Carbapenem Resistance and ESBL-Producing Enterobacteriaceae in Patients with Urological Infections from 2012 to 2021 in Three Korean Hospitals
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Data Harmonization Using OMOP CDM
2.3. Case Definition and Data Extraction
2.4. Antibiotic Susceptibility Testing
2.5. ESBL Detection
2.6. Panel Exclusions
2.7. Outcomes and Statistical Analysis
2.8. Ethical Considerations
3. Results
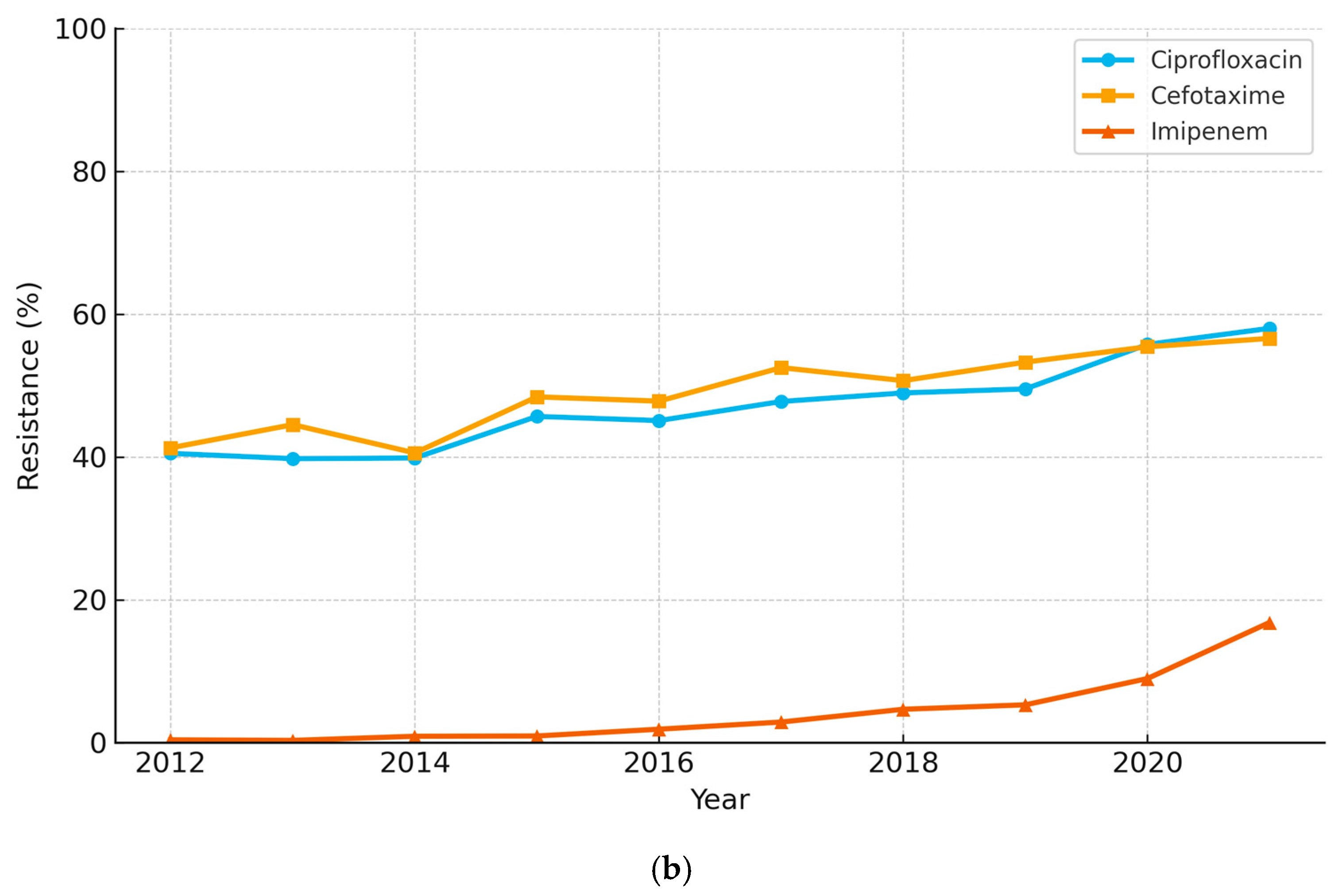
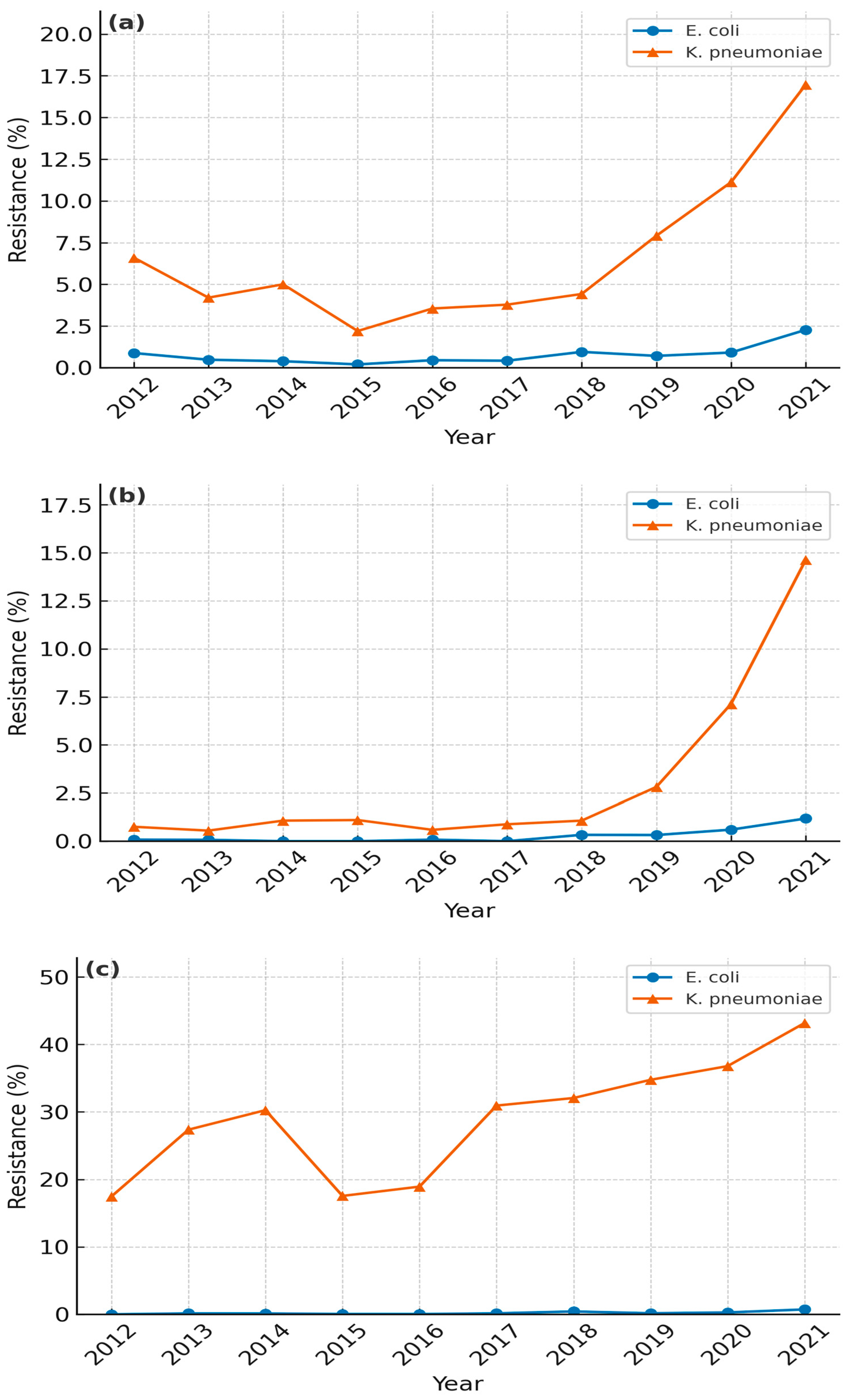
3.1. E. coli Resistance Trends
3.2. K. pneumoniae Resistance Trends
3.3. Sensitivity Analysis Excluding 2020–2021 and 2012–2013
3.4. Hospital-Level Analysis
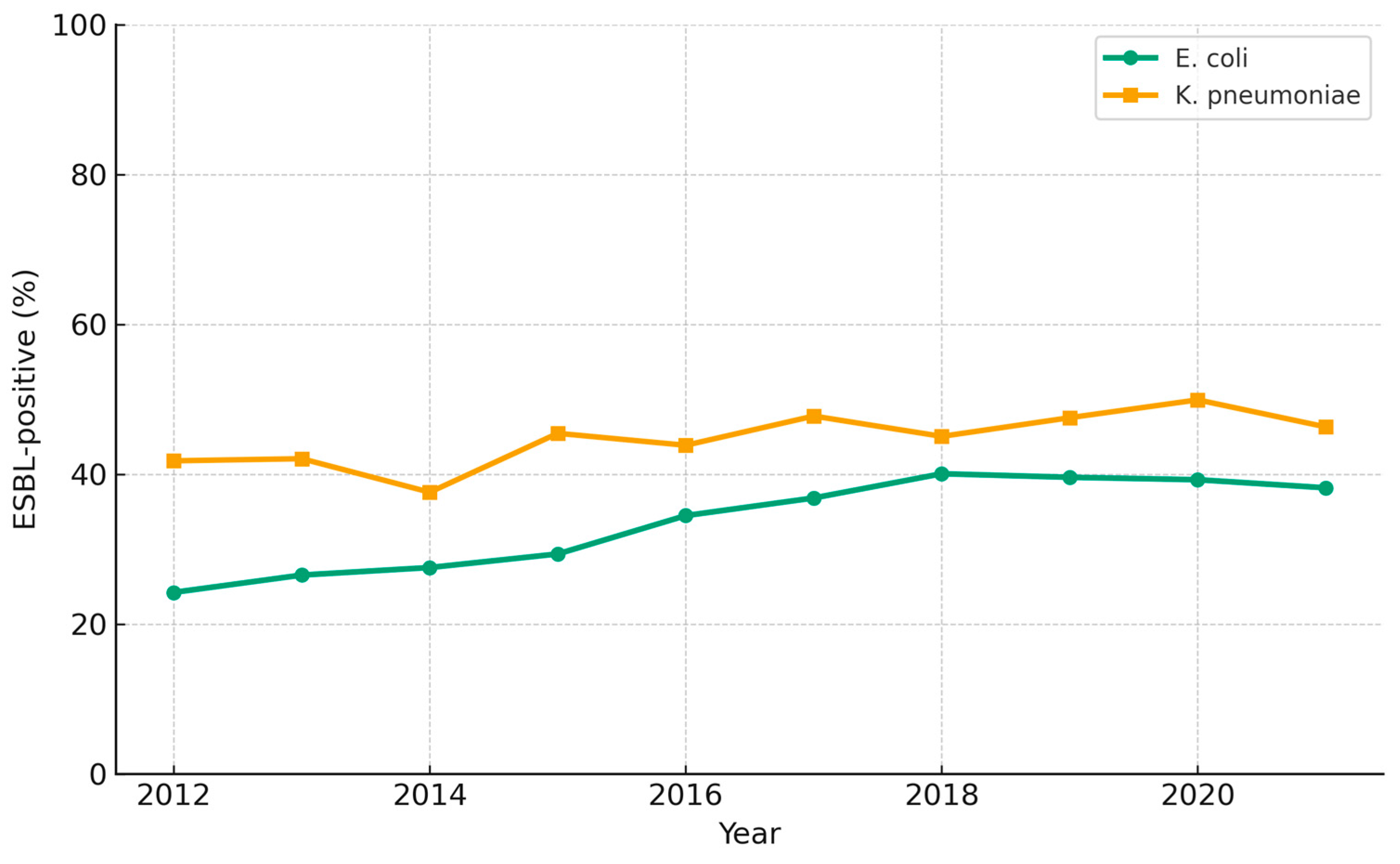
3.5. ESBL-Producing E. coli and K. pneumoniae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medina, M.; Castillo-Pino, E. An Introduction to the Epidemiology and Burden of Urinary Tract Infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef]
- Foxman, B. Epidemiology of Urinary Tract Infections: Incidence, Morbidity, and Economic Costs. Disease-a-Month 2003, 49, 53–70. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Pau, B.-N.; Vicenç, F.; Belén, V.; Antonia, A.; Oscar, L.; Benito, A.; Carles, P. A Cohort Study of Risk Factors That Influence Empirical Treatment of Patients with Acute Pyelonephritis. Antimicrob. Agents Chemother. 2017, 61, e01317-17. [Google Scholar] [CrossRef]
- Choi, J.H. The Meaning and Impact of Appropriate Use of Antibiotics. Infect. Chemother. 2012, 44, 331–337. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- WHO. Library Cataloguing-in-Publication Data Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015; ISBN 9789241509763. [Google Scholar]
- Bonkat, G.; Pickard, R.; Bartoletti, R.; Bruyère, F.; Geerlings, S.; Wagenlehner, F.; Wullt, B.; Pradere, B.; Veeratterapillay, R. Urological Infections. Arnh. Eur. Assoc. Urol. 2018. Available online: https://uroweb.org/guidelines/urological-infections (accessed on 8 August 2025).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Ghafourian, S.; Sadeghifard, N.; Soheili, S.; Sekawi, Z. Extended Spectrum Beta-Lactamases: Definition, Classification and Epidemiology. Curr. Issues Mol. Biol. 2015, 17, 11–22. [Google Scholar] [CrossRef]
- Bradford, P.A. Extended-Spectrum β-Lactamases in the 21st Century: Characterization, Epidemiology, and Detection of This Important Resistance Threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-Spectrum β-Lactamases: A Clinical Update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Gniadkowski, M. Evolution and Epidemiology of Extended-Spectrum b-Lactamases (ESBLs) and ESBL-Producing Microorganisms. Clin. Microbiol. Infect. 2001, 7, 597–608. [Google Scholar] [CrossRef]
- Kim, J.H.; Sun, H.Y.; Kim, T.H.; Shim, S.R.; Doo, S.W.; Yang, W.J.; Lee, E.J.; Song, Y.S. Prevalence of Antibiotic Susceptibility and Resistance of Escherichia Coli in Acute Uncomplicated Cystitis in Korea Systematic Review and Meta-Analysis. Medicine 2016, 95, e4663. [Google Scholar] [CrossRef]
- Kang, S.; Lee, S.Y.; Seo, S.H.; Lee, J.; Moon, D.C.; Yoo, J.S.; Choi, Y.H. Antimicrobial Resistance of Major Clinical Pathogens Isolated from General Hospitals in Korea: Results from the 2nd Phase (2020–2022) Kor-GLASS. Public Health Wkly. Rep. 2024, 17, 1034–1054. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Lu, Y.; Shen, W.; Wang, W.; Gao, J.; Gao, J.; Shao, P.; Zhou, Z. Molecular Epidemiology and Genetic Dynamics of Carbapenem-Resistant Hypervirulent Klebsiella Pneumoniae in China. Front. Cell Infect. Microbiol. 2025, 15, 1529929. [Google Scholar] [CrossRef]
- Bae, M.H.; Kim, M.S.; Kim, T.S.; Kim, S.; Yong, D.; Ha, G.Y.; Ryoo, N.H.; Uh, Y.; Shin, J.H.; Lee, H.S.; et al. Changing Epidemiology of Pathogenic Bacteria Over the Past 20 Years in Korea. J. Korean Med. Sci. 2023, 38, e73. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Doi, Y. The Global Epidemiology of Carbapenemase-Producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance Surveillance in Europe, 2022–2020 Data; ECDC: Solna, Sweden, 2023; Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2022-2020-data (accessed on 8 August 2025).
- Antimicrobial Resistance Surveillance in Europe 2023: 2021 Data; Publications Office of the European Union: Luxembourg, 2023; ISBN 9789294986122.
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240062702.
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024, ciae403. [Google Scholar] [CrossRef]
- Mathers, A.J.; Peirano, G.; Pitout, J.D.D. The Role of Epidemic Resistance Plasmids and International High- Risk Clones in the Spread of Multidrug-Resistant Enterobacteriaceae. Clin. Microbiol. Rev. 2015, 28, 565–591. [Google Scholar] [CrossRef]
- Codjoe, F.S.; Donkor, E.S. Carbapenem Resistance: A Review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef]
- Jung Wook, K.; Song Mee, B.; Sung Young, L.; Jung Sik, Y.; Soo Young, C. Trends of Antimicrobial Resistance Rates of Major Clinical Pathogens Isolated from General Hospitals in Korea in 2016–2019: Results from Kor-GLASS. Public Health Wkly. Rep. 2021, 14, 2007–2024. [Google Scholar]
- European Association of Urology (EAU). EAU Guidelines on Urological Infections; EAU: Arnhem, The Netherlands, 2023. [Google Scholar]
- Yu, S.H.; Jung, S.I.; Lee, S.J.; Oh, M.M.; Choi, J.B.; Choi, C., II; Kim, Y.J.; Park, D.J.; Bae, S.; Min, S.K. Nationwide Surveillance of Antimicrobial Resistance for Uncomplicated Cystitis in 2023: Conducted by the Korean Association of Urogenital Tract Infection and Inflammation. Investig. Clin. Urol. 2025, 66, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Korea Disease Control and Prevention Agency; Korean Society for Antimicrobial Therapy. Guidelines for the Antibiotic Use for Carbapenem-Resistant Enterobacterales (CRE) Infections; Korea Disease Control and Prevention Agency: Cheongju, Republic of Korea, 2024. [Google Scholar]
- Ahn, S.T.; Han, D.E.; Lee, D.H.; Kim, J.W.; Park, H.S.; Moon, D.G.; Oh, M.M. Single-Dose Amikacin plus 7 Days of Amoxicillin/ Clavulanate to Treat Acute Cystitis Caused by Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli: A Retrospective Cohort Study. Investig. Clin. Urol. 2021, 62, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Ryu, S.; Jeong, G.Y.; Kang, K.M.; Yoo, H. Introduction to the Antimicrobial Stewardship Program: Its Current Status and Policy Direction in the Republic of Korea. Public Health Wkly. Rep. 2022, 15, 2971–3003. [Google Scholar] [CrossRef]
- Kim, Y.A.; Park, Y.S.; Youk, T.; Lee, H.; Lee, K. Changes in Antimicrobial Usage Patterns in Korea: 12-Year Analysis Based on Database of the National Health Insurance Service-National Sample Cohort. Sci. Rep. 2018, 8, 12210. [Google Scholar] [CrossRef]
- Shin, D.H.; Kang, M.; Song, K.H.; Jung, J.; Kim, E.S.; Kim, H. Bin A Call for Antimicrobial Stewardship in Patients with COVID-19: A Nationwide Cohort Study in Korea. Clin. Microbiol. Infect. 2021, 27, 653–655. [Google Scholar] [CrossRef]
- Barišić, V.; Kovačević, T.; Travar, M.; Golić Jelić, A.; Kovačević, P.; Milaković, D.; Škrbić, R. A Retrospective Study of the Impact of the COVID-19 Pandemic on the Utilization and Quality of Antibiotic Use in a Tertiary Care Teaching Hospital in Low-Resource Settings. Antibiotics 2025, 14, 535. [Google Scholar] [CrossRef]
- Farzana, R.; Harbarth, S.J.; Yu, L.M.; Carretto, E.; Moore, C.E.; Feasey, N.A.; Gales, A.C.; Galal, U.; Ergonul, O.; Yong, D.; et al. The Impact of the COVID-19 Pandemic on Antimicrobial Usage: An International Patient-Level Cohort Study. JAC Antimicrob. Resist. 2025, 7, dlaf037. [Google Scholar] [CrossRef]

| Species | Year | Ciprofloxacin (Total) | Ciprofloxacin (ESBL+) | Cefotaxime (Total) | Cefotaxime (ESBL+) | Ertapenem (Total) | Ertapenem (ESBL+) |
|---|---|---|---|---|---|---|---|
| E. coli | 2017 | 51.83 | 78.46 | 39.54 | 98.98 | 0.35 | 0.42 |
| E. coli | 2019 | 58.46 | 79.2 | 42.45 | 98.71 | 0.51 | 0.71 |
| E. coli | 2021 | 60 | 83.31 | 40.21 | 99.59 | 1.21 | 2.27 |
| K. pneumoniae | 2017 | 47.75 | 81.38 | 52.49 | 99.75 | 4.94 | 3.78 |
| K. pneumoniae | 2019 | 49.51 | 78.67 | 53.26 | 98.34 | 7.96 | 7.91 |
| K. pneumoniae | 2021 | 57.99 | 87.98 | 56.58 | 98.88 | 18.09 | 16.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.B.; Ahn, S.T.; Joo, H.J.; Kim, J.W.; Oh, M.M. Carbapenem Resistance and ESBL-Producing Enterobacteriaceae in Patients with Urological Infections from 2012 to 2021 in Three Korean Hospitals. Diagnostics 2025, 15, 2004. https://doi.org/10.3390/diagnostics15162004
Jo SB, Ahn ST, Joo HJ, Kim JW, Oh MM. Carbapenem Resistance and ESBL-Producing Enterobacteriaceae in Patients with Urological Infections from 2012 to 2021 in Three Korean Hospitals. Diagnostics. 2025; 15(16):2004. https://doi.org/10.3390/diagnostics15162004
Chicago/Turabian StyleJo, Seon Beom, Sun Tae Ahn, Hyung Joon Joo, Jong Wook Kim, and Mi Mi Oh. 2025. "Carbapenem Resistance and ESBL-Producing Enterobacteriaceae in Patients with Urological Infections from 2012 to 2021 in Three Korean Hospitals" Diagnostics 15, no. 16: 2004. https://doi.org/10.3390/diagnostics15162004
APA StyleJo, S. B., Ahn, S. T., Joo, H. J., Kim, J. W., & Oh, M. M. (2025). Carbapenem Resistance and ESBL-Producing Enterobacteriaceae in Patients with Urological Infections from 2012 to 2021 in Three Korean Hospitals. Diagnostics, 15(16), 2004. https://doi.org/10.3390/diagnostics15162004







