M. P., Zuntini, R., Shah, K., De Marco, L., Zanetti, E., Pagliai, L., Blasi, I., Carossino, E., Ferretti, A., Mandato, V. D., & Aguzzoli, L. (2025). A Partial Hydatidiform Mole in an Ovarian Ectopic Pregnancy: An Exceptional Occurrence. Diagnostics, 15(16), 2024. https://doi.org/10.3390/diagnostics15162024






