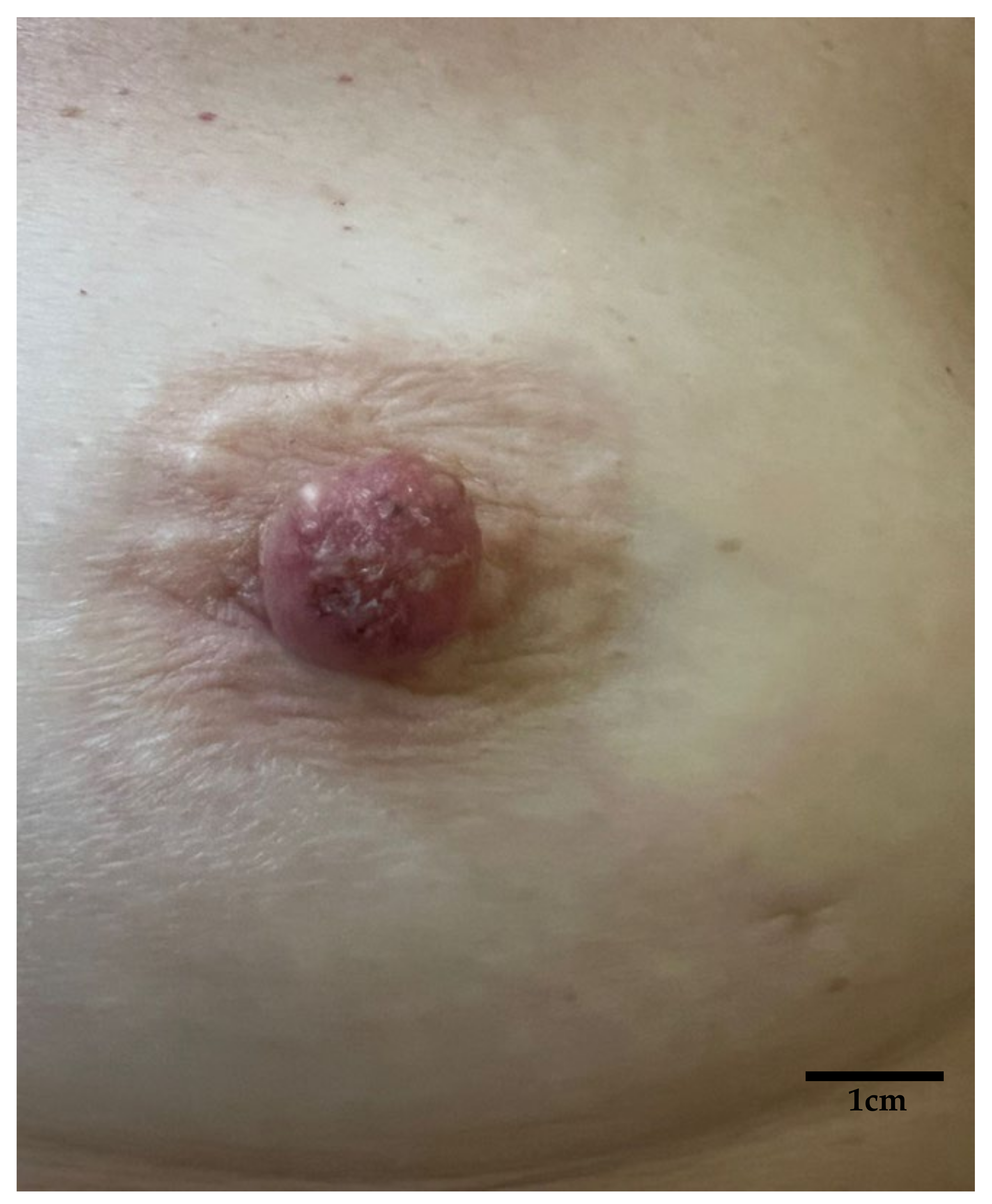
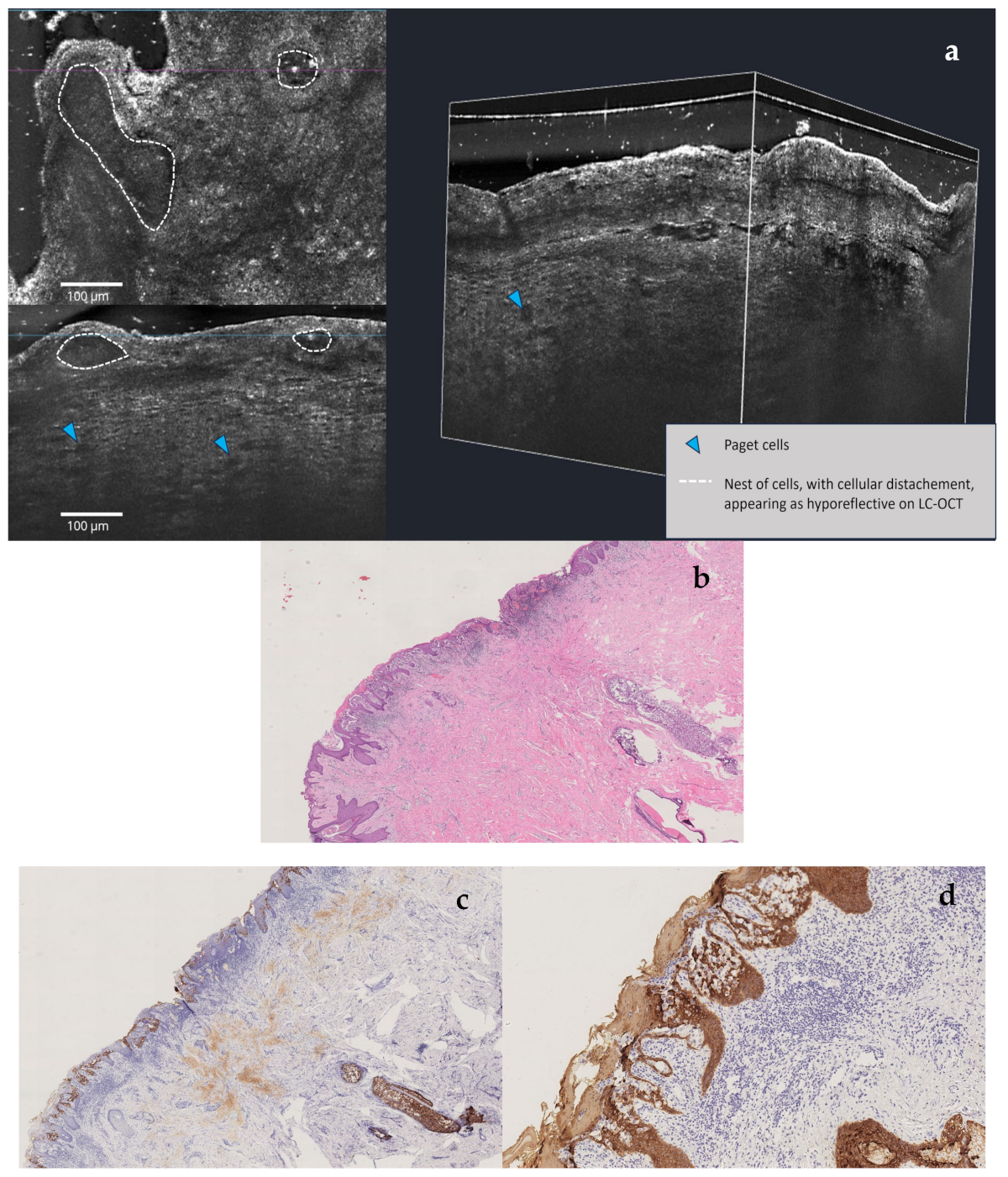
Multimodal Imaging Detection of Difficult Mammary Paget Disease: Dermoscopy, Reflectance Confocal Microscopy, and Line-Field Confocal–Optical Coherence Tomography
Abstract




Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPD | Mammary Paget disease |
| LC-OCT | Line-field confocal–optical coherence tomography |
| RCM | Reflectance confocal microscopy |
| NAC | Nipple areola complex |
| DCIS | Ductal carcinoma in situ |
| IDC | Invasive ductal carcinoma |
| LOQ | Lower outer quadrant |
| VABB | Vacuum-assisted breast biopsy |
| PgR | Progesterone Receptor |
| OSNA | One-step nucleic acid analysis amplification |
References
- Markarian, S.; Holmes, D.R. Mammary Paget’s Disease: An Update. Cancers 2022, 14, 2422. [Google Scholar] [CrossRef] [PubMed]
- Scott-Emuakpor, R.; Reza-Soltani, S.; Altaf, S.; NR, K.; Kołodziej, F.; Sil-Zavaleta, S.; Nalla, M.; Ullah, M.N.; Qureshi, M.R.; Ahmadi, Y.; et al. Mammary Paget’s Disease Mimicking Benign and Malignant Dermatological Conditions: Clinical Challenges and Diagnostic Considerations. Cureus 2024, 16, e65378. [Google Scholar] [CrossRef] [PubMed]
- Hudson-Phillips, S.; Cox, K.; Patel, P.; Al Sarakbi, W. Paget’s disease of the breast: Diagnosis and management. Br. J. Hosp. Med. 2023, 84, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Errichetti, E.; Kyrgidis, A.; Stolz, W.; Puig, S.; Malvehy, J.; Zalaudek, I.; Moscarella, E.; Longo, C.; Blum, A.; et al. Dermoscopic features of mammary Paget’s disease: A retrospective case-control study by the International Dermoscopy Society. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Errichetti, E.; Avellini, C.; Pegolo, E.; De Francesco, V. Dermoscopy as a Supportive Instrument in the Early Recognition of Erosive Adenomatosis of the Nipple and Mammary Paget’s Disease. Ann. Dermatol. 2017, 29, 365. [Google Scholar] [CrossRef] [PubMed]
- Crignis, G.S.N.D.; Abreu, L.D.; Buçard, A.M.; Barcaui, C.B. Polarized dermoscopy of mammary Paget disease. Bras. Dermatol. 2013, 88, 290–292. [Google Scholar] [CrossRef] [PubMed]
- D’Erme, A.M.; Iozzo, R.; Viacava, P.; De Luca, F.; Janowska, A.; Dini, V.; Romanelli, M.; Fidanzi, C.; Bagnoni, G. Pigmentary Mammary Paget Disease: Clinical, dermoscopical and histological challenge. Dermatol. Rep. 2021, 13, 9235. [Google Scholar] [CrossRef] [PubMed]
- Yanagishita, T.; Tamada, Y.; Tanaka, M.; Kasugai, C.; Takahashi, E.; Matsumoto, Y.; Watanabe, D. Pigmented mammary Paget disease mimicking melanoma on dermatoscopy. J. Am. Acad. Dermatol. 2011, 64, e114–e116. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance confocal microscopy. J. Am. Acad. Dermatol. 2021, 84, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Wellenhof, R.; Wurm, E.M.; Ahlgrimm-Siess, V.; Richtig, E.; Koller, S.; Smolle, J.; Gerger, A. Reflectance Confocal Microscopy—State-of-Art and Research Overview. Semin. Cutan. Med. Surg. 2009, 28, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Markowitz, O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018, 4, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Brunetti, T.; Cartocci, A.; Tognetti, L.; Suppa, M.; Malvehy, J.; Perez-Anker, J.; Puig, S.; Perrot, J.L.; Rubegni, P. Diagnostic Accuracy of Line-Field Confocal Optical Coherence Tomography for the Diagnosis of Skin Carcinomas. Diagnostics 2023, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Richtig, E.; Ahlgrimm-Siess, V.; Arzberger, E.; Hofmann-Wellenhof, R. Noninvasive differentiation between mamillary eczema and Paget disease by in vivo reflectance confocal microscopy on the basis of two case reports. Br. J. Dermatol. 2011, 165, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Levecq, O.; Azimani, H.; Siret, D.; Barut, A.; Suppa, M.; Del Marmol, V.; Malvehy, J.; Cinotti, E.; Rubegni, P.; et al. Line-field confocal optical coherence tomography for high-resolution noninvasive imaging of skin tumors. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Donelli, C.; Suppa, M.; Tognetti, L.; Perrot, J.L.; Calabrese, L.; Pérez-Anker, J.; Malvehy, J.; Rubegni, P.; Cinotti, E. Line-Field Confocal Optical Coherence Tomography for the Diagnosis of Skin Carcinomas: Real-Life Data over Three Years. Curr. Oncol. 2023, 30, 8853–8864. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantisani, C.; Caruso, G.; Taliano, A.; Longo, C.; Rizzuto, G.; D’Andrea, V.; Pietkiewicz, P.; Bortone, G.; Gargano, L.; Suppa, M.; et al. Multimodal Imaging Detection of Difficult Mammary Paget Disease: Dermoscopy, Reflectance Confocal Microscopy, and Line-Field Confocal–Optical Coherence Tomography. Diagnostics 2025, 15, 1898. https://doi.org/10.3390/diagnostics15151898
Cantisani C, Caruso G, Taliano A, Longo C, Rizzuto G, D’Andrea V, Pietkiewicz P, Bortone G, Gargano L, Suppa M, et al. Multimodal Imaging Detection of Difficult Mammary Paget Disease: Dermoscopy, Reflectance Confocal Microscopy, and Line-Field Confocal–Optical Coherence Tomography. Diagnostics. 2025; 15(15):1898. https://doi.org/10.3390/diagnostics15151898
Chicago/Turabian StyleCantisani, Carmen, Gianluca Caruso, Alberto Taliano, Caterina Longo, Giuseppe Rizzuto, Vito D’Andrea, Pawel Pietkiewicz, Giulio Bortone, Luca Gargano, Mariano Suppa, and et al. 2025. "Multimodal Imaging Detection of Difficult Mammary Paget Disease: Dermoscopy, Reflectance Confocal Microscopy, and Line-Field Confocal–Optical Coherence Tomography" Diagnostics 15, no. 15: 1898. https://doi.org/10.3390/diagnostics15151898
APA StyleCantisani, C., Caruso, G., Taliano, A., Longo, C., Rizzuto, G., D’Andrea, V., Pietkiewicz, P., Bortone, G., Gargano, L., Suppa, M., & Pellacani, G. (2025). Multimodal Imaging Detection of Difficult Mammary Paget Disease: Dermoscopy, Reflectance Confocal Microscopy, and Line-Field Confocal–Optical Coherence Tomography. Diagnostics, 15(15), 1898. https://doi.org/10.3390/diagnostics15151898








