Recent Advances in Magnetic Resonance Imaging for the Diagnosis of Liver Cancer: A Comprehensive Review
Abstract
1. Introduction
2. Methodology
3. The Pathological Features of Cancer and Their Visualization by MRI
4. Modern Approaches in Cancer Imaging Diagnosis
5. MRI in Liver Cancer Diagnosis
5.1. MRI in Diagnosis of Hepatocellular Carcinoma (HCC)
5.2. Comparison of MRI with Traditional Diagnostic Techniques
5.3. Current Development and Future Trends in MRI for Liver Cancer
5.4. The Role of AI and Radiomics in Liver Cancer MRI
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NMR | Nuclear Magnetic Resonance |
| DWI | Diffusion-Weighted Imaging |
| FNH | Focal Nodular Hyperplasia |
| AFP | Alpha-Fetoprotein |
| HCC | Hepatocellular Carcinoma |
| ICC | Intrahepatic Cholangiocarcinoma |
| DCE-MRI | Dynamic Contrast-Enhanced Magnetic Resonance Imaging |
| MRE | Magnetic Resonance Elastography |
| US | Ultrasound |
| CT | Computed Tomography |
| MRS | Magnetic Resonance Spectroscopy |
| AI | Artificial Intelligence |
| AMRI | Abbreviated Magnetic Resonance Imaging |
| LI-RADS | Liver Imaging Reporting and Data System |
References
- Guan, M.-C.; Wang, M.-D.; Liu, S.-Y.; Ouyang, W.; Liang, L.; Pawlik, T.M.; Xu, Q.-R.; Huang, D.-S.; Shen, F.; Zhu, H. Early diagnosis and therapeutic strategies for hepatocellular carcinoma: From bench to bedside. World J. Gastrointest. Oncol. 2021, 13, 197–215. [Google Scholar] [CrossRef]
- Augustine, R.; Al Mamun, A.; Hasan, A.; Salam, S.A.; Chandrasekaran, R.; Ahmed, R.; Thakor, A.S. Imaging cancer cells with nanostructures: Prospects of nanotechnology driven non-invasive cancer diagnosis. Adv. Colloid Interface Sci. 2021, 294, 102457. [Google Scholar] [CrossRef]
- Kim, J.; Min, J.H.; Kim, S.K.; Shin, S.-Y.; Lee, M.W. Detection of hepatocellular carcinoma in contrast-enhanced magnetic resonance imaging using deep learning classifier: A multi-center retrospective study. Sci. Rep. 2020, 10, 9458. [Google Scholar] [CrossRef]
- Lee, S.-K.; Oh, S.; Kim, H.-S.; Song, B.-P. Radio-frequency vector magnetic field mapping in magnetic resonance imaging. IEEE Trans. Med. Imaging 2020, 40, 963–973. [Google Scholar] [CrossRef]
- Palagi, L. Relaxometric/Structural Characterization and Preclinical Development of MRI Contrast Agents Based on Paramagnetic Metals (Gd, Fe). Ph.D. Thesis, Università di Torino, Turin, Italy, 2024. [Google Scholar]
- Zavala Bojorquez, J.A.; Jodoin, P.-M.; Bricq, S.; Walker, P.M.; Brunotte, F.; Lalande, A. Automatic classification of tissues on pelvic MRI based on relaxation times and support vector machine. PLoS ONE 2019, 14, e0211944. [Google Scholar] [CrossRef]
- Waheed, S.; Tahir, M.J.; Ullah, I.; Alwalid, O.; Irshad, S.G.; Asghar, M.S.; Yousaf, Z. The impact of dependence on advanced imaging techniques on the current radiology practice. Ann. Med. Surg. 2022, 78, 103708. [Google Scholar] [CrossRef]
- Eastwood, K.-A.; Mohan, A.R. Imaging in pregnancy. Obstet. Gynaecol. 2019, 21, 255–262. [Google Scholar] [CrossRef]
- Granata, V.; Bicchierai, G.; Fusco, R.; Cozzi, D.; Grazzini, G.; Danti, G.; De Muzio, F.; Maggialetti, N.; Smorchkova, O.; D’Elia, M. Diagnostic protocols in oncology: Workup and treatment planning. Part 2: Abbreviated MR protocol. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6499–6528. [Google Scholar] [PubMed]
- Banerjee, A.; Blasiak, B.; Dash, A.; Tomanek, B.; van Veggel, F.C.J.M.; Trudel, S. High-field magnetic resonance imaging: Challenges, advantages, and opportunities for novel contrast agents. Chem. Phys. Rev. 2022, 3, 011304. [Google Scholar] [CrossRef]
- Finkelstein, D.; Foremny, G.; Singer, A.; Clifford, P.; Pretell-Mazzini, J.; Kerr, D.A.; Subhawong, T.K. Differential diagnosis of T2 hypointense masses in musculoskeletal MRI. Skelet. Radiol. 2021, 50, 1981–1994. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, W. Radial Planes in Hip Magnetic Resonance Imaging: Techniques, Applications, and Perspectives. J. Magn. Reson. Imaging 2024, 60, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Mati, W. Advances in magnetic resonance imaging (MRI). In Advances in Medical and Surgical Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 121–142. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Advances in the Diagnosis and Evaluation of Disabling Physical Health Conditions; National Academies Press: Washington, DC, USA, 2023. [Google Scholar]
- Hussain, S.; Mubeen, I.; Ullah, N.; Shah, S.S.U.D.; Khan, B.A.; Zahoor, M.; Ullah, R.; Khan, F.A.; Sultan, M.A. Modern diagnostic imaging technique applications and risk factors in the medical field: A review. Biomed Res. Int. 2022, 2022, 5164970. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Roy, S.; Wang, P.; Li, Z.; Qiu, X.; Zhang, Y.; Yuan, J.; Guo, B. Unveiling the future: Advancements in MRI imaging for neurodegenerative disorders. Ageing Res. Rev. 2024, 95, 102230. [Google Scholar] [CrossRef] [PubMed]
- Bednarova, I.; Bednarova, S. Musculoskeletal and Bone Imaging. In The Radiology Survival Kit: What You Need to Know USMLE Clinis; Springer: Cham, Switzerland, 2022; pp. 203–257. [Google Scholar]
- Haj-Mirzaian, A.; Kadivar, A.; Kamel, I.R.; Zaheer, A. Updates on imaging of liver tumors. Curr. Oncol. Rep. 2020, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Latysheva, A. Diffusion and Perfusion-Weighted Imaging in the Structural and Functional Characterization of Diffuse Gliomas for Differential Diagnosis and Treatment Planning. Ph.D. Thesis, University of Oslo, Oslo, Norway, 2020. [Google Scholar]
- Jagannathan, N.R. Potential of magnetic resonance (MR) methods in clinical cancer research. In Biomedical Translational Research: Technologies for Improving Healthcare; Springer: Berlin/Heidelberg, Germany, 2022; pp. 339–360. [Google Scholar]
- Seng, D.W.R.; Kwek, E.B.K. Is routine MRI necessary to exclude pathological fractures in patients with an oncological history? Arch. Orthop. Trauma Surg. 2018, 138, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Sack, I. Magnetic resonance elastography from fundamental soft-tissue mechanics to diagnostic imaging. Nat. Rev. Phys. 2023, 5, 25–42. [Google Scholar] [CrossRef]
- Trinh, A.; Wintermark, M.; Iv, M. Clinical review of computed tomography and MR perfusion imaging in neuro-oncology. Radiol. Clin. N. Am. 2021, 59, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Nishie, A.; Asayama, Y.; Ishigami, K.; Ushijima, Y.; Kakihara, D.; Nakayama, T.; Morita, K.; Ishimatsu, K.; Honda, H. Hyperintense liver masses at hepatobiliary phase gadoxetic Acid–enhanced MRI: Imaging appearances and clinical importance. Radiographics 2020, 40, 72–94. [Google Scholar] [CrossRef] [PubMed]
- Nadarevic, T.; Colli, A.; Giljaca, V.; Fraquelli, M.; Casazza, G.; Manzotti, C.; Štimac, D.; Miletic, D. Magnetic resonance imaging for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst. Rev. 2022, 5, CD014798. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, J.D.; Yoo, C.C.; Lai, K.K.Y.; Braun, J.; McGovern, D.P.B.; Xie, Y.; Pandol, S.J.; Lu, S.C.; Li, D. Magnetic resonance imaging for characterization of hepatocellular carcinoma metabolism. Front. Physiol. 2022, 13, 1056511. [Google Scholar] [CrossRef] [PubMed]
- LaViolette, P.S. Advanced physiologic imaging: Diffusion–theory and applications. In Glioma Imaging: Physiologic, Metabolic, and Molecular Approaches; Springer: Cham, Switzerland, 2020; pp. 93–108. [Google Scholar]
- Hoffmann, E.; Gerwing, M.; Krähling, T.; Hansen, U.; Kronenberg, K.; Masthoff, M.; Geyer, C.; Höltke, C.; Wachsmuth, L.; Schinner, R. Vascular response patterns to targeted therapies in murine breast cancer models with divergent degrees of malignancy. Breast Cancer Res. 2023, 25, 56. [Google Scholar] [CrossRef] [PubMed]
- Chartampilas, E.; Rafailidis, V.; Georgopoulou, V.; Kalarakis, G.; Hatzidakis, A.; Prassopoulos, P. Current imaging diagnosis of hepatocellular carcinoma. Cancers 2022, 14, 3997. [Google Scholar] [CrossRef] [PubMed]
- Kalor, A.; Girometti, R.; Maheshwari, E.; Kierans, A.S.; Pugliesi, R.A.; Buros, C.; Furlan, A. Update on MR contrast agents for liver imaging: What to use and when. Radiol. Clin. 2022, 60, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-Y.; Park, M.-S.; Aljoqiman, K.S.; Choi, J.-Y.; Kim, M.-J. Gadoxetic acid-enhanced magnetic resonance imaging: Hepatocellular carcinoma and mimickers. Clin. Mol. Hepatol. 2019, 25, 223. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Jarnagin, W.; El Dika, I.; D’Angelica, M.; Lowery, M.; Brown, K.; Ludwig, E.; Kemeny, N.; Covey, A.; Crane, C.H. Liver and bile duct cancer. In Abeloff’s Clinical Oncology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1314–1341. [Google Scholar]
- Kwok, H.M.; Chau, C.M.; Lee, H.C.H.; Wong, T.; Chan, H.F.; Luk, W.H.; Yung, W.T.A.; Cheng, L.F.; Ma, K.F.J. Gadoxetic acid in hepatocellular carcinoma and liver metastases: Pearls and pitfalls. Clin. Radiol. 2023, 78, 715–723. [Google Scholar] [CrossRef] [PubMed]
- De Raffele, E. Benign Liver Tumours. In Liver Diseases: A Multidisciplinary Textbook; Springer: Cham, Switzerland, 2020; pp. 319–340. [Google Scholar]
- Sung, S.; Heymann, J.J.; Crapanzano, J.P.; Moreira, A.L.; Shu, C.; Bulman, W.A.; Saqi, A. Lung cancer cytology and small biopsy specimens: Diagnosis, predictive biomarker testing, acquisition, triage, and management. J. Am. Soc. Cytopathol. 2020, 9, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Chatura, K.R.; Sowmya, S.V.; Prasad, K.; Lakshminarayana, S.; Ali, F.M.; Awan, K.H.; Patil, S. Procedures and pitfalls in incisional biopsies of oral squamous cell carcinoma with respect to histopathological diagnosis. Disease-a-Month 2020, 66, 101035. [Google Scholar] [CrossRef] [PubMed]
- Hanif, H.; Ali, M.J.; Susheela, A.T.; Khan, I.W.; Luna-Cuadros, M.A.; Khan, M.M.; Lau, D.T.-Y. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Colli, A.; Nadarevic, T.; Miletic, D.; Giljaca, V.; Fraquelli, M.; Štimac, D.; Casazza, G. Abdominal ultrasound and alpha-foetoprotein for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst. Rev. 2021, 4, CD013346. [Google Scholar] [CrossRef] [PubMed]
- Sparchez, Z.; Craciun, R.; Caraiani, C.; Horhat, A.; Nenu, I.; Procopet, B.; Sparchez, M.; Stefanescu, H.; Mocan, T. Ultrasound or sectional imaging techniques as screening tools for hepatocellular carcinoma: Fall forward or move forward? J. Clin. Med. 2021, 10, 903. [Google Scholar] [CrossRef]
- Koutras, A.; Perros, P.; Prokopakis, I.; Ntounis, T.; Fasoulakis, Z.; Pittokopitou, S.; Samara, A.A.; Valsamaki, A.; Douligeris, A.; Mortaki, A. Advantages and limitations of ultrasound as a screening test for ovarian cancer. Diagnostics 2023, 13, 2078. [Google Scholar] [CrossRef]
- Shao, Y.-H.; Tsai, K.; Kim, S.; Wu, Y.-J.; Demissie, K. Exposure to tomographic scans and cancer risks. JNCI cancer Spectr. 2020, 4, pkz072. [Google Scholar] [CrossRef] [PubMed]
- Vernuccio, F.; Porrello, G.; Cannella, R.; Vernuccio, L.; Midiri, M.; Giannitrapani, L.; Soresi, M.; Brancatelli, G. Benign and malignant mimickers of infiltrative hepatocellular carcinoma: Tips and tricks for differential diagnosis on CT and MRI. Clin. Imaging 2021, 70, 33–45. [Google Scholar] [CrossRef]
- Lother, D.; Robert, M.; Elwood, E.; Smith, S.; Tunariu, N.; Johnston, S.R.D.; Parton, M.; Bhaludin, B.; Millard, T.; Downey, K. Imaging in metastatic breast cancer, CT, PET/CT, MRI, WB-DWI, CCA: Review and new perspectives. Cancer Imaging 2023, 23, 53. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, X.; Liang, Y.; Wen, H.; Wen, X.; Gu, M.; Ren, C.; Li, K.; Yu, L.; Lu, L. Diagnostic performance of diffusion MRI for differentiating benign and malignant nonfatty musculoskeletal soft tissue tumors: A systematic review and meta-analysis. J. Cancer 2021, 12, 7399–7412. [Google Scholar] [CrossRef]
- Jahng, G.-H.; Park, S.; Ryu, C.-W.; Cho, Z.-H. Magnetic Resonance Imaging: Historical Overview, Technical Developments, and Clinical Applications. Prog. Med. Phys. 2020, 31, 35–53. [Google Scholar] [CrossRef]
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef]
- Candita, G.; Rossi, S.; Cwiklinska, K.; Fanni, S.C.; Cioni, D.; Lencioni, R.; Neri, E. Imaging diagnosis of hepatocellular carcinoma: A state-of-the-art review. Diagnostics 2023, 13, 625. [Google Scholar] [CrossRef] [PubMed]
- Osho, A.; Rich, N.E.; Singal, A.G. Role of imaging in management of hepatocellular carcinoma: Surveillance, diagnosis, and treatment response. Hepatoma Res. 2020, 6, 55. [Google Scholar] [CrossRef]
- Kim, P.H.; Hwang, J.-Y.; Choi, Y.H.; Yoon, H.M.; Lee, C.W. Safety of gadoxetate disodium for hepatobiliary MRI in children and adolescents. Radiology 2024, 311, e232462. [Google Scholar] [CrossRef]
- Schooler, G.R.; Hull, N.C.; Lee, E.Y. Hepatobiliary MRI contrast agents: Pattern recognition approach to pediatric focal hepatic lesions. Am. J. Roentgenol. 2020, 214, 976–986. [Google Scholar] [CrossRef]
- Li, J.; Zhao, S.; Ling, Z.; Li, D.; Jia, G.; Zhao, C.; Lin, X.; Dai, Y.; Jiang, H.; Wang, S. Dual-Energy Computed Tomography Imaging in Early-Stage Hepatocellular Carcinoma: A Preliminary Study. Contrast Media Mol. Imaging 2022, 2022, 2146343. [Google Scholar] [CrossRef]
- Zen, Y. Intrahepatic cholangiocarcinoma: Typical features, uncommon variants, and controversial related entities. Hum. Pathol. 2023, 132, 197–207. [Google Scholar] [CrossRef]
- Neureiter, D.; Tornesello, M.L.; Bekric, D. Novel Therapeutic Approaches for Biliary Tract Cancer and Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2023, 11, 1320084. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, G.; Zhang, J.; Xu, C.; Zhu, F.; Xu, P. DCE-MRI based radiomics nomogram for preoperatively differentiating combined hepatocellular-cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma. Eur. Radiol. 2022, 32, 5004–5015. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Ottaiano, A.; Nasti, G.; La Porta, M.; Danti, G.; Cappabianca, S. Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: How radiologist should assess MR features. Radiol. Med. 2021, 126, 1584–1600. [Google Scholar] [CrossRef]
- Choi, H.H.; Rodgers, S.K.; Khurana, A.; Nelson, L.W.; Kamaya, A. Role of Ultrasound for Chronic Liver Disease and Hepatocellular Carcinoma Surveillance. Magn. Reson. Imaging Clin. N. Am. 2021, 29, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H. Current role of ultrasound in the diagnosis of hepatocellular carcinoma. J. Med. Ultrason. 2020, 47, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Nadarevic, T.; Giljaca, V.; Colli, A.; Fraquelli, M.; Casazza, G.; Miletic, D.; Štimac, D. Computed tomography for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst. Rev. 2021, 10, CD013362. [Google Scholar] [PubMed]
- Nayantara, P.V.; Kamath, S.; Manjunath, K.N.; Rajagopal, K. V Computer-aided diagnosis of liver lesions using CT images: A systematic review. Comput. Biol. Med. 2020, 127, 104035. [Google Scholar] [CrossRef]
- Vernuccio, F.; Cannella, R.; Bartolotta, T.V.; Galia, M.; Tang, A.; Brancatelli, G. Advances in liver US, CT, and MRI: Moving toward the future. Eur. Radiol. Exp. 2021, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Riche, M.; Amelot, A.; Peyre, M.; Capelle, L.; Carpentier, A.; Mathon, B. Complications after frame-based stereotactic brain biopsy: A systematic review. Neurosurg. Rev. 2021, 44, 301–307. [Google Scholar] [CrossRef]
- Wu, L.; Shen, Y.; Li, F. Non-invasive diagnosis of liver fibrosis: A review of current imaging modalities. Gastroenterol. Hepatol. 2020, 43, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Hormuth, D.A.; Sorace, A.G.; Virostko, J.; Abramson, R.G.; Bhujwalla, Z.M.; Enriquez-Navas, P.; Gillies, R.; Hazle, J.D.; Mason, R.P.; Quarles, C.C. Translating preclinical MRI methods to clinical oncology. J. Magn. Reson. Imaging 2019, 50, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Fliedner, F.P.; Engel, T.B.; El-Ali, H.H.; Hansen, A.E.; Kjaer, A. Diffusion weighted magnetic resonance imaging (DW-MRI) as a non-invasive, tissue cellularity marker to monitor cancer treatment response. BMC Cancer 2020, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Olson, M.C.; Samir, A.E.; Venkatesh, S.K. Liver fibrosis assessment: MR and US elastography. Abdom. Radiol. 2022, 47, 3037–3050. [Google Scholar] [CrossRef]
- Shenoy-Bhangle, A.; Baliyan, V.; Kordbacheh, H.; Guimaraes, A.R.; Kambadakone, A. Diffusion weighted magnetic resonance imaging of liver: Principles, clinical applications and recent updates. World J. Hepatol. 2017, 9, 1081–1091. [Google Scholar] [CrossRef]
- Li, J.; Venkatesh, S.K.; Yin, M. Advances in magnetic resonance elastography of liver. Magn. Reson. Imaging Clin. N. Am. 2020, 28, 331–340. [Google Scholar] [CrossRef]
- Hoodeshenas, S.; Yin, M.; Venkatesh, S.K. Magnetic resonance elastography of liver: Current update. Top. Magn. Reson. Imaging 2018, 27, 319–333. [Google Scholar] [CrossRef]
- Serai, S.D. Basics of magnetic resonance imaging and quantitative parameters T1, T2, T2*, T1rho and diffusion-weighted imaging. Pediatr. Radiol. 2022, 52, 217–227. [Google Scholar] [CrossRef]
- Zong, R.; Ma, X.; Shi, Y.; Geng, L. The assessment of pathological response to neoadjuvant chemotherapy in muscle-invasive bladder cancer patients with DCE-MRI and DWI: A systematic review and meta-analysis. Br. J. Radiol. 2023, 96, 20230239. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.; Chapiro, J.; Paradis, V.; Seraphin, T.P.; Kather, J.N. Artificial intelligence in liver diseases: Improving diagnostics, prognostics and response prediction. JHEP Rep. 2022, 4, 100443. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Zhong, C.; Liang, Z.; Zhang, Y.; Wu, B.; Xu, F.; Cong, L.; Wu, S.; Tian, Y. Six application scenarios of artificial intelligence in the precise diagnosis and treatment of liver cancer. Artif. Intell. Rev. 2021, 54, 5307–5346. [Google Scholar] [CrossRef]
- Hill, C.E.; Biasiolli, L.; Robson, M.D.; Grau, V.; Pavlides, M. Emerging artificial intelligence applications in liver magnetic resonance imaging. World J. Gastroenterol. 2021, 27, 6825–6843. [Google Scholar] [CrossRef]
- Messina, C.; Bignone, R.; Bruno, A.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Coppolino, P.; De Robertis, R.; Gentili, F. Diffusion-weighted imaging in oncology: An update. Cancers 2020, 12, 1493. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, I.S.; Song, Y.S.; Kim, J.I.; Choi, K.; Song, J.W. Diagnostic performance of diffusion-weighted (DWI) and dynamic contrast-enhanced (DCE) MRI for the differentiation of benign from malignant soft-tissue tumors. J. Magn. Reson. Imaging 2019, 50, 798–809. [Google Scholar] [CrossRef]
- Mathew, R.P.; Venkatesh, S.K. Imaging of hepatic fibrosis. Curr. Gastroenterol. Rep. 2018, 20, 45. [Google Scholar] [CrossRef]
- Shrivastava, D.; Srivastava, K.C.; Ganji, K.K.; Alam, M.K.; Al Zoubi, I.; Sghaireen, M.G. Quantitative assessment of gingival inflammation in patients undergoing nonsurgical periodontal therapy using photometric CIELab analysis. Biomed. Res. Int. 2021, 2021, 6615603. [Google Scholar] [CrossRef]
- Jiang, Y.; Edwards, A.V.; Newstead, G.M. Artificial intelligence applied to breast MRI for improved diagnosis. Radiology 2021, 298, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.C.; Connell, A.; Simonetto, D.A.; Hughes, C.; Shah, V.H. Application of artificial intelligence for the diagnosis and treatment of liver diseases. Hepatology 2021, 73, 2546–2563. [Google Scholar] [CrossRef]
- Lombardo, E.; Dhont, J.; Page, D.; Garibaldi, C.; Künzel, L.A.; Hurkmans, C.; Tijssen, R.H.N.; Paganelli, C.; Liu, P.Z.Y.; Keall, P.J. Real-time motion management in MRI-guided radiotherapy: Current status and AI-enabled prospects. Radiother. Oncol. 2023, 190, 109970. [Google Scholar] [CrossRef]
- Fahmy, D.; Alksas, A.; Elnakib, A.; Mahmoud, A.; Kandil, H.; Khalil, A.; Ghazal, M.; van Bogaert, E.; Contractor, S.; El-Baz, A. The role of radiomics and AI technologies in the segmentation, detection, and management of hepatocellular carcinoma. Cancers 2022, 14, 6123. [Google Scholar] [CrossRef] [PubMed]
- Vrettos, K.; Triantafyllou, M.; Marias, K.; Karantanas, A.H.; Klontzas, M.E. Artificial intelligence-driven radiomics: Developing valuable radiomics signatures with the use of artificial intelligence. BJR Artif. Intell. 2024, 1, ubae011. [Google Scholar] [CrossRef]
- Wu, L.; Lai, Q.; Li, S.; Wu, S.; Li, Y.; Huang, J.; Zeng, Q.; Wei, D. Artificial intelligence in predicting recurrence after first-line treatment of liver cancer: A systematic review and meta-analysis. BMC Med. Imaging 2024, 24, 263. [Google Scholar] [CrossRef]
- Fekri-Ershad, S.; Alsaffar, M.F. Developing a tuned three-layer perceptron fed with trained deep convolutional neural networks for cervical cancer diagnosis. Diagnostics 2023, 13, 686. [Google Scholar] [CrossRef]
- Fekri-Ershad, S. Cell phenotype classification using multi threshold uniform local ternary patterns in fluorescence microscope images. Multimed. Tools Appl. 2021, 80, 12103–12116. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Belli, A.; Romano, C.; Ottaiano, A.; Nasti, G. Magnetic resonance features of liver mucinous colorectal metastases: What the radiologist should know. J. Clin. Med. 2022, 11, 2221. [Google Scholar] [CrossRef]
- Elsayes, K.M.; Hooker, J.C.; Agrons, M.M.; Kielar, A.Z.; Tang, A.; Fowler, K.J.; Chernyak, V.; Bashir, M.R.; Kono, Y.; Do, R.K. 2017 version of LI-RADS for CT and MR imaging: An update. Radiographics 2017, 37, 1994–2017. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Tan, N.; Xiong, S.; Luo, W.; Xia, H.; Luo, B. Deep learning methods in medical image-based hepatocellular carcinoma diagnosis: A systematic review and meta-analysis. Cancers 2023, 15, 5701. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Wen, Z.-Y.; Liu, X.-Y.; Ma, Z.-H.; Liu, Y.-E.; Cao, X.-Y.; Hou, L.; Xie, H. Current status and prospect of treatments for recurrent hepatocellular carcinoma. World J. Hepatol. 2023, 15, 129–150. [Google Scholar] [CrossRef] [PubMed]
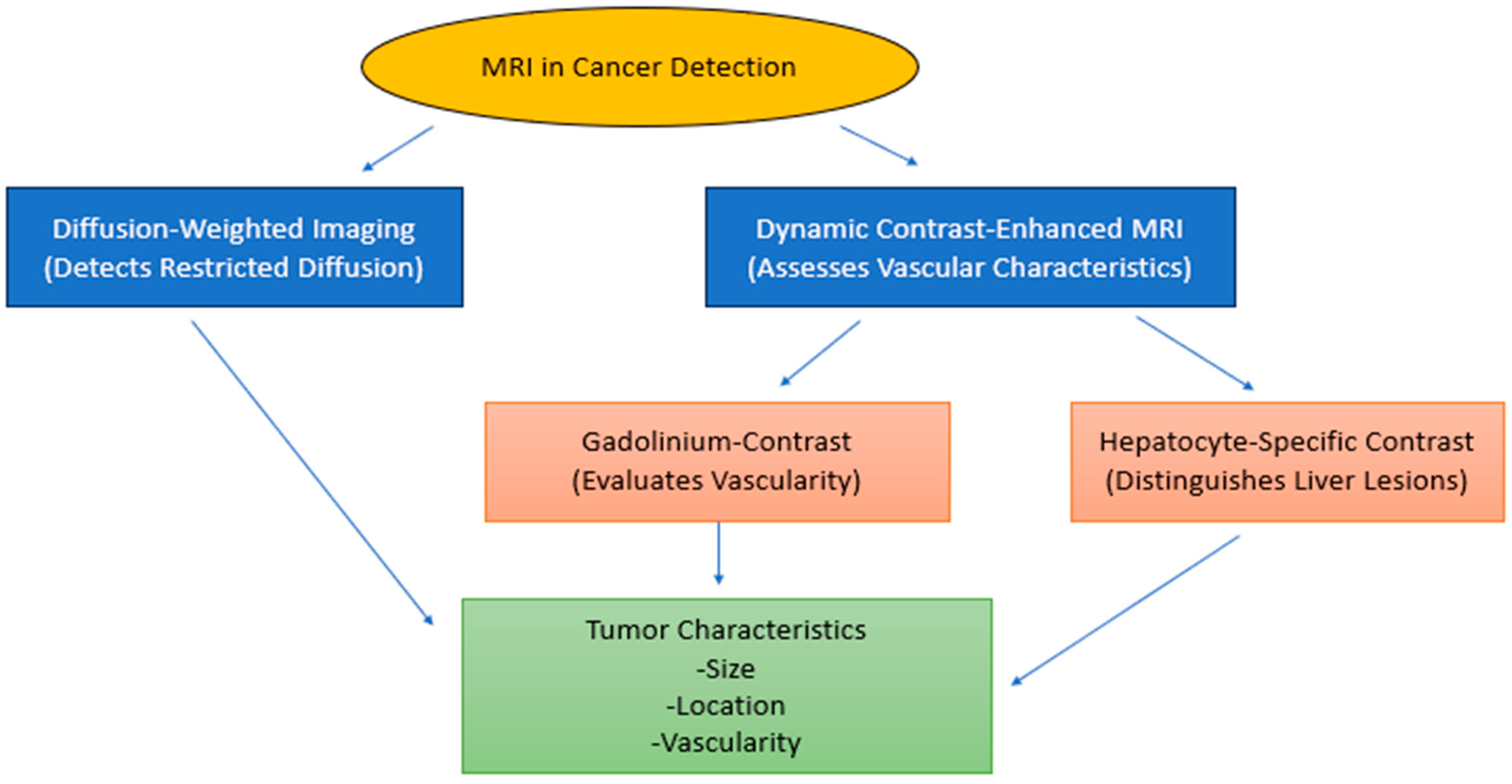
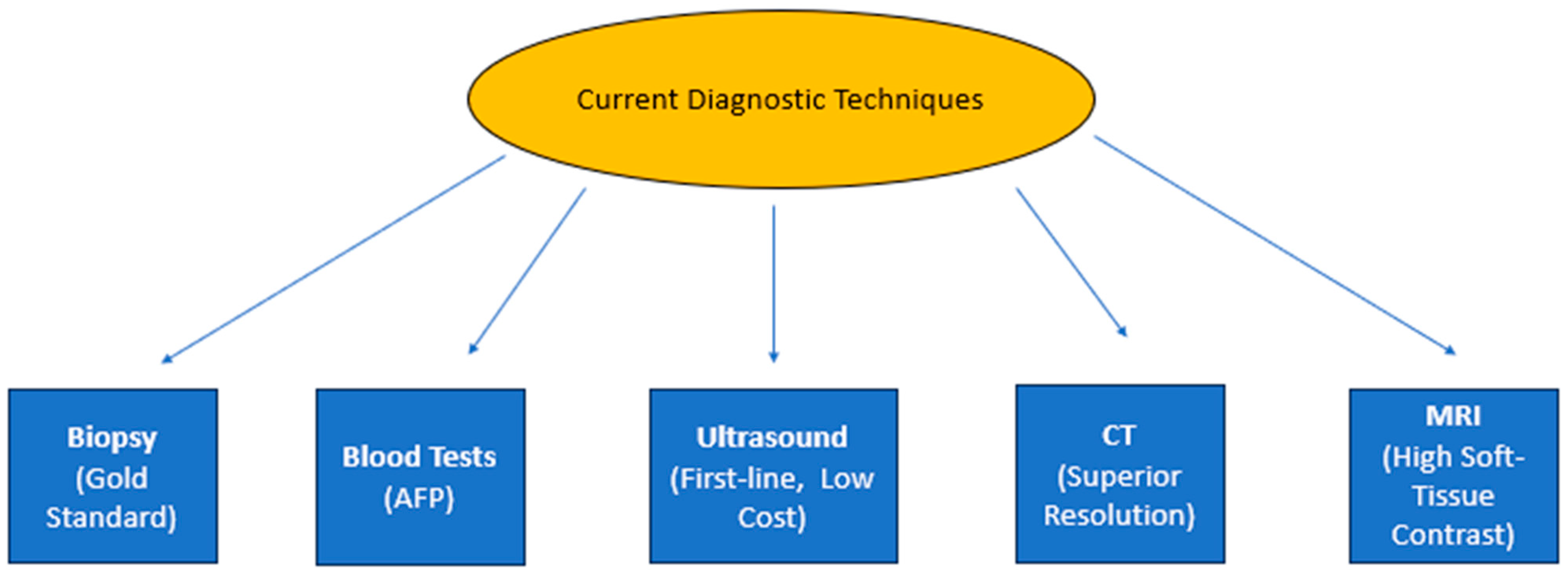
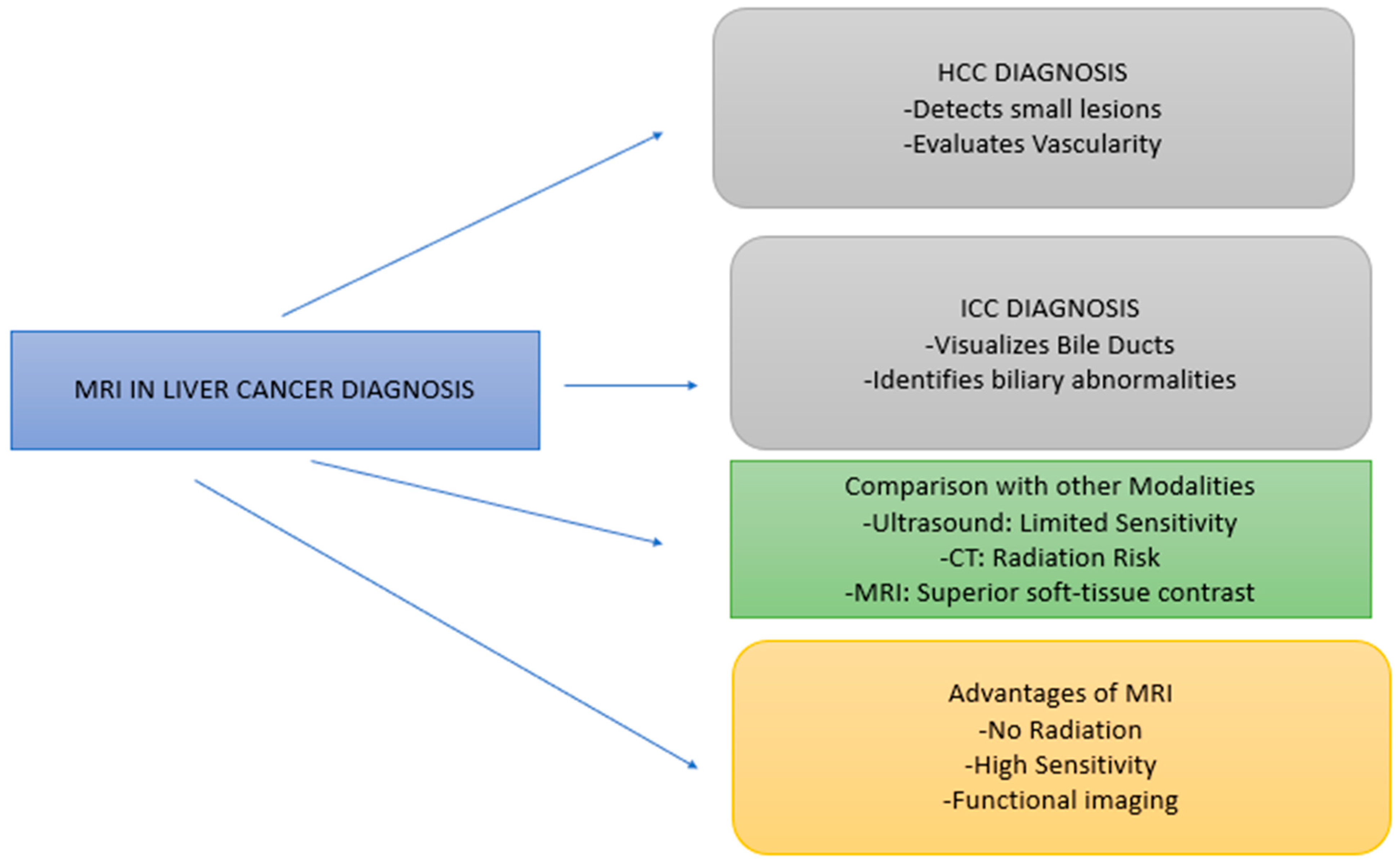

| MRI Technique | Purpose | Findings |
|---|---|---|
| Diffusion-weighted imaging (DWI) | Assessing tissue cellularity. | Identifies highly cellular tumors and small dependent lesions |
| Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) | Evaluating the amount, frequency, and ratio of blood circulation within the body. | It provides information on the nature of the tumor’s vascularization and enhancement characteristics. |
| Magnetic resonance elastography (MRE) | Measuring tissue stiffness. | Discriminates between benign and malignant growth due to the hardness of the tumor. |
| Hepatobiliary phase imaging (using agents like Gd-EOB-DTPA) | Looking for abnormalities associated with the liver and the ducts of the gland. | Enhances visibility of tumor masses in the liver as well as bile ducts. |
| Study | Modality of Imaging | Sensitivity (%) | Specificity (%) | Key Notes |
|---|---|---|---|---|
| [31] | Contrast-enhanced MRI | 90–94 | 89–93 | Deep learning classifier improves HCC detection accuracy |
| [56] | Ultrasound (US) | 60–75 | 70–85 | Recommended for HCC surveillance in chronic liver disease |
| [38] | US + Alpha-fetoprotein | ~65 | ~82 | Cochrane meta-analysis supports combined approach for early diagnosis |
| [55] | Abbreviated MRI (AMRI) | 83–88 | 87–91 | Comparable to full MRI; useful for rapid HCC screening |
| Study | AI Model | Input Type | Performance | Key Notes |
|---|---|---|---|---|
| [3] | Deep CNN Classifier | Contrast-enhanced MRI | AUC~0.91 | Multi-center study using CNN for lesion classification |
| [81] | Radiomics + AI | MRI Segmentation Maps | Variable | AI used for segmentation and detection in HCC |
| [83] | Prelim ML Models (Meta-analysis) | Clinical and Imaging Characteristics | AUC~0.89 | AI model is a valuable instrument in the prediction of reoccurrence after HCC treatment |
| [79] | General AI Applications in HCC | Mixed (MRI + Clinical Data) | Not stated | Review of the diagnostic and therapeutic AI applications in the sphere of liver diseases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshomrani, F. Recent Advances in Magnetic Resonance Imaging for the Diagnosis of Liver Cancer: A Comprehensive Review. Diagnostics 2025, 15, 2016. https://doi.org/10.3390/diagnostics15162016
Alshomrani F. Recent Advances in Magnetic Resonance Imaging for the Diagnosis of Liver Cancer: A Comprehensive Review. Diagnostics. 2025; 15(16):2016. https://doi.org/10.3390/diagnostics15162016
Chicago/Turabian StyleAlshomrani, Faisal. 2025. "Recent Advances in Magnetic Resonance Imaging for the Diagnosis of Liver Cancer: A Comprehensive Review" Diagnostics 15, no. 16: 2016. https://doi.org/10.3390/diagnostics15162016
APA StyleAlshomrani, F. (2025). Recent Advances in Magnetic Resonance Imaging for the Diagnosis of Liver Cancer: A Comprehensive Review. Diagnostics, 15(16), 2016. https://doi.org/10.3390/diagnostics15162016





