From Large-Scale Characterization to Subgroup-Specific Predictive Modeling: A Study on the Diagnostic Value of Liver Stiffness Measurements in Focal Liver Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Features of Liver Stiffness Measurements in Liver Lesions
2.2. Diagnostic Value of Liver Stiffness Measurements in AFP and HBsAg-Negative Cohort
2.3. Statistical Analysis
3. Results
3.1. Liver Stiffness Measurements in 8817 Liver Lesions
3.1.1. Liver Stiffness Measurements in Various Liver Lesion Types in Benign and Malignant Lesions
3.1.2. Liver Stiffness Measurements of 8817 Liver Lesions Under Subgroups of Serology
- Subgroups based on HBsAg (Figure 3)
- Subgroups based on AFP (Figure 4)
3.2. Diagnostic Value of Liver Stiffness Measurements in AFP and HBsAg Negative Cohort
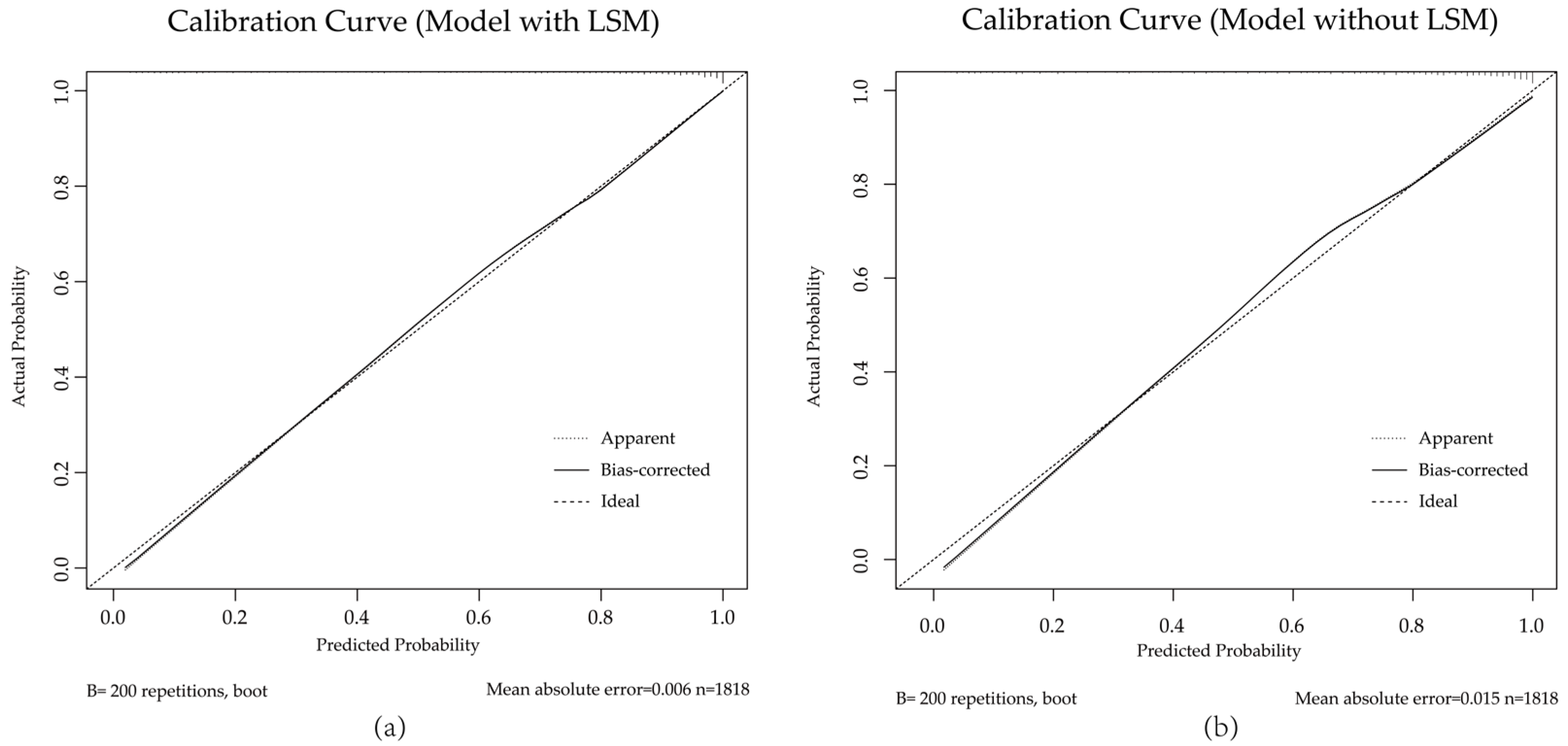
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozturk, A.; Olson, M.C.; Samir, A.E.; Venkatesh, S.K. Liver fibrosis assessment: MR and US elastography. Abdom. Radiol. 2022, 47, 3037–3050. [Google Scholar]
- Bai, X.; Pu, C.; Zhen, W.; Huang, Y.; Zhang, Q.; Li, Z.; Zhang, Y.; Xu, R.; Yao, Z.; Wu, W.; et al. Identifying liver cirrhosis in patients with chronic hepatitis B: An interpretable machine learning algorithm based on LSM. Ann. Med. 2025, 57, 2477294. [Google Scholar]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar]
- Liang, J.; Ampuero, J.; Castell, J.; Zhang, Q.; Zhang, S.; Chen, Y.; Romero-Gómez, M. Clinical application of Magnetic resonance elastography in hepatocellular carcinoma: From diagnosis to prognosis. Ann. Hepatol. 2023, 28, 100889. [Google Scholar]
- Guo, J.; Jiang, D.; Qian, Y.; Yu, J.; Gu, Y.J.; Zhou, Y.Q.; Zhang, H.P. Differential diagnosis of different types of solid focal liver lesions using two-dimensional shear wave elastography. World J. Gastroenterol. 2022, 28, 4716–4725. [Google Scholar]
- Dominguez, A.; Fino, D.; Spina, J.C.; Moyano Brandi, N.; Capó, J.; Noceti, M.; Ariza, P.P.; Moura Cunha, G. Assessment of SE-MRE-derived shear stiffness at 3.0 Tesla for solid liver tumors characterization. Abdom. Radiol. 2021, 46, 1904–1911. [Google Scholar]
- Frenette, C.; Mendiratta-Lala, M.; Salgia, R.; Wong, R.J.; Sauer, B.G.; Pillai, A. ACG Clinical Guideline: Focal Liver Lesions. Am. J. Gastroenterol. 2024, 119, 1235–1271. [Google Scholar]
- Guo, Q.; Zhu, X.; Beeraka, N.M.; Zhao, R.; Li, S.; Li, F.; Mahesh, P.A.; Nikolenko, V.N.; Fan, R.; Liu, J. Projected epidemiological trends and burden of liver cancer by 2040 based on GBD, CI5plus, and WHO data. Sci. Rep. 2024, 14, 28131. [Google Scholar]
- Zhan, Z.; Chen, B.; Huang, R.; Lin, W.; Lan, S.; Yao, X.; Huang, S.; Lin, W.; Xu, S.; Zhou, S.; et al. Long-term trends and future projections of liver cancer burden in China from 1990 to 2030. Sci. Rep. 2025, 15, 13120. [Google Scholar]
- Chen, D.S.; Sung, J.L.; Sheu, J.C.; Lai, M.Y.; How, S.W.; Hsu, H.C.; Lee, C.S.; Wei, T.C. Serum alpha-fetoprotein in the early stage of human hepatocellular carcinoma. Gastroenterology 1984, 86, 1404–1409. [Google Scholar]
- Liu, Y.; Jiang, W.; Li, X.; Zhao, H.; Wang, S. The Diagnostic Performance of AFP, AFP-L3, DCP, CA199, and Their Combination for Primary Liver Cancer. J. Hepatocell. Carcinoma 2025, 12, 513–526. [Google Scholar]
- Wen, B.; Te, L.; Bai, C.; Jiang, W.; Zuo, D.; Hao, Q.; Wang, J.; Ren, L. Relative contribution of hepatitis B and C viruses in primary liver cancer in China: A systematic review and meta-analysis. J. Infect. 2024, 89, 106298. [Google Scholar]
- Atwell, T.D.; Smith, R.L.; Hesley, G.K.; Callstrom, M.R.; Schleck, C.D.; Harmsen, W.S.; Charboneau, J.W.; Welch, T.J. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am. J. Roentgenol. 2010, 194, 784–789. [Google Scholar]
- Baradaran Najar, A.; Gilbert, G.; Karam, E.; Volniansky, A.; Fohlen, A.; Barat, M.; Montagnon, E.; Castel, H.; Giard, J.-M.; Nguyen, B.N.; et al. MR Elastography for Classification of Focal Liver Lesions Using Viscoelastic Parameters: A Pilot Study Based on Intrinsic and Extrinsic Activations. J. Magn. Reson. Imaging 2025, 61, 2525–2540. [Google Scholar]
- Patil, S.D.; Kamat, H.A.; Nimbal, V.; Sajjan, S.; Muchandi, R.; Patil, S.; Kanamadi, S. Triple-Phase Computed Tomography and Sonoelastographic Evaluation in Focal Hepatic Lesions. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. 4), S3706–S3708. [Google Scholar]
- Nagar, V.A.; Ye, J.R.; Ng, W.H.; Chan, Y.H.; Hui, F.; Lee, C.K.; Lim, C.C. Diffusion-weighted MR imaging: Diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am. J. Neuroradiol. 2008, 29, 1147–1152. [Google Scholar]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar]
- Singh, S.; Fujii, L.L.; Murad, M.H.; Wang, Z.; Asrani, S.K.; Ehman, R.L.; Kamath, P.S.; Talwalkar, J.A. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1573–1584.e2. [Google Scholar]
- Keskin, S.; Babaoglu, O.; Keskin, Z. An investigation of the efficacy of shear wave elastography in the characterization of benign and malignant liver lesions. Pol. J. Radiol. 2022, 87, e462–e468. [Google Scholar]
- Masuzaki, R.; Tateishi, R.; Yoshida, H.; Sato, T.; Ohki, T.; Goto, T.; Yoshida, H.; Sato, S.; Sugioka, Y.; Ikeda, H.; et al. Assessing liver tumor stiffness by transient elastography. Hepatol. Int. 2007, 1, 394–397. [Google Scholar]
- Hennedige, T.P.; Hallinan, J.T.; Leung, F.P.; Teo, L.L.; Iyer, S.; Wang, G.; Chang, S.; Madhavan, K.K.; Wee, A.; Venkatesh, S.K. Comparison of magnetic resonance elastography and diffusion-weighted imaging for differentiating benign and malignant liver lesions. Eur. Radiol. 2016, 26, 398–406. [Google Scholar]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127 (Suppl. 1), S35–S50. [Google Scholar]
- Baglieri, J.; Brenner, D.A.; Kisseleva, T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1723. [Google Scholar]
- Kim, J.R.; Jang, K.T.; Jang, J.Y. Intraductal papillary neoplasm of the bile duct: Review of updated clinicopathological and imaging characteristics. Br. J. Surg. 2023, 110, 1229–1240. [Google Scholar]
- Krawczyk, M.; Ziarkiewicz-Wróblewska, B.; Podgórska, J.; Grzybowski, J.; Gierej, B.; Krawczyk, P.; Grąt, M.; Kornasiewicz, O.; Skalski, M.; Wróblewski, T. Intraductal papillary neoplasm of the bile duct—A comprehensive review. Adv. Med. Sci. 2021, 66, 138–147. [Google Scholar]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar]
- Cordero-Espinoza, L.; Huch, M. The balancing act of the liver: Tissue regeneration versus fibrosis. J. Clin. Investig. 2018, 128, 85–96. [Google Scholar]
- Hoffmann, C.; Djerir, N.E.H.; Danckaert, A.; Fernandes, J.; Roux, P.; Charrueau, C.; Lachagès, A.M.; Charlotte, F.; Brocheriou, I.; Clément, K.; et al. Hepatic stellate cell hypertrophy is associated with metabolic liver fibrosis. Sci. Rep. 2020, 10, 3850. [Google Scholar]
- Liu, Y.R.; Lin, B.B.; Zeng, D.W.; Zhu, Y.Y.; Chen, J.; Zheng, Q.; Dong, J.; Jiang, J.J. Alpha-fetoprotein level as a biomarker of liver fibrosis status: A cross-sectional study of 619 consecutive patients with chronic hepatitis B. BMC Gastroenterol. 2014, 14, 145. [Google Scholar]
- Kim, C.Y.; Kim, B.R.; Lee, S.S.; Jeon, D.H.; Lee, C.M.; Kim, W.S.; Cho, H.C.; Kim, J.J.; Lee, J.M.; Kim, H.J.; et al. Clinical features of hepatitis B and C virus infections, with high α-fetoprotein levels but not hepatocellular carcinoma. Medicine 2017, 96, e5844. [Google Scholar]
- El Raziky, M.; Attia, D.; El Akel, W.; Shaker, O.; Khatab, H.; Abdo, S.; Elsharkawy, A.; Esmat, G. Hepatic fibrosis and serum alpha-fetoprotein (AFP) as predictors of response to HCV treatment and factors associated with serum AFP normalisation after treatment. Arab. J. Gastroenterol. 2013, 14, 94–98. [Google Scholar]
- Wang, J.; Zhang, P.; Liao, J.; Zhu, Y.; Liu, X.; Tang, H. Association of α-fetoprotein levels with liver stiffness measurement in outpatients with chronic hepatitis B. Biosci. Rep. 2021, 41, BSR20203048. [Google Scholar]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar]
- Shan, L.; Wang, F.; Xue, W.; Zhai, D.; Liu, J.; Lv, X. New insights into fibrotic signaling in hepatocellular carcinoma. Front. Oncol. 2023, 13, 1196298. [Google Scholar]
- Lin, Y.; Wang, Q.; Feng, M.; Lao, J.; Wu, C.; Luo, H.; Ji, L.; Xia, Y. A cost-effective predictive tool for AFP-negative focal hepatic lesions of retrospective study: Enhancing clinical triage and decision-making. PeerJ 2025, 13, e19150. [Google Scholar]
- Gad, M.A.M.; Eraky, T.E.; Omar, H.M.; Abosheaishaa, H.M. Role of real-time shear-wave elastogarphy in differentiating hepatocellular carcinoma from other hepatic focal lesions. Eur. J. Gastroenterol. Hepatol. 2021, 33, 407–414. [Google Scholar]
- Liebman, H.A.; Furie, B.C.; Tong, M.J.; Blanchard, R.A.; Lo, K.J.; Lee, S.D.; Coleman, M.S.; Furie, B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N. Engl. J. Med. 1984, 310, 1427–1431. [Google Scholar]
- Feng, H.; Li, B.; Li, Z.; Wei, Q.; Ren, L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer 2021, 21, 401. [Google Scholar]
- Yang, M.; Zhang, H.; Zhang, J.; Yao, X. Risk factors and prevention of liver cancer: A bibliometric and visual analysis. Medicine 2023, 102, e35740. [Google Scholar]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. Excli J. 2016, 15, 817–828. [Google Scholar]
- Tibshirani, R. The lasso method for variable selection in the Cox model. Stat. Med. 1997, 16, 385–395. [Google Scholar]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar]







| Number (%) | LSM (kPa) | ||
|---|---|---|---|
| Median (Min.–Max.) | Mean ± SD | ||
| Cirrhotic nodules * | 64 (0.73) | 14.90 (7.50–36.90) | 15.58 ± 4.72 |
| Malignant * | 7902 (89.62) | 11.39 (3.90–88.60) | 12.02 ± 4.73 |
| Benign * | 851 (9.65) | 6.67 (3.40–29.20) | 7.46 ± 2.80 |
| Number (%) | LSM (kPa) | ||
|---|---|---|---|
| Median (Min.–Max.) | Mean ± SD | ||
| Malignant | 7902 | ||
| HCC *# | 5853 (74.0) | 12.40 (3.90–88.60) | 12.87 ± 4.73 |
| CCA * | 892 (11.29) | 9.53 (4.00–41.70) | 10.51 ± 4.17 |
| cHCC-CC *# | 139 (1.76) | 11.55 (4.20–24.20) | 12.05 ± 4.07 |
| MET * | 1018 (12.88) | 7.83 (4.00–30.90) | 8.44 ± 2.91 |
| Benign | 851 | ||
| AML | 74 (8.70) | 6.53 (3.40–12.50) | 6.87 ± 1.92 |
| FNH ** | 198 (23.27) | 6.20 (3.60–21.50) | 7.10 ± 2.83 |
| IPNB | 16 (1.88) | 7.27 (4.40–13.10) | 8.05 ± 2.80 |
| HCA | 34 (4.00) | 6.30 (4.60–10.80) | 6.84 ± 1.68 |
| HIPT | 16 (1.88) | 7.85 (5.10–11.40) | 7.66 ± 1.72 |
| HHs | 305 (35.84) | 6.66 (3.60–22.40) | 7.59 ± 2.96 |
| SHC ** | 208 (24.44) | 6.96 (4.10–29.20) | 7.88 ± 2.94 |
| Cirrhotic nodules | 64 (0.73) | 14.90 (7.50–36.90) | 15.58 ± 4.72 |
| Training (n = 1818) | Validation (n = 453) | p Value | |
|---|---|---|---|
| Age | 59.69 (14.04) | 59.85 (14.78) | 0.883 |
| Gender (%) | 0.561 | ||
| Male | 1157 (63.6%) | 281 (62.0%) | |
| Female | 661 (36.4%) | 172 (38.0%) | |
| LSM (kPa) | 9.88 (4.44) | 9.75 (4.26) | 0.577 |
| DCP (μg/L) | 844.07 (4704.99) | 726.92 (4761.34) | 0.636 |
| PLT (×109/L) | 195.93 (79.33) | 200.59 (80.45) | 0.265 |
| TBIL (μmol/L) | 21.40 (41.53) | 24.09 (52.67) | 0.244 |
| ALT (U/L) | 36.08 (56.84) | 35.89 (57.08) | 0.947 |
| AST(U/L) | 34.30 (48.95) | 32.08 (34.17) | 0.364 |
| GGT (U/L) | 118.88 (233.07) | 138.66 (281.62) | 0.122 |
| PT (s) | 11.88 (1.23) | 11.88 (1.14) | 0.959 |
| INR | 1.05 (0.11) | 1.05 (0.10) | 0.827 |
| Full Model (LSM) OR (95% CI) | Comparison Model (No LSM) OR (95% CI) | |
|---|---|---|
| LSM | 1.27 (0.03) *** | |
| age | 1.08 (0.01) *** | 1.10 (0.01) *** |
| gender | 1.79 (0.14) *** | 2.06 (0.14) *** |
| AST > 40 U/L | 5.87 (0.33) *** | 7.03 (0.31) *** |
| GGT > 50 U/L | 2.68 (0.15) *** | 2.95 (0.15) *** |
| DCP > 20 μg/L | 1.89 (0.15) *** | 2.35 (0.15) *** |
| PLT < 100 × 109/L | 3.12 (0.33) *** | |
| PT > 13 s | 2.51 (0.32) ** | |
| INR > 1 | 1.57 (0.14) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Guo, Y.-L.; Lv, Q.-Y.; Wang, Z.; Zhou, J.; Hu, J. From Large-Scale Characterization to Subgroup-Specific Predictive Modeling: A Study on the Diagnostic Value of Liver Stiffness Measurements in Focal Liver Lesions. Diagnostics 2025, 15, 1986. https://doi.org/10.3390/diagnostics15161986
Xu Y, Guo Y-L, Lv Q-Y, Wang Z, Zhou J, Hu J. From Large-Scale Characterization to Subgroup-Specific Predictive Modeling: A Study on the Diagnostic Value of Liver Stiffness Measurements in Focal Liver Lesions. Diagnostics. 2025; 15(16):1986. https://doi.org/10.3390/diagnostics15161986
Chicago/Turabian StyleXu, Ying, Ying-Long Guo, Qian-Yu Lv, Zheng Wang, Jian Zhou, and Jie Hu. 2025. "From Large-Scale Characterization to Subgroup-Specific Predictive Modeling: A Study on the Diagnostic Value of Liver Stiffness Measurements in Focal Liver Lesions" Diagnostics 15, no. 16: 1986. https://doi.org/10.3390/diagnostics15161986
APA StyleXu, Y., Guo, Y.-L., Lv, Q.-Y., Wang, Z., Zhou, J., & Hu, J. (2025). From Large-Scale Characterization to Subgroup-Specific Predictive Modeling: A Study on the Diagnostic Value of Liver Stiffness Measurements in Focal Liver Lesions. Diagnostics, 15(16), 1986. https://doi.org/10.3390/diagnostics15161986





