GenPad: A Highly Efficient Roadmap for the Development of a New Rapid, Highly Sensitive, and Portable Point-of-Care Testing System for Nucleic Acid Diagnostics in Japan
Abstract
1. Introduction
2. The Fight Against the New Coronavirus Started with the “Diamond Princess” Incident
3. System for Dealing with Emerging Infectious Diseases
4. Development of Eprimer-SmartAmp Technology—The Basic Chemical Reaction for the GenPad Platform
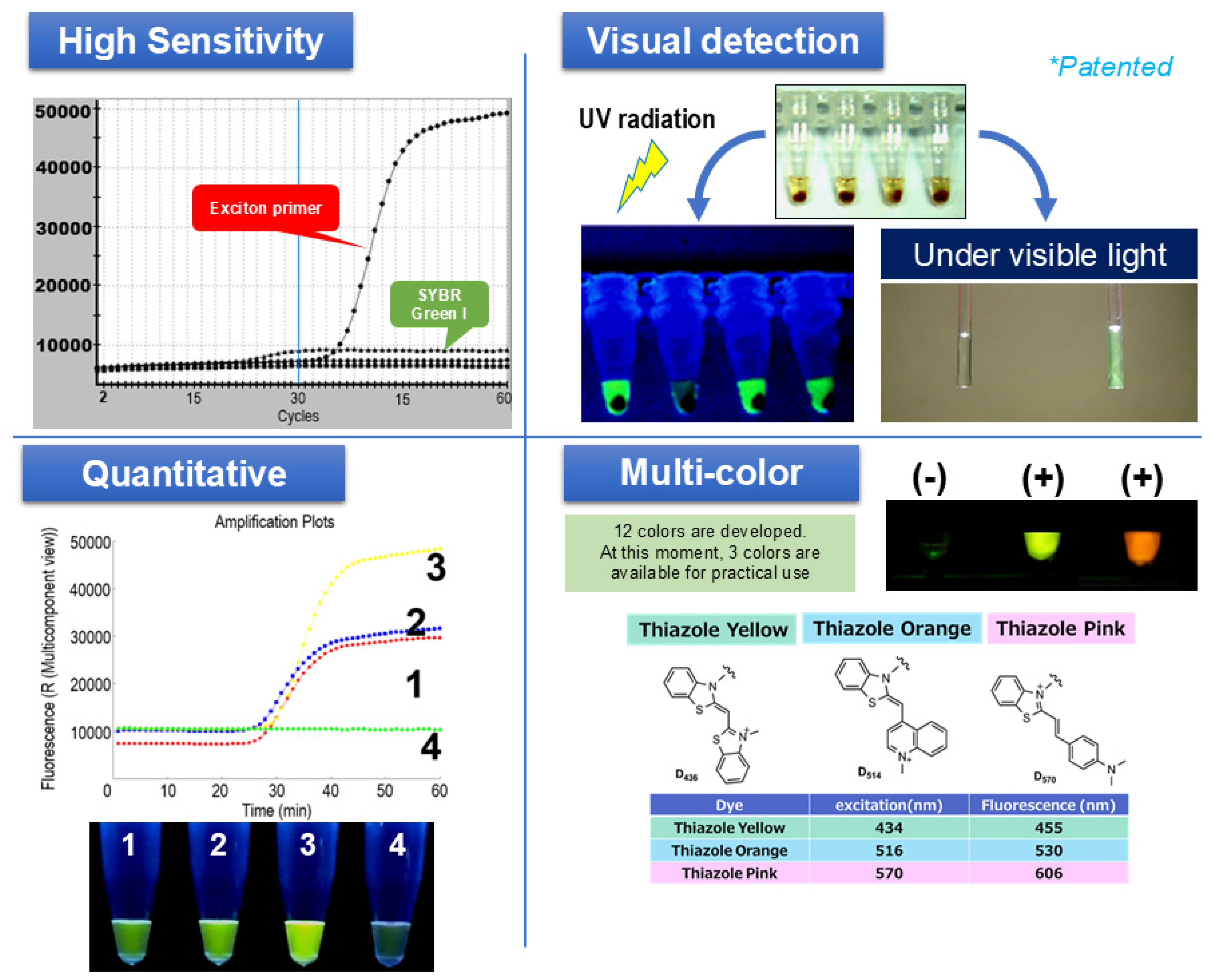
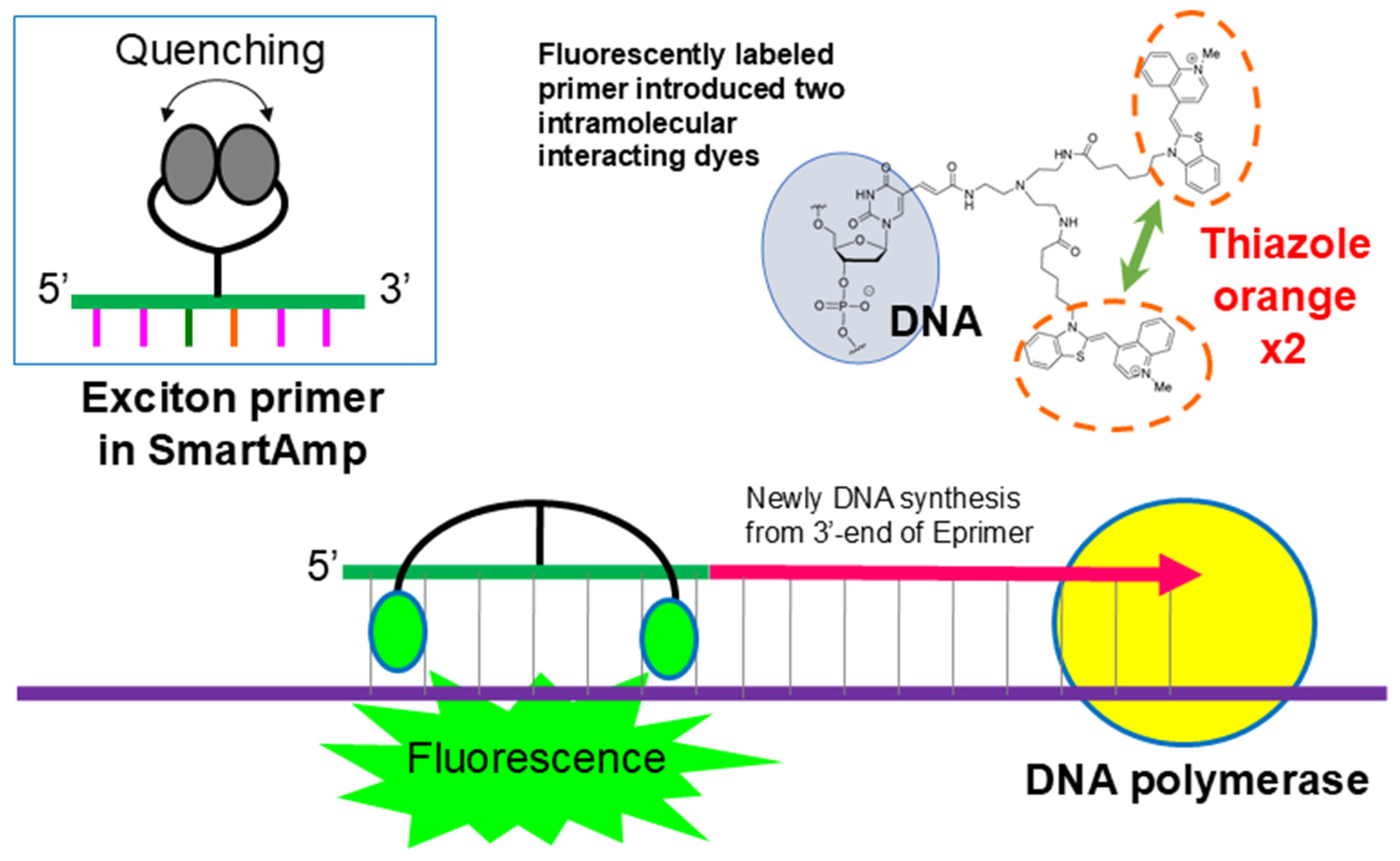
4.1. Principle and Features of SmartAmp as a Sensor for Nucleic Acids
Principle of SmartAmp Amplification and the Turn-Back Primer
4.2. Conditions Required for Sample Pretreatment
4.3. COVID-19 SmartAmp Reagent: Sensitivity and LOD
5. Development of GenPad POCT Automated Platform
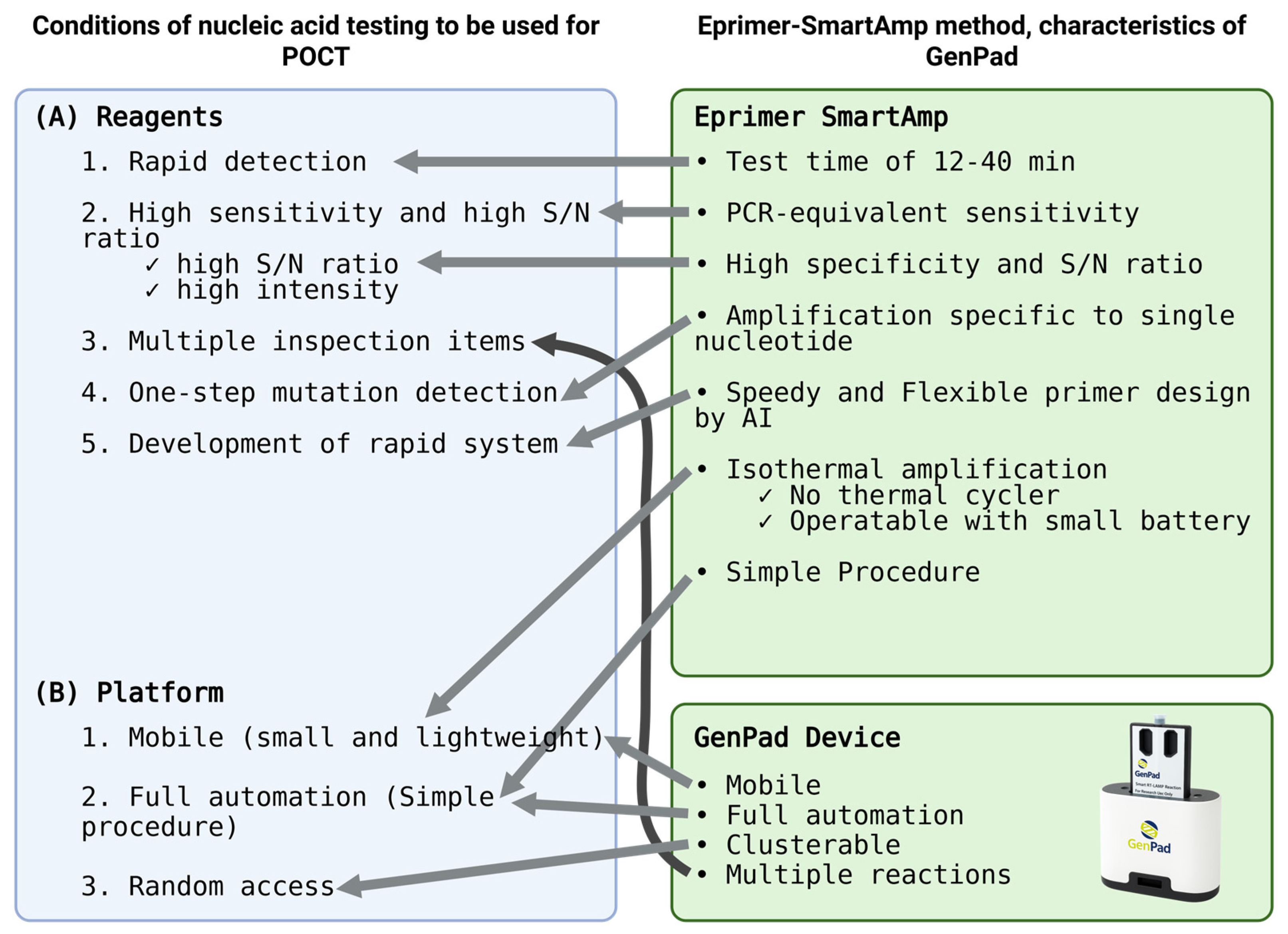
5.1. Conditions Required for the Platform
5.2. Characteristics of GenPad
5.2.1. Cartridge Sensitivity and Number of Reaction Slots
5.2.2. Internal Structure of the GenPad Device
5.2.3. Detection Time
5.2.4. Power Consumption and Portability
5.2.5. Detection of Single-Base Mutations
5.2.6. Collection of Specimens
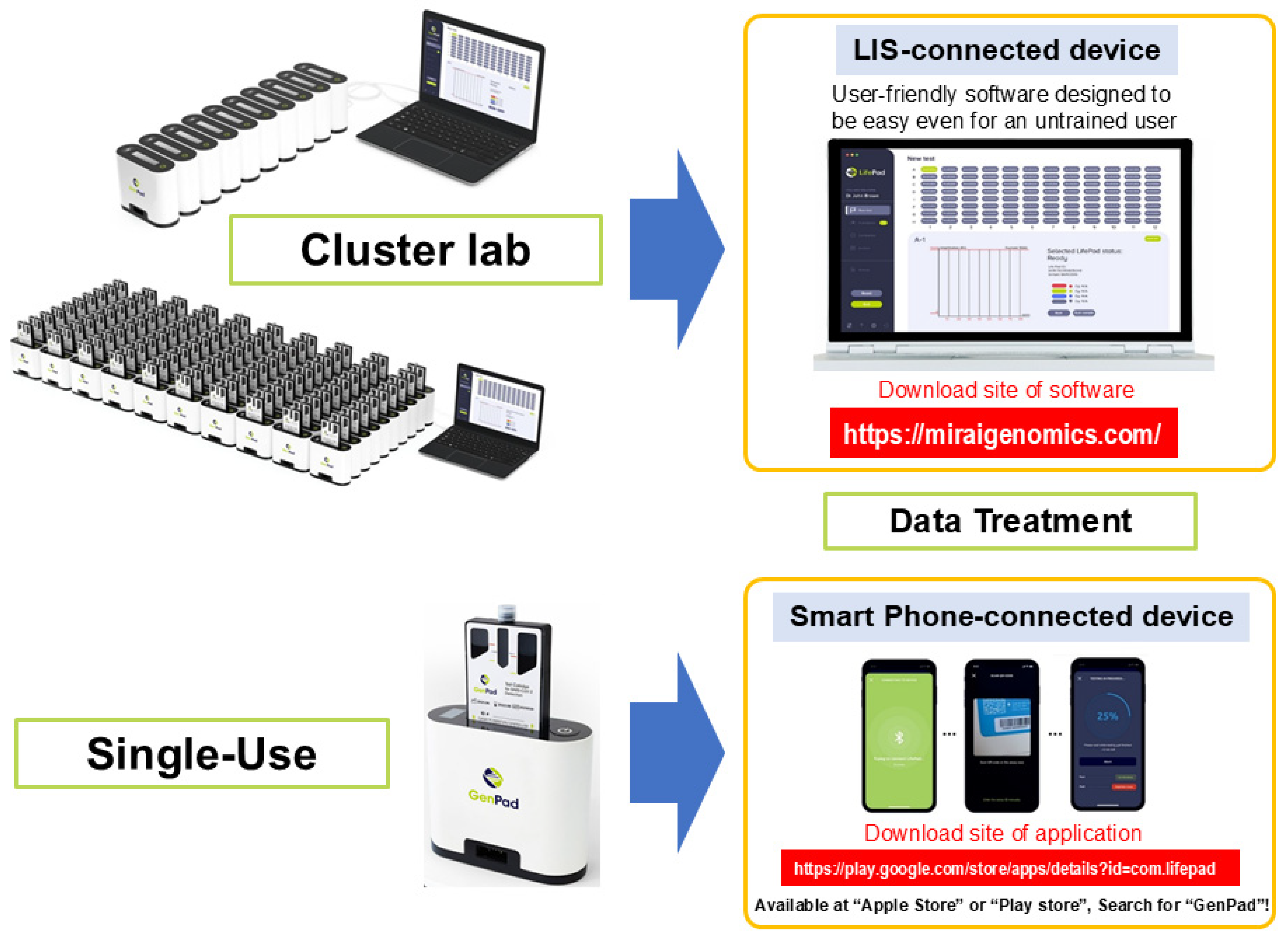
5.2.7. Closed System and Complete Automation
5.3. GenPad Clustering and Data Display
5.4. Comparison of GenPad and Other Testing Platforms
6. Potential Social Impact of GenPad-Like Systems
7. GenPad Is an Essential Element in the Construction of a Smart Medical City
7.1. Smart Medical City
7.2. Telemedicine and GenPad
7.3. Potential Extension to Cancer Mutation Testing
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lakshmanan, K.; Liu, B.M. Impact of Point-of-Care Testing on Diagnosis, Treatment, and Surveillance of Vaccine-Preventable Viral Infections. Diagnostics 2025, 15, 123. [Google Scholar] [CrossRef]
- Kuroiwa, Y. It Started with the Diamond Princess! Team Kanagawa 250 Days of Truth; IDP Publishing: Singapore, 2007. [Google Scholar]
- Bharadwaj, M.; Bengtson, M.; Golverdingen, M.; Waling, L.; Dekker, C. Diagnosing point-of-care diagnostics for neglected tropical diseases. PLoS Negl. Trop. Dis. 2021, 15, e0009405. [Google Scholar] [CrossRef]
- Hussein, H.A.; Hassan, R.Y.A.; Chino, M.; Febbraio, F. Point-of-Care Diagnostics of COVID-19: From Current Work to Future Perspectives. Sensors 2020, 20, 4289. [Google Scholar] [CrossRef]
- Ahmed, S.; Jafri, R. Point of Care Tests—The Future of Diagnostic Medicine. EJIFCC 2024, 35, 140–141. [Google Scholar]
- Dinnes, J.; Deeks, J.J.; Adriano, A.; Berhane, S.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; Beese, S.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020, 8, CD013705. [Google Scholar] [PubMed]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef]
- Sonnleitner, S.T.; Sonnleitner, S.; Hinterbichler, E.; Halbfurter, H.; Kopecky, D.B.C.; Koblmüller, S.; Sturmbauer, C.; Posch, W.; Walder, G. The mutational dynamics of the SARS-CoV-2 virus in serial passages in vitro. Virol. Sin. 2022, 37, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Klein, J.; Robertson, A.J.; Peña-Hernández, M.A.; Lin, M.J.; Roychoudhury, P.; Lu, P.; Fournier, J.; Ferguson, D.; Bakhash, S.A.K.M.; et al. De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: A case report. Nat. Commun. 2022, 13, 1547. [Google Scholar] [CrossRef]
- Chen, H.; Liu, K.; Li, Z.; Wang, P. Point of care testing for infectious diseases. Clin. Chim. Acta 2019, 493, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Mitani, Y.; Lezhava, A.; Kawai, Y.; Kikuchi, T.; Oguchi-Katayama, A.; Kogo, Y.; Itoh, M.; Miyagi, T.; Takakura, H.; Hoshi, K.; et al. Rapid SNP diagnostics using asymmetric isothermal amplification and a new mismatch-suppression technology. Nat. Methods 2007, 4, 257–262. [Google Scholar] [CrossRef]
- Esbin, M.N.; Whitney, O.N.; Chong, S.; Maurer, A.; Darzacq, X.; Tjian, R. Overcoming the bottleneck to widespread testing: A rapid review of nucleic acid testing approaches for COVID-19 detection. RNA 2020, 26, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Hayashizaki, Y. Development of a new rapid, high-sensitive, and portable Point of Care Test system for nucleic acif detection. TEN: Tsunami Earth Netw. 2023, 4, 22–34. (In Japanese) [Google Scholar]
- Chang, Y.; Zhang, M.; Liu, G.; Wu, X.; Yan, Q.; Yang, C.; Liu, L.; Feng, Y.; Xia, X. Rapid and sensitive detection of Mycobacterium tuberculosis using nested multi-enzyme isothermal rapid amplification in a single reaction. Microbiol Spectr. 2024, 12, e0088724. [Google Scholar] [CrossRef]
- Binnicker, M.J. Nucleic Acid Amplification Assays for Viral Detection and Characterization. J. Clin. Microbiol. 2022, 60, e0109422. [Google Scholar]
- Fragkou, P.C.; Moschopoulos, C.D.; Dimopoulou, D.; Ong, D.S.Y.; Dimopoulou, K.; Nelson, P.P.; Schweitzer, V.A.; Janocha, H.; Karofylakis, E.; Papathanasiou, K.A.; et al. Performance of point-of care molecular and antigen-based tests for SARS-CoV-2: A living systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S49–S52. [Google Scholar]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Chin, C.D.; Laksanasopin, T.; Cheung, Y.K.; Steinmiller, D.; Linder, V.; Parsa, H.; Wang, J.; Moore, H.; Rouse, R.; Umviligihozo, G.; et al. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat. Med. 2011, 17, 1015–1019. [Google Scholar] [CrossRef]
- Roberts, J.D.; Wells, G.A.; Le May, M.R.; Labinaz, M.; Glover, C.; Froeschl, M.; Dick, A.; Marquis, J.F.; O’Brien, E.; Goncalves, S.; et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): A prospective, randomised, proof-of-concept trial. Lancet 2012, 379, 1705–1711. [Google Scholar] [CrossRef]
- Hoshi, K.; Takakura, H.; Mitani, Y.; Tatsumi, K.; Momiyama, N.; Ichikawa, Y.; Togo, S.; Miyagi, T.; Kawai, Y.; Kogo, Y.; et al. Rapid detection of epidermal growth factor receptor mutations in lung cancer by the Smart-Amplification Process. Clin. Cancer Res. 2007, 13, 4974–4983. [Google Scholar] [CrossRef]
- Lezhava, A.; Ishidao, T.; Ishizu, Y.; Naito, K.; Hanami, T.; Katayama, A.; Kogo, Y.; Soma, T.; Ikeda, S.; Murakami, K.; et al. Exciton-Primer Mediated SNP Detection in SmartAmp2 Reactions. Hum. Mutat. 2010, 31, 208–217. [Google Scholar] [CrossRef]
- Ikeda, S.; Kubota, T.; Yuki, M.; Okamoto, A. Exciton-Controlled Hybridization-Sensitive Fluorescent Probes: Multicolor Detection of Nucleic Acids. Angew. Chem. 2009, 121, 6602–6606. [Google Scholar] [CrossRef]
- Mao, R.; Wu, X.; Miao, Q.; Cai, T. Asymmetric stem-loop-mediated isothermal amplification of nucleic acids for DNA diagnostic assays by simple modification of canonical PCR primers. Front. Bioeng. Biotechnol. 2022, 10, 931770. [Google Scholar] [CrossRef]
- Delobel, D.; Furutani, Y.; Nagoshi, S.; Tsubota, A.; Miyasaka, A.; Watashi, K.; Wakita, T.; Matsuura, T.; Usui, K. SEB genotyping: SmartAmp-Eprimer binary code genotyping for complex, highly variable targets applied to HBV. BMC Infect. Dis. 2022, 22, 516. [Google Scholar] [CrossRef]
- Nagasawa, S.; Mori, A.; Hirata, Y.; Motomura, A.; Ishii, N.; Okaba, K.; Horioka, K.; Makino, Y.; Nakajima, M.; Torimitsu, S.; et al. SmartAmp method can rapidly detect SARS-CoV-2 in dead bodies. Forensic Sci. Int. 2022, 331, 111168. [Google Scholar] [CrossRef]
- Asai, N.; Nakamura, A.; Sakanashi, D.; Koita, I.; Ohashi, W.; Kawamoto, Y.; Miyazaki, N.; Ohno, T.; Yamada, A.; Chida, S.; et al. Comparative study of SmartAmp assay and reverse transcription-polymerase chain reaction by saliva specimen for the diagnosing COVID-19. J. Infect. Chemothe. 2022, 28, 120–123. [Google Scholar] [CrossRef]
- Kawai, Y.; Kimura, Y.; Lezhava, A.; Kanamori, H.; Usui, K.; Hanami, T.; Soma, T.; Morlighem, J.É.; Saga, S.; Ishizu, Y.; et al. One-step detection of the 2009 pandemic influenza A(H1N1) virus by the RT-SmartAmp assay and its clinical validation. PLoS ONE 2012, 7, e30236. [Google Scholar] [CrossRef] [PubMed]
- Baudhuin, L.M.; Train, L.J.; Goodman, S.G.; Lane, G.E.; Lennon, R.J.; Mathew, V.; Murthy, V.; Nazif, T.M.; So, D.Y.F.; Sweeney, J.P.; et al. Point of care CYP2C19 genotyping after percutaneous coronary intervention. Pharmacogenomics J. 2022, 22, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Tao, Y.; Fajardo, E.; Reipold, E.I.; Chou, R.; Tucker, J.D.; Easterbrook, P. Diagnostic Accuracy of Point-of-Care HCV Viral Load Assays for HCV Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Kabbale, K.D.; Nsengimaana, B.; Semakuba, F.D.; Kagurusi, B.A.; Mwubaha, C.; Wiringilimaana, I.; Katairo, T.; Kiyaga, S.; Mbabazi, M.; Gonahasa, S.; et al. Field evaluation of the Bioline Malaria Ag P.f/Pan Rapid Diagnostic Test: Causes of Microscopy Discordance and Performance in Uganda. Res. Sq. 2025, 206, 1260–1266. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Xpert® Xpress SARS-CoV-2—Instructions for Use (Laboratory), Rev. G. Available online: https://www.fda.gov/media/136314 (accessed on 23 July 2025).
- Fluxergy Test Kit COVID-19—Specification Sheet and Instructions for Use. Available online: https://www.fluxergy.com/assay/covid-19 (accessed on 23 July 2025).
- QIAstat-Dx Syndromic Testing—Product Page. Available online: https://www.qiagen.com/ca/products/diagnostics-and-clinical-research/infectious-disease/qiastat-dx-syndromic-testing/qiastat-dx-ca (accessed on 23 July 2025).
- Visby Medical Respiratory Health Test—Product Page 2024. Available online: https://www.visbymedical.com/respiratory-health-test/ (accessed on 23 July 2025).
- Renzoni, A.; Perez, F.; Ngo Nsoga, M.T.; Yerly, S.; Boehm, E.; Gayet-Ageron, A.; Kaiser, L.; Schibler, M. Analytical Evaluation of Visby Medical RT-PCR Portable Device for Rapid Detection of SARS-CoV-2. Diagnostics 2021, 11, 813. [Google Scholar] [CrossRef]
- Baca, J.T.; Severns, V.; Lovato, D.; Branch, D.W.; Larson, R.S. Rapid Detection of Ebola Virus with a Reagent-Free, Point-of-Care Biosensor. Sensors 2015, 15, 8605–8614. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Ma, X.; Sheng, N.; Qi, X.; Chu, Y.; Song, Q.; Zou, B.; Zhou, G. Point-of-care DNA testing by automatically and sequentially performing extraction, amplification and identification in a closed-type cassette. Sens. Actuators B Chem. 2021, 327, 128919. [Google Scholar] [CrossRef] [PubMed]
- Salopuro, T.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Tuomilehto, J.; Laakso, M.; Uusitupa, M. Common variants in beta2- and beta3-adrenergic receptor genes and uncoupling protein 1 as predictors of the risk for type 2 diabetes and body weight changes. The Finnish Diabetes Prevention Study. Clin. Genet. 2004, 66, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Shimizu, K.; Nakamura, K.; Nakamura, T.; Mitani, Y.; Obayashi, K.; Fujita, Y.; Kakegawa, S.; Miyamae, Y.; Kaira, K.; et al. Usefulness of peptide nucleic acid (PNA)-clamp smart amplification process version 2 (SmartAmp2) for clinical diagnosis of KRAS codon 12 mutations in lung adenocarcinoma: Comparison of PNA-clamp SmartAmp2 and PCR-related methods. J. Mol. Diagn. 2010, 12, 118–124. [Google Scholar] [CrossRef]
- Tanaka, N.; Sakamoto, K.; Okabe, H.; Fujioka, A.; Yamamura, K.; Nakagawa, F.; Nagase, H.; Yokogawa, T.; Oguchi, K.; Ishida, K.; et al. Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol. Rep. 2014, 32, 2319–2326. [Google Scholar] [CrossRef]
- Tsukihara, H.; Nakagawa, F.; Sakamoto, K.; Ishida, K.; Tanaka, N.; Okabe, H.; Uchida, J.; Matsuo, K.; Takechi, T. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol. Rep. 2015, 33, 2135–2142. [Google Scholar] [CrossRef]
| Purpose/Action | Description of Application | Expected Outcome/Benefit | Application Field |
|---|---|---|---|
| Determination of positive/negative | Positive cases that were not detectable by on-site antigen testing were successfully identified. Compared to the usual PCR testing, it provides results much earlier; since conventional PCR tests are outsourced to a laboratory, it takes a while to receive results. | Preventing the spread of infection | Elderly facilities Medical institutes |
| Prepared for the occurrence of fever cases in sports competitions. | Sports | ||
| Rapid testing was successfully performed for employees who had close contact with positive cases. | Workplaces | ||
| Confirmation of negative | Causes other than COVID-19 were successfully identified, allowing for the provision of an appropriate treatment based on high-sensitivity COVID-19-negative confirmation. | Applying an appropriate treatment | Medical institutes |
| Negative results were confirmed even in cases where infected individuals or close contacts were present in the vicinity. | Obtaining peace of mind: Maintaining daily life and social activities as much as possible | Users of elderly care facilities Workplaces | |
| It was possible to verify a negative transition prior to reintegration into society following illness and recovery. | Preventing the spread of infection | Workplaces | |
| Testing of all participants | A testing procedure was conducted for all the participants of a sporting event. | Preventing the spread of infection | Sports |
| Method | Time to Result (Min) | LOD (Copies or rxn/mL) | |
|---|---|---|---|
| GenPad | Isothermal (SmartAmp) | 10–40 pos/≤40 neg | 10–50 rxn/ml |
| Xpert® Xpress SARS-CoV-2 | Microfluidic RT-PCR | 30–60 | ≈100 copies/mL |
| Fluxergy Analyzer COVID-19 | Microfluidic direct RT-PCR | ≈65 | 0.3 TCID50/mL * |
| QIAstat-Dx Respiratory SARS-CoV-2 Panel | Multiplex microfluidic RT-PCR | 60 | 500 copies/mL |
| Veros™ SARS-CoV-2 (Visby) | Isothermal (RT-LAMP) | 30 | 100 copies/swab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusev, O. GenPad: A Highly Efficient Roadmap for the Development of a New Rapid, Highly Sensitive, and Portable Point-of-Care Testing System for Nucleic Acid Diagnostics in Japan. Diagnostics 2025, 15, 2020. https://doi.org/10.3390/diagnostics15162020
Gusev O. GenPad: A Highly Efficient Roadmap for the Development of a New Rapid, Highly Sensitive, and Portable Point-of-Care Testing System for Nucleic Acid Diagnostics in Japan. Diagnostics. 2025; 15(16):2020. https://doi.org/10.3390/diagnostics15162020
Chicago/Turabian StyleGusev, Oleg. 2025. "GenPad: A Highly Efficient Roadmap for the Development of a New Rapid, Highly Sensitive, and Portable Point-of-Care Testing System for Nucleic Acid Diagnostics in Japan" Diagnostics 15, no. 16: 2020. https://doi.org/10.3390/diagnostics15162020
APA StyleGusev, O. (2025). GenPad: A Highly Efficient Roadmap for the Development of a New Rapid, Highly Sensitive, and Portable Point-of-Care Testing System for Nucleic Acid Diagnostics in Japan. Diagnostics, 15(16), 2020. https://doi.org/10.3390/diagnostics15162020





