Red Blood Cells and Human Aging: Exploring Their Biomarker Potential
Abstract
1. Introduction
2. Defining Biomarkers of Aging
3. Aging and Red Cell Production and Regulation
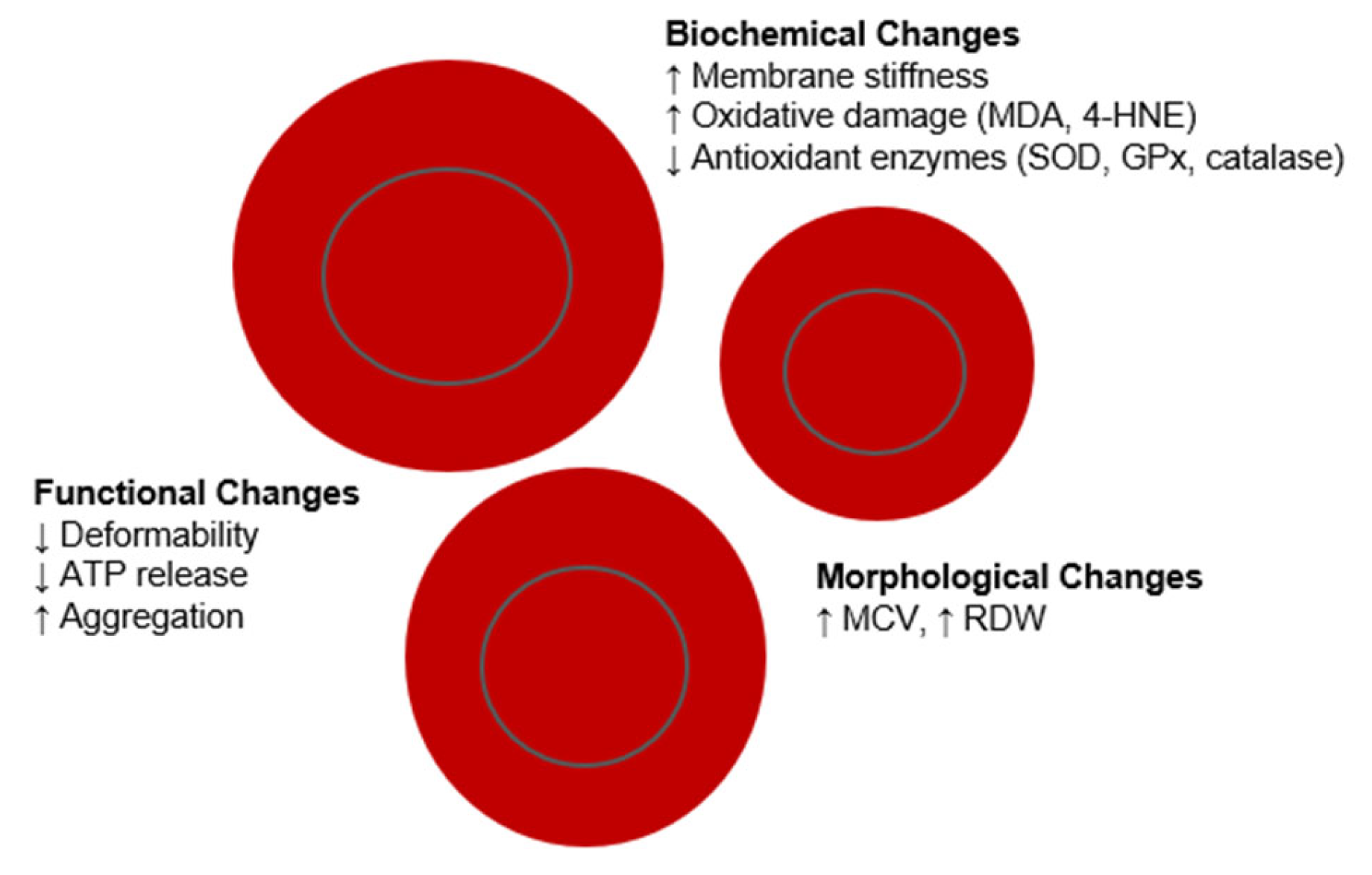
4. Age-Related Changes in Red Blood Cells
5. Functional Alterations of Red Blood Cells with Age
6. Biochemical Modifications in Red Blood Cells During Organismal Aging
7. Comparative Assessment of Red Blood Cells and Other Aging Biomarkers
8. Limitations and Challenges in Utilizing Red Blood Cells as Aging Biomarkers
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Zhu, Z.; Wang, L.; Li, C.; Sun, L.; Wang, W.; Gong, W. Biomarkers of Aging and Relevant Evaluation Techniques: A Comprehensive Review. Aging Dis. 2024, 15, 977–1005. [Google Scholar] [CrossRef] [PubMed]
- Prudinnik, D.S.; Kussanova, A.; Vorobjev, I.A.; Tikhonov, A.; Ataullakhanov, F.I.; Barteneva, N.S. Deformability of Heterogeneous Red Blood Cells in Aging and Related Pathologies. Aging Dis. 2025, 16, 1242–1264. [Google Scholar] [CrossRef] [PubMed]
- Colloca, G.; Di Capua, B.; Bellieni, A.; Fusco, D.; Ciciarello, F.; Tagliaferri, L.; Valentini, V.; Balducci, L. Biological and functional biomarkers of aging: Definition, characteristics, and how they can impact everyday cancer treatment. Curr. Oncol. Rep. 2020, 22, 115. [Google Scholar] [CrossRef]
- Rollandi, G.A.; Chiesa, A.; Sacchi, N.; Castagnetta, M.; Puntoni, M.; Amaro, A.; Banelli, B.; Pfeffer, U. Biological age versus chronological age in the prevention of age associated diseases. OBM Geriatr. 2019, 3, 051. [Google Scholar] [CrossRef]
- Wagner, K.-H.; Cameron-Smith, D.; Wessner, B.; Franzke, B. Biomarkers of aging: From function to molecular biology. Nutrients 2016, 8, 338. [Google Scholar] [CrossRef]
- Bai, X. Biomarkers of Aging. Adv. Exp. Med. Biol. 2018, 1086, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Bryk, A.H.; Wiśniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Dzieciatkowska, M.; Nemkov, T.; Hansen, K.C. Red blood cell proteomics update: Is there more to discover? Blood Transfus. 2017, 15, 182–187. [Google Scholar] [CrossRef]
- Palii, C.G.; Cheng, Q.; Gillespie, M.A.; Shannon, P.; Mazurczyk, M.; Napolitani, G.; Price, N.D.; Ranish, J.A.; Morrissey, E.; Higgs, D.R. Single-cell proteomics reveal that quantitative changes in co-expressed lineage-specific transcription factors determine cell fate. Cell Stem Cell 2019, 24, 812–820.e5. [Google Scholar] [CrossRef]
- Hartmann, A.; Hartmann, C.; Secci, R.; Hermann, A.; Fuellen, G.; Walter, M. Ranking biomarkers of aging by citation profiling and effort scoring. Front. Genet. 2021, 12, 686320. [Google Scholar] [CrossRef]
- Tang, P.; Wang, H. Regulation of erythropoiesis: Emerging concepts and therapeutic implications. Hematology 2023, 28, 2250645. [Google Scholar] [CrossRef]
- Singh, S.; Jakubison, B.; Keller, J.R. Protection of hematopoietic stem cells from stress-induced exhaustion and aging. Curr. Opin. Hematol. 2020, 27, 225–231. [Google Scholar] [CrossRef]
- Price, E.A. Aging and erythropoiesis: Current state of knowledge. Blood Cells Mol. Dis. 2008, 41, 158–165. [Google Scholar] [CrossRef]
- Aaron, N.; Costa, S.; Rosen, C.J.; Qiang, L. The Implications of Bone Marrow Adipose Tissue on Inflammaging. Front. Endocrinol. 2022, 13, 853765. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Morceau, F.; Dicato, M.; Diederich, M. Pro-inflammatory cytokine-mediated anemia: Regarding molecular mechanisms of erythropoiesis. Mediat. Inflamm. 2009, 2009, 405016. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Hou, S.C.; Shen, C.K. Erythroid gene suppression by NF-kappa B. J. Biol. Chem. 2003, 278, 19534–19540. [Google Scholar] [CrossRef]
- Caria, C.A.; Faà, V.; Ristaldi, M.S. Krüppel-Like Factor 1: A Pivotal Gene Regulator in Erythropoiesis. Cells 2022, 11, 3069. [Google Scholar] [CrossRef]
- Iriarte-Gahete, M.; Tarancon-Diez, L.; Garrido-Rodríguez, V.; Leal, M.; Pacheco, Y.M. Absolute and functional iron deficiency: Biomarkers, impact on immune system, and therapy. Blood Rev. 2024, 68, 101227. [Google Scholar] [CrossRef]
- Yacoub, M.F.; Ferwiz, H.F.; Said, F. Effect of Interleukin and Hepcidin in Anemia of Chronic Diseases. Anemia 2020, 2020, 3041738. [Google Scholar] [CrossRef]
- Maggio, M.; Guralnik, J.M.; Longo, D.L.; Ferrucci, L. Interleukin-6 in aging and chronic disease: A magnificent pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 575–584. [Google Scholar] [CrossRef]
- Ferrucci, L.; Semba, R.D.; Guralnik, J.M.; Ershler, W.B.; Bandinelli, S.; Patel, K.V.; Sun, K.; Woodman, R.C.; Andrews, N.C.; Cotter, R.J.; et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood 2010, 115, 3810–3816. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Hu, L.; Cheng, T. How age affects human hematopoietic stem and progenitor cells and the strategies to mitigate aging. Exp. Hematol. 2025, 143, 104711. [Google Scholar] [CrossRef]
- Du, Y.; Jin, M.; Liu, Q.; Zhao, J.; Song, A.; Li, W.; Chang, H.; Ma, F.; Huang, G. Association of Red Blood Cell Indices with Mild Cognitive Impairment in Chinese Elderly Individuals: A Matched Case-control Study. Curr. Alzheimer Res. 2020, 17, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.; Ershler, W.; Artz, A.; Lazo-Langner, A.; Walston, J.; Pahor, M.; Ferrucci, L.; Evans, W.J. Unexplained anemia of aging: Etiology, health consequences, and diagnostic criteria. J. Am. Geriatr. Soc. 2022, 70, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, H.; Park, J.W.; Son, B.R.; Park, J.H.; Jang, L.C.; Lee, J.G. Age-related changes in mean corpuscular volumes in patients without anaemia: An analysis of large-volume data from a single institute. J. Cell. Mol. Med. 2022, 26, 3548–3556. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Cai, L.; Ren, C.; Xiong, T.; Jin, L.; Nong, S.; Chen, Q.; Li, Y.; Cong, Y.; et al. Erythropoiesis changes with increasing age in the elderly Chinese. Int. J. Lab. Hematol. 2021, 43, 1168–1173. [Google Scholar] [CrossRef]
- Goldberg, I.; Cohen, E.; Gafter-Gvili, A.; Shochat, T.; Kugler, E.; Margalit, I.; Goldberg, E.; Raanani, P.; Krause, I. A Longitudinal Assessment of the Natural Change in Haemoglobin, Haematocrit, and Mean Corpuscular Volume with Age. Acta Haematol. 2023, 146, 206–213. [Google Scholar] [CrossRef]
- de Freitas, M.V.; Marquez-Bernardes, L.F.; de Arvelos, L.R.; Paraíso, L.F.; Gonçalves e Oliveira, A.F.M.; Mascarenhas Netto, R.d.C.; Neto, M.B.; Garrote-Filho, M.S.; de Souza, P.C.A.; Penha-Silva, N. Influence of age on the correlations of hematological and biochemical variables with the stability of erythrocyte membrane in relation to sodium dodecyl sulfate. Hematology 2014, 19, 424–430. [Google Scholar] [CrossRef]
- Danese, E.; Lippi, G.; Montagnana, M. Red blood cell distribution width and cardiovascular diseases. J. Thorac. Dis. 2015, 7, E402–E411. [Google Scholar] [CrossRef]
- Pilling, L.C.; Atkins, J.L.; Duff, M.O.; Beaumont, R.N.; Jones, S.E.; Tyrrell, J.; Kuo, C.L.; Ruth, K.S.; Tuke, M.A.; Yaghootkar, H.; et al. Red blood cell distribution width: Genetic evidence for aging pathways in 116,666 volunteers. PLoS ONE 2017, 12, e0185083. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Lui, L.Y.; Browner, W.S.; Cauley, J.A.; Ensrud, K.E.; Kado, D.M.; Orwoll, E.S.; Schousboe, J.T.; Cummings, S.R. Association Between Variation in Red Cell Size and Multiple Aging-Related Outcomes. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Gialluisi, A.; Tirozzi, A.; Costanzo, S.; De Bartolo, M.I.; Belvisi, D.; Magnacca, S.; De Curtis, A.; Falciglia, S.; Ricci, M.; Cerletti, C.; et al. Blood-based biological ageing and red cell distribution width are associated with prevalent Parkinson’s disease: Findings from a large Italian population cohort. Front. Endocrinol. 2024, 15, 1376545. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, Z.A.; Ünal, A.; Yiğiter, R.; Yesil, Y.; Kuyumcu, M.E.; Neyal, M.; Kepekçi, Y. Is increased red cell distribution width (RDW) indicating the inflammation in Alzheimer’s disease (AD)? Arch. Gerontol. Geriatr. 2013, 56, 50–54. [Google Scholar] [CrossRef]
- Bertolotti, M.; Pirotti, T.; Castellani Tarabini, G.I.; Lancellotti, G.; Cuccorese, M.; Trenti, T.; Mussi, C. Modifications in hemoglobin levels associated with age in an outpatient population from northern italy. Sci. Rep. 2025, 15, 8960. [Google Scholar] [CrossRef]
- Yu, F.; Chen, C.; Liu, W.; Zhao, Z.; Fan, Y.; Li, Z.; Huang, W.; Xie, T.; Luo, C.; Yao, Z. Longevity Humans Have Youthful Erythrocyte Function and Metabolic Signatures. Aging Cell 2025, 24, e14482. [Google Scholar] [CrossRef]
- Kaminsky, Y.G.; Reddy, V.P.; Ashraf, G.M.; Ahmad, A.; Benberin, V.V.; Kosenko, E.A.; Aliev, G. Age-related defects in erythrocyte 2,3-diphosphoglycerate metabolism in dementia. Aging Dis. 2013, 4, 244–255. [Google Scholar] [CrossRef]
- García-Río, F.; Villamor, A.; Gómez-Mendieta, A.; Lores, V.; Rojo, B.; Ramírez, T.; Villamor, J. The progressive effects of ageing on chemosensitivity in healthy subjects. Respir. Med. 2007, 101, 2192–2198. [Google Scholar] [CrossRef]
- Mohandas, N.; Gallagher, P.G. Red cell membrane: Past, present, and future. Blood 2008, 112, 3939–3948. [Google Scholar] [CrossRef]
- Namvar, A.; Blanch, A.J.; Dixon, M.W.; Carmo, O.M.S.; Liu, B.; Tiash, S.; Looker, O.; Andrew, D.; Chan, L.J.; Tham, W.H.; et al. Surface area-to-volume ratio, not cellular viscoelasticity, is the major determinant of red blood cell traversal through small channels. Cell. Microbiol. 2021, 23, e13270. [Google Scholar] [CrossRef] [PubMed]
- McMahon, T.J. Red blood cell deformability, vasoactive mediators, and adhesion. Front. Physiol. 2019, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Carallo, C.; Irace, C.; De Franceschi, M.; Coppoletta, F.; Tiriolo, R.; Scicchitano, C.; Scavelli, F.; Gnasso, A. The effect of aging on blood and plasma viscosity. An 11.6 years follow-up study. Clin. Hemorheol. Microcirc. 2011, 47, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Racine, M.L.; Dinenno, F.A. Reduced deformability contributes to impaired deoxygenation-induced ATP release from red blood cells of older adult humans. J. Physiol. 2019, 597, 4503–4519. [Google Scholar] [CrossRef]
- Stevenson, A.; Lopez, D.; Khoo, P.; Kalaria, R.N.; Mukaetova-Ladinska, E.B. Exploring erythrocytes as blood biomarkers for Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 60, 845–857. [Google Scholar] [CrossRef]
- Goi, G.; Cazzola, R.; Tringali, C.; Massaccesi, L.; Volpe, S.R.; Rondanelli, M.; Ferrari, E.; Herrera, C.J.; Cestaro, B.; Lombardo, A.; et al. Erythrocyte membrane alterations during ageing affect beta-D-glucuronidase and neutral sialidase in elderly healthy subjects. Exp. Gerontol. 2005, 40, 219–225. [Google Scholar] [CrossRef]
- Kosenko, E.A.; Aliev, G.; Tikhonova, L.A.; Li, Y.; Poghosyan, A.C.; Kaminsky, Y.G. Antioxidant status and energy state of erythrocytes in Alzheimer dementia: Probing for markers. CNS Neurol. Disord. Drug. Targets. 2012, 11, 926–932. [Google Scholar] [CrossRef]
- McMahon, T.J.; Darrow, C.C.; Hoehn, B.A.; Zhu, H. Generation and Export of Red Blood Cell ATP in Health and Disease. Front. Physiol. 2021, 12, 754638. [Google Scholar] [CrossRef]
- Daraghmeh, D.N.; Karaman, R. The Redox Process in Red Blood Cells: Balancing Oxidants and Antioxidants. Antioxidants 2025, 14, 36. [Google Scholar] [CrossRef]
- Radosinska, J.; Vrbjar, N. Erythrocyte Deformability and Na,K-ATPase Activity in Various Pathophysiological Situations and Their Protection by Selected Nutritional Antioxidants in Humans. Int. J. Mol. Sci. 2021, 22, 11924. [Google Scholar] [CrossRef]
- Maurya, P.K.; Prakash, S. Decreased activity of Ca++-ATPase and Na+/K+-ATPase during aging in humans. Appl. Biochem. Biotechnol. 2013, 170, 131–137. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Meiselman, H.J.; Baskurt, O.K. Blood rheology and aging. J. Geriatr. Cardiol. JGC 2013, 10, 291. [Google Scholar]
- Raberin, A.; Burtscher, J.; Connes, P.; Millet, G.P. Hypoxia and hemorheological properties in older individuals. Ageing Res. Rev. 2022, 79, 101650. [Google Scholar] [CrossRef]
- Mykhailova, O.; Brandon-Coatham, M.; Hemmatibardehshahi, S.; Yazdanbakhsh, M.; Olafson, C.; Yi, Q.L.; Kanias, T.; Acker, J.P. Donor age contributes more to the rheological properties of stored red blood cells than donor sex and biological age distribution. Blood Adv. 2025, 9, 673–686. [Google Scholar] [CrossRef]
- Long, J.; Wang, X.; Yuan, J.; Yang, J.; Zhou, J.; Chen, Y.; Hu, E.; Zhou, Y.; Min, X. Reference intervals of complete blood count parameters for individuals aged 80 to 89 years in Guizhou, China: A STROBE-compliant retrospective study. Medicine 2022, 101, e30859. [Google Scholar] [CrossRef]
- Hammi, H.; Perrotin, P.; Guillet, R.; Boynard, M. Determination of red blood cell aggregation in young and elderly subjects evaluated by ultrasound: Influence of dihydroergocryptine mesylate. Clin. Hemorheol. Microcirc. 1994, 14, 117–126. [Google Scholar] [CrossRef]
- Mehdi, M.M.; Singh, P.; Rizvi, S.I. Erythrocyte sialic acid content during aging in humans: Correlation with markers of oxidative stress. Dis. Markers 2012, 32, 179–186. [Google Scholar] [CrossRef]
- Yadav, S.; Deepika; Moar, K.; Kumar, A.; Khola, N.; Pant, A.; Kakde, G.S.; Maurya, P.K. Reconsidering red blood cells as the diagnostic potential for neurodegenerative disorders. Biol. Cell 2024, 116, e2400019. [Google Scholar] [CrossRef]
- Schneider, T.R.; Stöckli, L.; Felbecker, A.; Nirmalraj, P.N. Protein fibril aggregation on red blood cells: A potential biomarker to distinguish neurodegenerative diseases from healthy aging. Brain Commun. 2024, 6, fcae180. [Google Scholar] [CrossRef]
- Maurya, P.K.; Kumar, P.; Chandra, P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J. Methodol. 2015, 5, 216–222. [Google Scholar] [CrossRef]
- Rizvi, S.I.; Maurya, P.K. Markers of oxidative stress in erythrocytes during aging in humans. Ann. N.Y. Acad. Sci. 2007, 1100, 373–382. [Google Scholar] [CrossRef]
- Hausman, D.B.; Fischer, J.G.; Johnson, M.A. Protein, lipid, and hematological biomarkers in centenarians: Definitions, interpretation and relationships with health. Maturitas 2012, 71, 205–212. [Google Scholar] [CrossRef]
- Caprari, P.; Scuteri, A.; Salvati, A.; Bauco, C.; Cantafora, A.; Masella, R.; Modesti, D.; Tarzia, A.; Marigliano, V. Aging and red blood cell membrane: A study of centenarians. Exp. Gerontol. 1999, 34, 47–57. [Google Scholar] [CrossRef]
- Kumar, A. Biomedical studies on lipid peroxidation and erythrocyte fragility during the process of aging. Asian Pac. J. Trop. Biomed. 2011, 1, 6–7. [Google Scholar] [CrossRef]
- Waitumbi, J.N.; Donvito, B.; Kisserli, A.; Cohen, J.H.; Stoute, J.A. Age-related changes in red blood cell complement regulatory proteins and susceptibility to severe malaria. J. Infect. Dis. 2004, 190, 1183–1191. [Google Scholar] [CrossRef]
- Bettiol, A.; Galora, S.; Argento, F.R.; Fini, E.; Emmi, G.; Mattioli, I.; Bagni, G.; Fiorillo, C.; Becatti, M. Erythrocyte oxidative stress and thrombosis. Expert Rev. Mol. Med. 2022, 24, e31. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxidative Med. Cell. Longev. 2010, 3, 2–12. [Google Scholar] [CrossRef]
- Kawamoto, E.M.; Vasconcelos, A.R.; Degaspari, S.; Böhmer, A.E.; Scavone, C.; Marcourakis, T. Age-related changes in nitric oxide activity, cyclic GMP, and TBARS levels in platelets and erythrocytes reflect the oxidative status in central nervous system. Age 2013, 35, 331–342. [Google Scholar] [CrossRef]
- Maurya, P.K.; Kumar, P.; Siddiqui, N.; Tripathi, P.; Rizvi, S.I. Age-associated changes in erythrocyte glutathione peroxidase activity: Correlation with total antioxidant potential. Indian J. Biochem. Biophys. 2010, 47, 319–321. [Google Scholar]
- Mathur, A.; Taurin, S.; Alshammary, S. New insights into methods to measure biological age: A literature review. Front. Aging 2024, 5, 1395649. [Google Scholar] [CrossRef]
- Mason, C.E.; Sierra, M.A.; Feng, H.J.; Bailey, S.M. Telomeres and aging: On and off the planet! Biogerontology 2024, 25, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2020, 11, 630186. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and telomere length: A general overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Sánchez, S.; Aubert, G.; Ripoll-Cladellas, A.; Henkelman, S.; Zhernakova, D.V.; Sinha, T.; Kurilshikov, A.; Cenit, M.C.; Jan Bonder, M.; Franke, L. Genetic, parental and lifestyle factors influence telomere length. Commun. Biol. 2022, 5, 565. [Google Scholar] [CrossRef]
- Ma, B.; Martínez, P.; Sánchez-Vázquez, R.; Blasco, M.A. Telomere dynamics in human pluripotent stem cells. Cell Cycle 2023, 22, 2505–2521. [Google Scholar] [CrossRef]
- Sanders, J.L.; Newman, A.B. Telomere length in epidemiology: A biomarker of aging, age-related disease, both, or neither? Epidemiol. Rev. 2013, 35, 112–131. [Google Scholar] [CrossRef]
- Yu, H.J.; Byun, Y.H.; Park, C.K. Techniques for assessing telomere length: A methodological review. Comput. Struct. Biotechnol. J. 2024, 23, 1489–1498. [Google Scholar] [CrossRef]
- Vera, E.; Blasco, M.A. Beyond average: Potential for measurement of short telomeres. Aging 2012, 4, 379–392. [Google Scholar] [CrossRef]
- Montpetit, A.J.; Alhareeri, A.A.; Montpetit, M.; Starkweather, A.R.; Elmore, L.W.; Filler, K.; Mohanraj, L.; Burton, C.W.; Menzies, V.S.; Lyon, D.E.; et al. Telomere length: A review of methods for measurement. Nurs. Res. 2014, 63, 289–299. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, Y. Epigenetic Clock: DNA Methylation in Aging. Stem. Cells. Int. 2020, 2020, 1047896. [Google Scholar] [CrossRef]
- Richardson, M.; Brandt, C.; Jain, N.; Li, J.L.; Demanelis, K.; Jasmine, F.; Kibriya, M.G.; Tong, L.; Pierce, B.L. Characterization of DNA methylation clock algorithms applied to diverse tissue types. Aging 2025, 17, 67–96. [Google Scholar] [CrossRef]
- Zhang, Z.; Reynolds, S.R.; Stolrow, H.G.; Chen, J.Q.; Christensen, B.C.; Salas, L.A. Deciphering the role of immune cell composition in epigenetic age acceleration: Insights from cell-type deconvolution applied to human blood epigenetic clocks. Aging Cell 2024, 23, e14071. [Google Scholar] [CrossRef] [PubMed]
- Gems, D.; Virk, R.S.; de Magalhães, J.P. Epigenetic clocks and programmatic aging. Ageing Res. Rev. 2024, 101, 102546. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.J.; Konwar, C.; Asiimwe, R.; Dinh, L.; Razzaghian, H.R.; de Goede, O.; MacIsaac, J.L.; Morin, A.M.; Kolsun, K.P.; Gervin, K.; et al. DNA methylation differences between cord and adult white blood cells reflect postnatal immune cell maturation. Commun. Biol. 2025, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Bergstedt, J.; Azzou, S.A.K.; Tsuo, K.; Jaquaniello, A.; Urrutia, A.; Rotival, M.; Lin, D.T.S.; MacIsaac, J.L.; Kobor, M.S.; Albert, M.L.; et al. The immune factors driving DNA methylation variation in human blood. Nat. Commun. 2022, 13, 5895. [Google Scholar] [CrossRef]
- Khodasevich, D.; Gladish, N.; Daredia, S.; Bozack, A.K.; Shen, H.; Nwanaji-Enwerem, J.C.; Needham, B.L.; Rehkopf, D.H.; Cardenas, A. Influence of race, ethnicity, and sex on the performance of epigenetic predictors of phenotypic traits. Clin. Epigenet. 2025, 17, 59. [Google Scholar] [CrossRef]
- Watkins, S.H.; Testa, C.; Chen, J.T.; De Vivo, I.; Simpkin, A.J.; Tilling, K.; Diez Roux, A.V.; Davey Smith, G.; Waterman, P.D.; Suderman, M.; et al. Epigenetic clocks and research implications of the lack of data on whom they have been developed: A review of reported and missing sociodemographic characteristics. Environ. Epigenet. 2023, 9, dvad005. [Google Scholar] [CrossRef]
- Teschendorff, A.E.; Horvath, S. Epigenetic ageing clocks: Statistical methods and emerging computational challenges. Nat. Rev. Genet. 2025, 26, 350–368. [Google Scholar] [CrossRef]
- Bell, C.G.; Lowe, R.; Adams, P.D.; Baccarelli, A.A.; Beck, S.; Bell, J.T.; Christensen, B.C.; Gladyshev, V.N.; Heijmans, B.T.; Horvath, S.; et al. DNA methylation aging clocks: Challenges and recommendations. Genome Biol. 2019, 20, 249. [Google Scholar] [CrossRef]
- Stier, A.; Reichert, S.; Criscuolo, F.; Bize, P. Red blood cells open promising avenues for longitudinal studies of ageing in laboratory, non-model and wild animals. Exp. Gerontol. 2015, 71, 118–134. [Google Scholar] [CrossRef]
- Foy, B.H.; Petherbridge, R.; Roth, M.; Mow, C.; Patel, H.R.; Patel, C.H.; Ho, S.N.; Lam, E.; Karczewski, K.J.; Tozzo, V.; et al. Hematologic setpoints are a stable and patient-specific deep phenotype. Nature 2025, 637, 430–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, P.; Tang, H.; Chen, K.; Chen, Y.; Xu, D. Biological variations of hematologic parameters determined by UniCel DxH 800 hematology analyzer. Arch. Pathol. Lab. Med. 2013, 137, 1106–1110. [Google Scholar] [CrossRef][Green Version]
- Obeidi, N.; Safavi, E.; Emami, H. Evaluation of the effect of temperature and time of incubation on complete blood count (CBC) tests. Afr. J. Biotechnol. 2012, 11, 1761–1763. [Google Scholar] [CrossRef]
| Feature | Young Individuals | Aged Individuals |
|---|---|---|
| HSC function | High self-renewal and proliferation capacity | Reduced self-renewal; stem cell exhaustion |
| EPO | High responsiveness to EPO | Diminished EPO receptor signaling and responsiveness |
| Bone marrow microenvironment | Supportive niche with low inflammation | Increased adiposity, chronic low-grade inflammation |
| Iron availability | Adequate absorption and utilization | Functional iron deficiency due to hepcidin elevation |
| Inflammatory cytokines (e.g., IL-1, TNF-α, IFN-γ) | Low baseline levels | Elevated levels; suppression of erythroid progenitors |
| Erythropoiesis efficiency | Robust production and differentiation | Impaired differentiation and increased risk of anemia |
| RBC Feature | Young Adults | Older Adults |
|---|---|---|
| RBC count | Normal/high | Decreased |
| Hb | Normal | Decreased |
| MCV | Normal | Slightly increased |
| RDW | Low (homogenous size) | Increased (heterogeneous size) |
| Deformability | High (good microcirculation) | Decreased (rigid, impaired flow) |
| ATP release | Adequate response to hypoxia | Reduced ATP-mediated vasodilation |
| Aggregation tendency | Low | Increased aggregation and blood viscosity |
| Oxygen carrying capacity | Efficient Hb saturation and delivery | Reduced capacity and altered Hb affinity |
| Membrane integrity | High fluidity, stable proteins | Increased lipid peroxidation, protein clustering |
| Antioxidant enzyme activity | High (protection against ROS) | Decreased, higher oxidative stress susceptibility |
| Biomarker | Primary Mechanism Associated with Aging | Key Advantages | Key Limitations |
|---|---|---|---|
| Telomere Length |
|
|
|
| DNA Methylation (Epigenetic Clocks) | Epigenetic drift and gene regulation |
|
|
| RBC indices | RBC integrity and function changes with age. |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriacou, R.P.; Shibeeb, S. Red Blood Cells and Human Aging: Exploring Their Biomarker Potential. Diagnostics 2025, 15, 1993. https://doi.org/10.3390/diagnostics15161993
Kyriacou RP, Shibeeb S. Red Blood Cells and Human Aging: Exploring Their Biomarker Potential. Diagnostics. 2025; 15(16):1993. https://doi.org/10.3390/diagnostics15161993
Chicago/Turabian StyleKyriacou, Roula P., and Sapha Shibeeb. 2025. "Red Blood Cells and Human Aging: Exploring Their Biomarker Potential" Diagnostics 15, no. 16: 1993. https://doi.org/10.3390/diagnostics15161993
APA StyleKyriacou, R. P., & Shibeeb, S. (2025). Red Blood Cells and Human Aging: Exploring Their Biomarker Potential. Diagnostics, 15(16), 1993. https://doi.org/10.3390/diagnostics15161993






