Prenatal Ultrasonographic Markers of Macrossomia and C-Peptide in Gestational Diabetes Mellitus: A Prospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Participant Selection
2.3. Data Collection
2.4. Ethical Considerations
2.5. Data Analysis
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordero, L.; Landon, M.B. Infant of the diabetic mother. Clin. Perinatol. 1993, 20, 635–648. [Google Scholar] [CrossRef]
- Kapoor, R.R.; Flanagan, S.E.; James, C.; Shield, J.; Ellard, S.; Hussain, K. Hyperinsulinaemic hypo-glycaemia. Arch. Dis. Child. 2009, 94, 450–457. [Google Scholar] [CrossRef]
- Saber, A.M.; Mohamed, M.A.; Sadek, A.A.; Mahmoud, R.A. Role of umbilical cord C-peptide levels in early prediction of hypoglycemia in infants of diabetic mothers. BMC Pediatr. 2021, 21, 85. [Google Scholar] [CrossRef]
- Vejrazkova, D.; Vankova, M.; Lukasova, P.; Vcelak, J.; Bendlova, B. Insights into the physiology of C-peptide. Physiol. Res. 2020, 69 (Suppl. S2), S237–S243. [Google Scholar] [CrossRef] [PubMed]
- Niknam, A.; Ramezani Tehrani, F.; Behboudi-Gandevani, S.; Rahmati, M.; Hedayati, M.; Abedini, M.; Firouzi, F.; Torkestani, F.; Zokaee, M.; Azizi, F. Umbilical cord blood concentration of connecting pep-tide (C-peptide) and pregnancy outcomes. BMC Pregnancy Childbirth 2022, 22, 764. [Google Scholar] [CrossRef] [PubMed]
- Aharon-Hananel, G.; Dori-Dayan, N.; Zemet, R.; Bakal, L.; Jabarin, A.; Levi, K.; Hemi, R.; Barhod, E.; Kordi-Patimer, O.; Mazaki-Tovi, S.; et al. The relationship between neona-tal hypoglycaemia and cord blood C-peptide levels in neonates of birthing individuals with type 1 diabetes. Diabetes Metab. Res. Rev. 2024, 40, e3714. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, J.J.; Chen, X.H.; Cao, L.; Wu, Y.; Zhu, L.J.; Lv, K.-T.; Ji, C.-B.; Guo, X.-R.; Jeong, J.-W. Measurement of fetal abdominal and subscapular subcutaneous tissue thickness during pregnancy to predict macrosomia: A pilot study. PLoS ONE 2014, 9, e93077. [Google Scholar] [CrossRef]
- Panunzi, C.; Cardinali, F.; Khalil, A.; Mustafa, H.J.; Spinillo, A.; Rizzo, G.; Flacco, M.E.; Maruotti, G.; D’Anto-nio, F. Ultrasound prediction of fetal macrosomia in pregnancies complicated by diabetes mellitus: A systematic review and meta-analysis. J. Perinat. Med. 2024, 52, 623–632. [Google Scholar] [CrossRef]
- Santolaya-Forgas, J.; Meyer, W.J.; Gauthier, D.W.; Kahn, D. Intrapartum fetal subcutaneous tissue/femur length ratio: An ultrasonographic clue to fetal macrosomia. Am. J. Obstet. Gynecol. 1994, 171, 1072–1075. [Google Scholar] [CrossRef]
- Dervisoglu, P.; Kosecik, M.; Kumbasar, S. Effects of gestational and pregestational diabetes mellitus on the foetal heart: A cross-sectional study. J. Obstet. Gynaecol. 2018, 38, 408–412. [Google Scholar] [CrossRef]
- Da Correggio, K.S.; Galluzzo, R.N.; von Wangenheim, A.; Werner, H.; Castro, P.T.; Araujo Júnior, E.; Onofre, A.S.C. Available evidence on fetal liver changes detected by ultrasound in pregnant women with gestational diabetes mellitus: Systematic review. J. Clin. Ultrasound 2024, 52, 1121–1128. [Google Scholar] [CrossRef]
- Roberts, A.B.; Mitchell, J.; Murphy, C.; Koya, H.; Cundy, T. Fetal liver length in diabetic pregnancy. Am. J. Obstet Gynecol. 1994, 170 Pt 1, 1308–1312. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System, Natality on CDC WONDER Online Database. Data Are from the Natality Records 2016–2021, as Compiled from Data Provided by the 57 Vital Statistics Jurisdictions Through the Vital Statistics Cooperative Program. Available online: http://wonder.cdc.gov/natality-expanded-current.html (accessed on 31 January 2023).
- Rajamoorthi, A.; LeDuc, C.A.; Thaker, V.V. The metabolic conditioning of obesity: A review of the pathogenesis of obesity and the epigenetic pathways that “program” obesity from conception. Front. Endocrinol. 2022, 13, 1032491. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of chronic adult disease. Acta Paediatr. Suppl. 2004, 93, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. J. Vasc. Interv. Radiol. 2018, 29, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Block, M.B.; Pildes, R.S.; Mossabhoy, N.A.; Steiner, D.F.; Rubenstein, A.H. C-peptide immunoreactivity (CPR): A new method for studying infants of insulin-treated diabetic mothers. Pediatrics 1974, 53, 923–928. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.J.; Lee, H.J.; Yu, E.H.; Yoon, H.J.; Kim, S.C. Correlation between fetal subcutaneous fat thickness and insulin resistance through cord blood analysis immediately after delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 302, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Uebel, K.; Pusch, K.; Gedrich, K.; Schneider, K.T.M.; Hauner, H.; Bader, B.L. Effect of maternal obesity with and without gestational diabetes on offspring subcutaneous and preperitoneal adipose tissue development from birth up to year-1. BMC Pregnancy Childbirth 2014, 14, 138. [Google Scholar] [CrossRef]
- Osmulski, M.E.; Yu, Y.; Kuang, A.; Josefson, J.L.; Hivert, M.F.; Scholtens, D.M.; Lowe, W.L. Subtypes of Gestational Diabetes Mellitus Are Differentially Associated With Newborn and Childhood Metabolic Outcomes. Diabetes Care 2025, 48, 390–399. [Google Scholar] [CrossRef]
- Whitelaw, A. Subcutaneous fat in newborn infants of diabetic mothers: An indication of quality of diabetic control. Lancet Lond. Engl. 1977, 1, 15–18. [Google Scholar] [CrossRef]
- Madsen, L.R.; Gibbons, K.S.; Ma, R.C.W.; Tam, W.H.; Catalano, P.M.; Sacks, D.A.; Lowe, J.; McIntyre, H.D. Do variations in insulin sensitivity and insulin secretion in pregnancy predict differences in obstetric and neonatal outcomes? Diabetologia 2021, 64, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Mein, B.J.; Benzie, R.J.; Ormerod, J.T.; Robledo, K.P.; Hibbert, E.J.; Nanan, R.K.; Duarte, M. Maternal diabetes independent of BMI is associated with altered accretion of adipose tissue in large for gestational age fetuses. PLoS ONE 2022, 17, e0268972. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, R.; Nodzenski, M.; Talbot, O.; Kuang, A.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Ilkayeva, O.R.; O’nEal, S.K.; Lowe, L.P.; et al. Maternal metabolites during pregnancy are associated with newborn outcomes and hyperinsulinaemia across ancestries. Diabetologia 2019, 62, 473–484. [Google Scholar] [CrossRef]
- Depla, A.L.; De Wit, L.; Steenhuis, T.J.; Slieker, M.G.; Voormolen, D.N.; Scheffer, P.G.; De Heus, R.; Van Rijn, B.B.; Bekker, M.N. Effect of maternal diabetes on fetal heart function on echocardiography: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2021, 57, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Oluklu, D.; Menekse Beser, D.; Uyan Hendem, D.; Yildirim, M.; Tugrul Ersak, D.; Turgut, E.; Sahin, D. The evaluation of fetal interventricular septum with M-mode and spectral tissue Doppler imaging in gestational diabetes mellitus: A case-control study. J. Perinat. Med. 2024, 52, 239–245. [Google Scholar] [CrossRef]
- Chandra, T.; Tripathi, S.; Tiwari, A.; Sonkar, G.; Agarwal, S.; Kumar, M.; Singh, S. Role of cord blood IGF-1 and maternal HbA1c levels to predict interventricular septal hypertrophy among infants of diabetic mothers: A case-control study. Early Hum. Dev. 2023, 179, 105751. [Google Scholar] [CrossRef]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef]
- Duygulu, D.; Mutlu Sütcüoğlu, B.; Turgut, E.; Özdemir, H.; Karçaaltıncaba, D. Prospective evaluation of ultrasonographic fetal cardiac morphometry and functions in the third trimester of pregnancies with gestational diabetes mellitus. J. Clin. Ultrasound 2024, 52, 1265–1272. [Google Scholar] [CrossRef]
- Garg, S.; Sharma, P.; Sharma, D.; Behera, V.; Durairaj, M.; Dhall, A. Use of fetal echocardiography for characterization of fetal cardiac structure in women with normal pregnancies and gestational diabetes mellitus. J. Ultrasound Med. 2014, 33, 1365–1369. [Google Scholar] [CrossRef]
- Naeiji, Z.; Gargar, S.S.; Pooransari, P.; Rahmati, N.; Mirzamoradi, M.; Eshraghi, N.; Ghaemi, M.; Arbabzadeh, T.; Masoumi, M.; Shamsinezhad, B.B.; et al. Association between fetal liver diameter and glycemic control in pregnant women with gestational diabetes: A pilot study. Diabetes Metab. Syndr. 2023, 17, 102853. [Google Scholar] [CrossRef]
- Garcia-Flores, J.; Cruceyra, M.; Cañamares, M.; Garicano, A.; Nieto, O.; Tamarit, I. Predictive value of fetal hepatic biometry for birth weight and cord blood markers in gestational diabetes. J. Perinatol. 2016, 36, 723–728. [Google Scholar] [CrossRef]
- Tekin, S.; Ocal, A.; Guleroglu, F.Y.; Ozgün Selcuk, C.G.; Eyisoy, O.G.; Ocal, E.U.B.; Cetin, A. Ultrasonographic Evaluation of Fetal Liver, Pancreas, Thymus, and Adrenal Glands in Diabetic Versus Non-Diabetic Pregnancies. J. Clin. Ultrasound 2025. ahead of print. [Google Scholar] [CrossRef]
- Boito, S.M.; Struijk, P.C.; Ursem, N.T.C.; Stijnen, T.; Wladimiroff, J.W. Assessment of fetal liver volume and umbilical venous volume flow in pregnancies complicated by insulin-dependent diabetes mellitus. BJOG 2003, 110, 1007–1013. [Google Scholar] [PubMed]
- Weschenfelder, F.; Baum, N.; Lehmann, T.; Schleußner, E.; Groten, T. The Relevance of Fetal Abdominal Subcutaneous Tissue Recording in Predicting Perinatal Outcome of GDM Pregnancies: A Retrospective Study. J. Clin. Med. 2020, 9, 3375. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Zayed, R.; Thomas, L.; Agarwal, M. Gestational diabetes mellitus: Fetal liver length measurements between 21 and 24 weeks’ gestation. J. Clin. Ultrasound 2007, 35, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Cozma, M.A.; Găman, M.A.; Dobrică, E.C.; Boroghină, S.C.; Iancu, M.A.; Crețoiu, S.M.; Simionescu, A.A. A Glimpse at the Size of the Fetal Liver-Is It Connected with the Evolution of Gestational Diabetes? Int. J. Mol. Sci. 2021, 22, 7866. [Google Scholar] [CrossRef]
- Huluta, I.; Wright, A.; Cosma, L.M.; Hamed, K.; Nicolaides, K.H.; Charakida, M. Fetal Cardiac Function at Midgestation in Women Who Subsequently Develop Gestational Diabetes. JAMA Pediatr. 2023, 177, 718. [Google Scholar] [CrossRef]
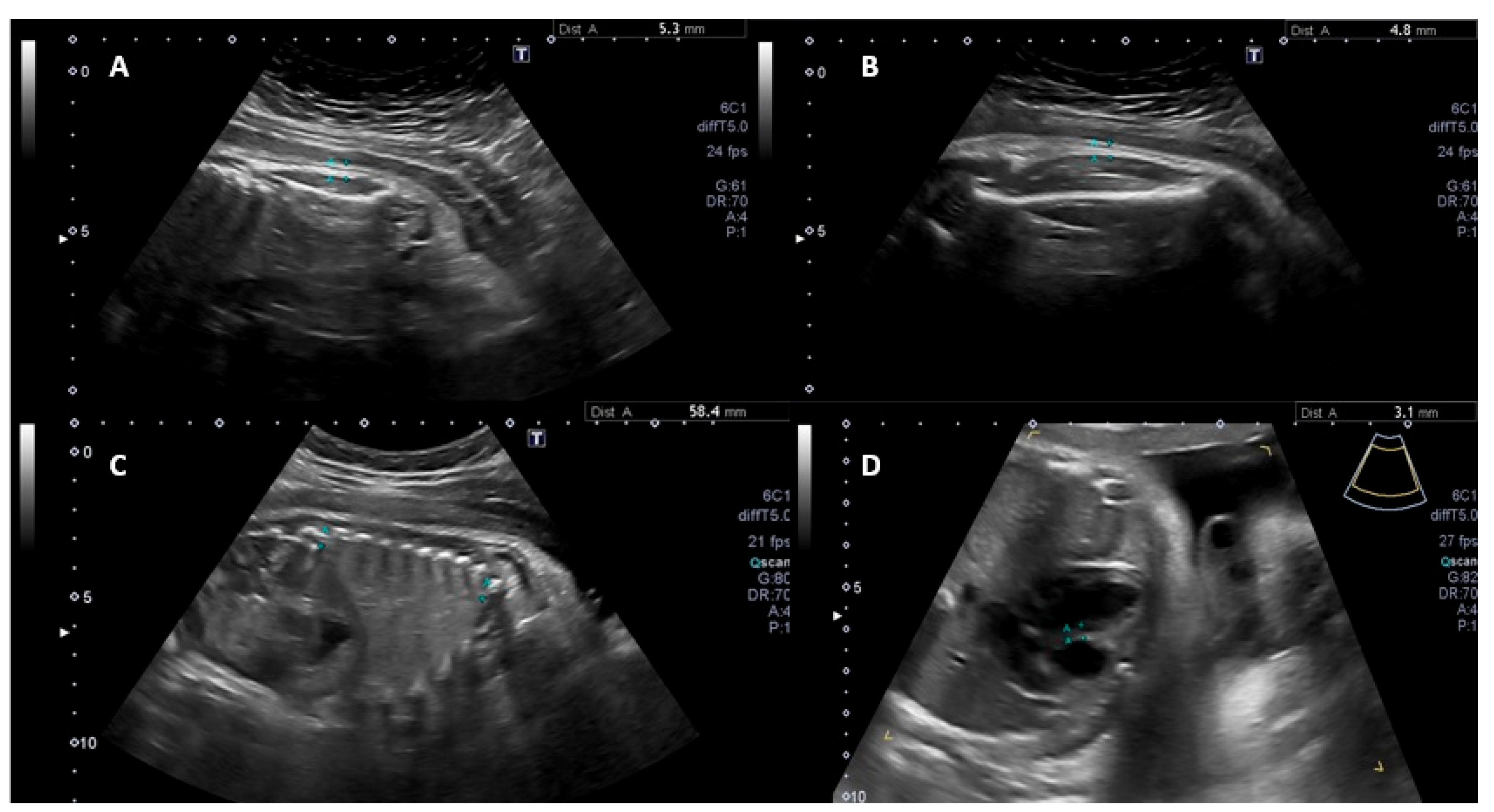
| Variables | Group | |
|---|---|---|
| Control (n = 95) | GDM (n = 128) | |
| Age (years) | 27.0 ± 5.8 | 29.0 ± 5.8 |
| BMI (Kg/m2) | 26.1 ± 5.2 | 29.4 ± 6.7 |
| BMI Categories (%) | ||
| Underweight | 3 (3.2) | 1 (0.8) |
| Normal weight | 38 (40.0) | 36 (28.1) |
| Overweight | 31 (32.6) | 38 (29.7) |
| Obesity class I | 16 (16.8) | 25 (19.5) |
| Obesity class II | 6 (6.3) | 18 (14.1) |
| Obesity class III | 1 (1.1) | 10 (7.8) |
| Race (%) | ||
| White | 71 (74.7) | 94 (73.4) |
| Non-White | 24 (25.3) | 34 (26.6) |
| Alcohol use (%) | ||
| No | 87 (91.6) | 125 (97.7) |
| Yes | 8 (8.4) | 3 (2.3) |
| Use of illicit drugs (%) | ||
| No | 95 (100.0) | 125 (97.7) |
| Yes | 0 (0.0) | 3 (2.3) |
| Smoking (%) | ||
| No | 86 (90.5) | 125 (97.7) |
| Yes | 9 (9.5) | 3 (2.3) |
| Physical activity (%) | ||
| No | 84 (88.4) | 96 (75.0) |
| Yes | 11 (11.6) | 32 (25.0) |
| Variables | n (%) |
|---|---|
| Gestational age at diagnosis | |
| 1st trimester | 50 (39.1) |
| 2nd trimester | 53 (41.4) |
| 3rd trimester | 25 (19.5) |
| Gestational age at initiation of insulin therapy * | |
| 2nd trimester | 15 (45.5) |
| 3rd trimester | 18 (54.5) |
| OGTT | |
| No | 75 (58.6) |
| Yes | 53 (41.4) |
| Adequate diet * | |
| No | 64 (55.2) |
| Yes | 52 (44.8) |
| Variables | Mean ± SD | Mean Difference | p-Value * | 95% CI of the Mean Difference |
|---|---|---|---|---|
| Thigh skinfold | ||||
| Control group | 5.5 ± 1.2 | −0.34 | 0.07 | −0.70; 0.02 |
| GDM group | 5.8 ± 1.4 | |||
| Abdominal skinfold | ||||
| Control group | 6.2 ± 1.3 | −0.70 | <0.01 | −1.05; −0.36 |
| GDM group | 6.9 ± 1.3 | |||
| Liver length | ||||
| Control group | 49.5 ± 7.2 | −3.79 | <0.01 | −5.70; −1.98 |
| GDM group | 53.3 ± 7.5 | |||
| Subscapular skinfold | ||||
| Control group | 6.6 ± 1.8 | −0.61 | 0.02 | −1.11; −0.13 |
| GDM group | 7.2 ± 1.8 | |||
| Interventricular septum | ||||
| Control group | 5.5 ± 1.0 | −0.65 | <0.01 | −0.92; −0.37 |
| GDM group | 6.1 ± 1.0 |
| Variables | Group | p-Value * | |
|---|---|---|---|
| Control (n = 95) | GDM (n = 128) | ||
| Thigh-to-femur skinfold ratio | 0.009 | ||
| Normal | 64 (50.0) | 64 (50.0) | |
| Altered | 31 (32.6) | 64 (67.4) | |
| Abdominal skinfold | 0.29 | ||
| Normal | 92 (43.4) | 120 (56.6) | |
| Altered | 3 (27.3) | 8 (72.7) | |
| Liver length | 0.02 | ||
| Normal | 91 (45.3) | 110 (54.7) | |
| Altered | 4 (18.2) | 18 (81.8) | |
| Subscapular skinfold | 0.03 | ||
| Normal | 83 (46.1) | 97 (53.9) | |
| Altered | 12 (27.9) | 31 (72.1) | |
| Interventricular septum | 0.008 | ||
| Normal | 20 (64.5) | 11 (35.5) | |
| Altered | 75 (39.1) | 117 (60.9) | |
| C-Peptide | Thigh Skinfold | Abdominal Skinfold | Liver Length | Subscapular Skinfold | Interventricular Septum |
|---|---|---|---|---|---|
| C-Peptide | – | ||||
| Thigh Skinfold | 0.30 | – | |||
| Abdominal Skinfold | 0.27 | 0.41 | – | ||
| Liver Length | 0.23 | 0.19 | 0.24 | – | |
| Subscapular Skinfold | 0.16 | 0.34 | 0.39 | 0.22 | – |
| Interventricular Septum | 0.10 | 0.31 | 0.44 | 0.19 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galluzzo, R.N.; Da Correggio, K.S.; von Wangenheim, A.; Callado, G.Y.; Werner, H.; Araujo Júnior, E.; Castro, P.T.; Calagna, G.; Onofre, A.S.C. Prenatal Ultrasonographic Markers of Macrossomia and C-Peptide in Gestational Diabetes Mellitus: A Prospective Cohort Study. Diagnostics 2025, 15, 1989. https://doi.org/10.3390/diagnostics15161989
Galluzzo RN, Da Correggio KS, von Wangenheim A, Callado GY, Werner H, Araujo Júnior E, Castro PT, Calagna G, Onofre ASC. Prenatal Ultrasonographic Markers of Macrossomia and C-Peptide in Gestational Diabetes Mellitus: A Prospective Cohort Study. Diagnostics. 2025; 15(16):1989. https://doi.org/10.3390/diagnostics15161989
Chicago/Turabian StyleGalluzzo, Roberto Noya, Karine Souza Da Correggio, Aldo von Wangenheim, Gustavo Yano Callado, Heron Werner, Edward Araujo Júnior, Pedro Teixeira Castro, Glória Calagna, and Alexandre Sherlley Casimiro Onofre. 2025. "Prenatal Ultrasonographic Markers of Macrossomia and C-Peptide in Gestational Diabetes Mellitus: A Prospective Cohort Study" Diagnostics 15, no. 16: 1989. https://doi.org/10.3390/diagnostics15161989
APA StyleGalluzzo, R. N., Da Correggio, K. S., von Wangenheim, A., Callado, G. Y., Werner, H., Araujo Júnior, E., Castro, P. T., Calagna, G., & Onofre, A. S. C. (2025). Prenatal Ultrasonographic Markers of Macrossomia and C-Peptide in Gestational Diabetes Mellitus: A Prospective Cohort Study. Diagnostics, 15(16), 1989. https://doi.org/10.3390/diagnostics15161989









