Early Detection of Autism Spectrum Disorder Through Automated Machine Learning
Abstract
1. Introduction
- Level 1 (Requiring Support): Affected individuals may have significant difficulty initiating social interactions and may have problems with organization and planning, but they are often able to function independently with some support.
- Level 2 (Requiring Substantial Support): There are obvious deficits in verbal and non-verbal communication. Social disablement are more severe, and inflexible behavior occurs frequently, even with support.
- Level 3 (Requiring Very Substantial Support): Affected individuals exhibit significant difficulties with verbal and nonverbal communication and extreme resistance to change. Their restricted and repetitive conduct significantly impairs their ability to function in all domains.
- Social impairment—such as sharing emotions, holding a conversation.
- Communication difficulties—can be verbal (expressed through language) or non-verbal (such as facial expressions, eye contact, and gestures).
- Repetitive and stereotyped behavior—repeating words or actions.
- To gather empirical data for our study, we conducted a survey utilizing the autism spectrum quotient in toddlers-10 (Q-CHAT-10) questionnaire across various rehabilitation centers in Pakistan. The questionnaire comprises ten items that capture behavioral characteristics associated with ASD and seven other variables, including demographic information (e.g., age, gender). After administering the survey, the resulting dataset was obtained.
- After collecting the dataset, we implemented an automated machine learning (AUTOML) pipeline using the TPOT (Tree-based Pipeline Optimization Tool) library for ASD detection. The TPOT automated the process of model selection and hyperparameter optimization.
- In order to ensure the validity of the TPOT on our dataset, we conducted a thorough verification process. This involved manually recreating the machine learning model using the exact parameters generated by the TPOT. By replicating the model creation manually, we aimed to verify the consistency and reliability of TPOT’s automated pipeline. To examine and compute the effectiveness of both the AUTOML generated and manually recreated model, we employed a rigorous comparative evaluation.
- Unlike manual model tuning, the TPOT automates the selection and optimization of ML pipelines through genetic programming, enabling the discovery of high-performing models with minimal human intervention. This makes it especially useful in healthcare scenarios where domain experts may not have ML expertise.
2. Literature Review
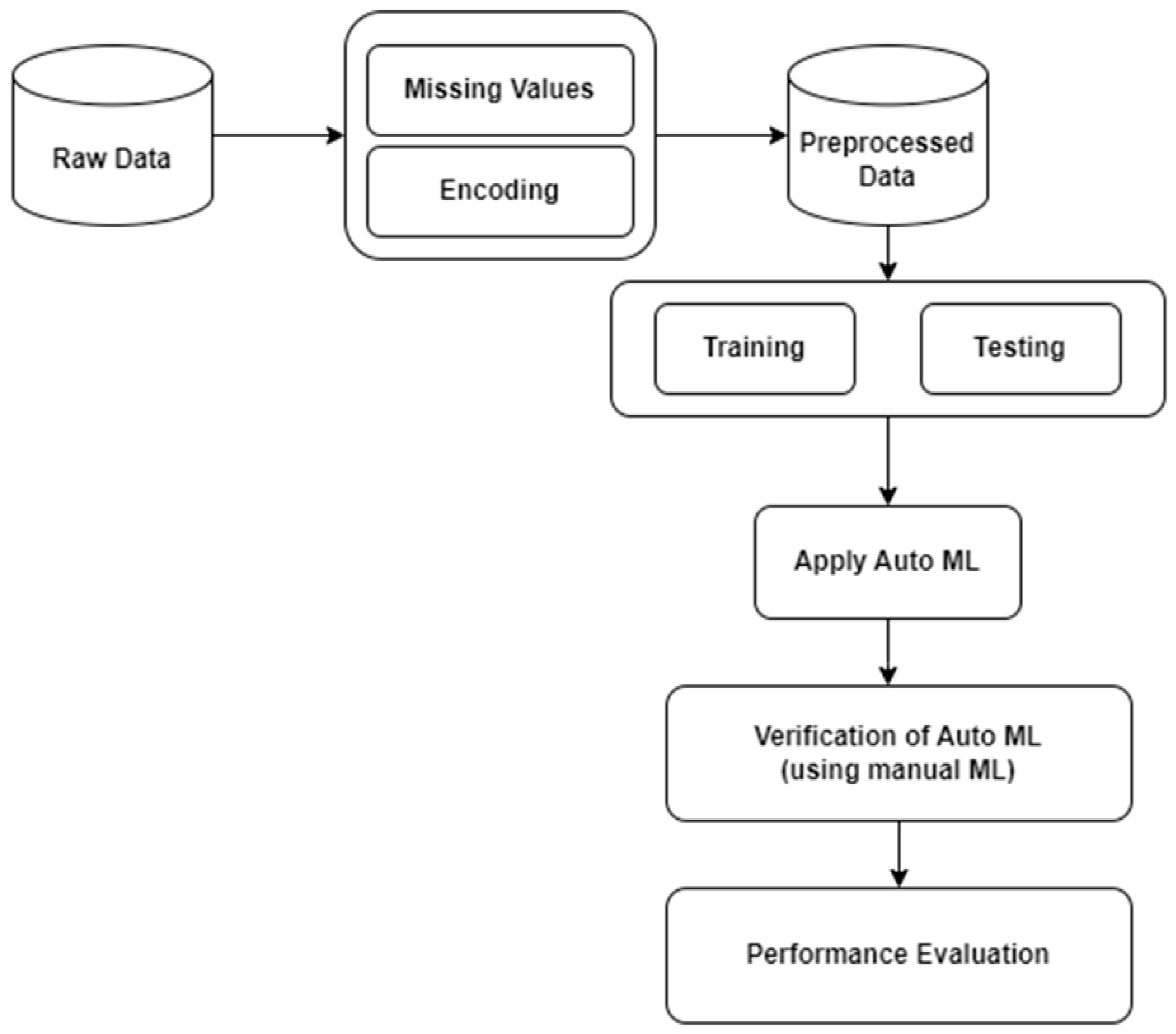
3. Proposed Framework
3.1. Data Collection
3.2. Data Preprocessing
3.3. Data Partitioning
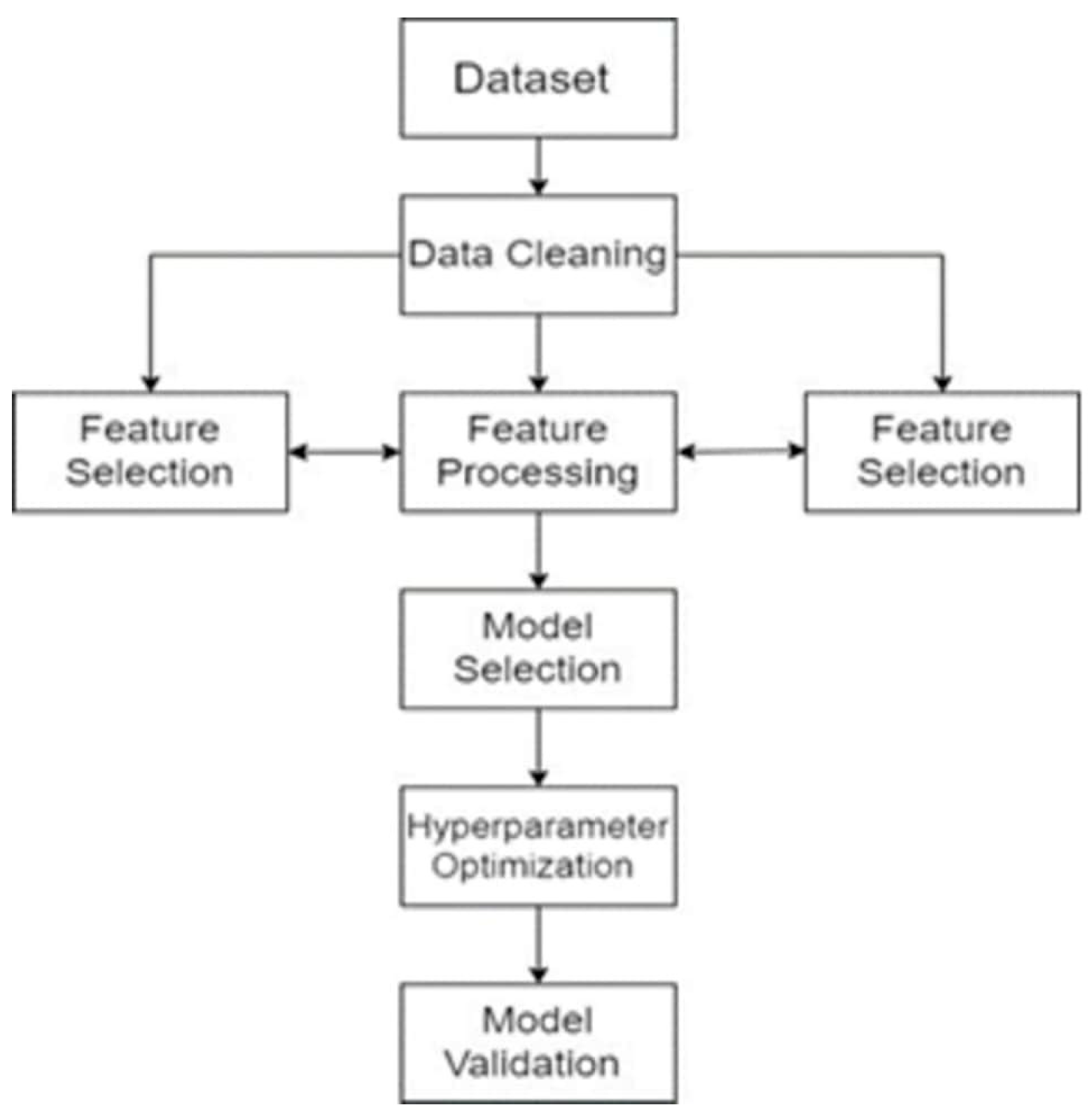
3.4. Model Development (AUTOML)
3.5. Tree-Based Pipeline Optimization Tool (TPOT)
3.5.1. Data Preparation
3.5.2. Feature Engineering
3.5.3. Pipeline Generation
3.5.4. Model Selection and Hyperparameter Tuning
3.5.5. Model Evaluation
3.5.6. Final Model Selection
3.6. Performance Evaluation Metrics
- Confusion Matrix
- Precision
- Recall
- F1-Score
- AUC-ROC Analysis
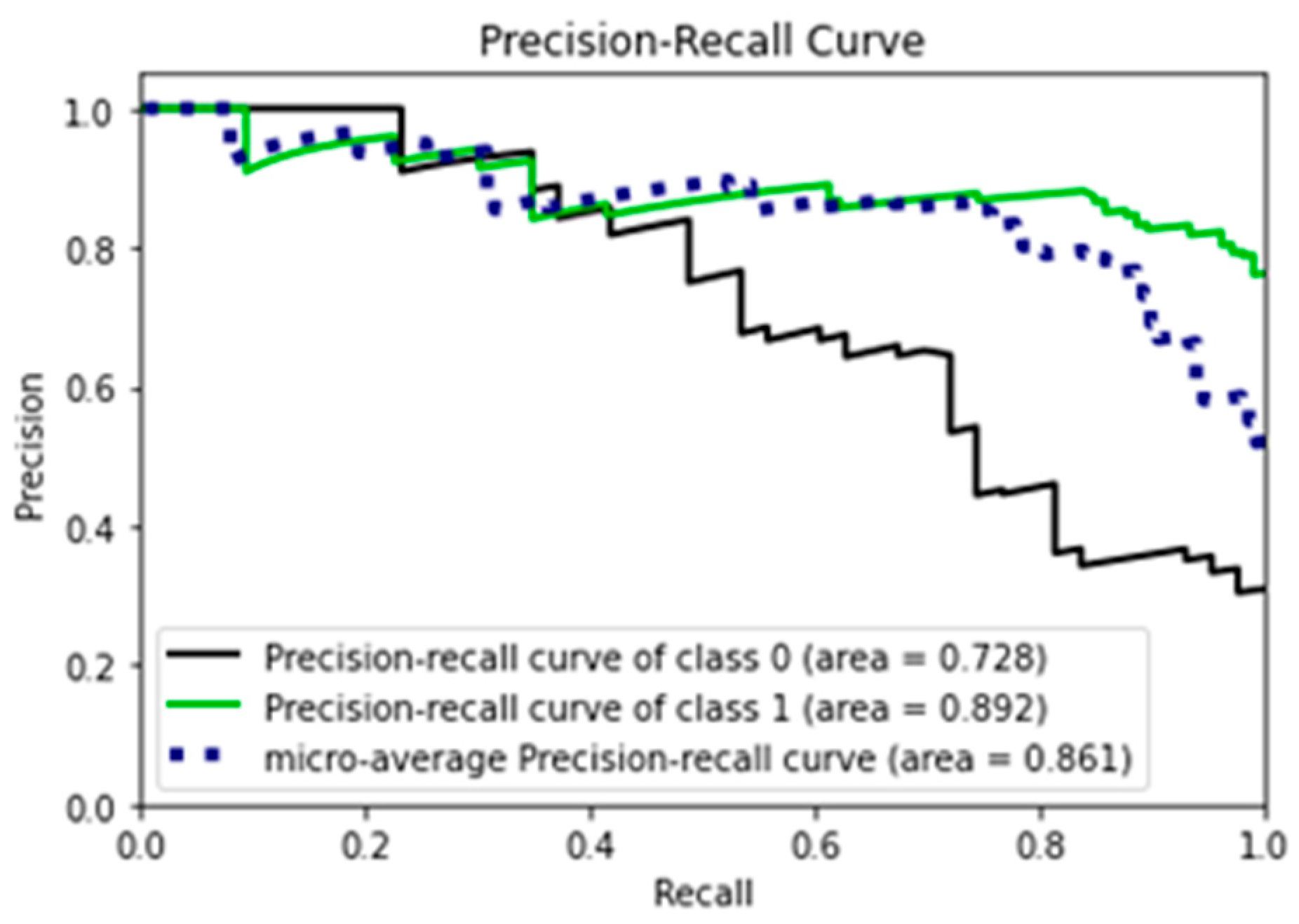
- Precision–Recall Curve
4. Results and Evaluations
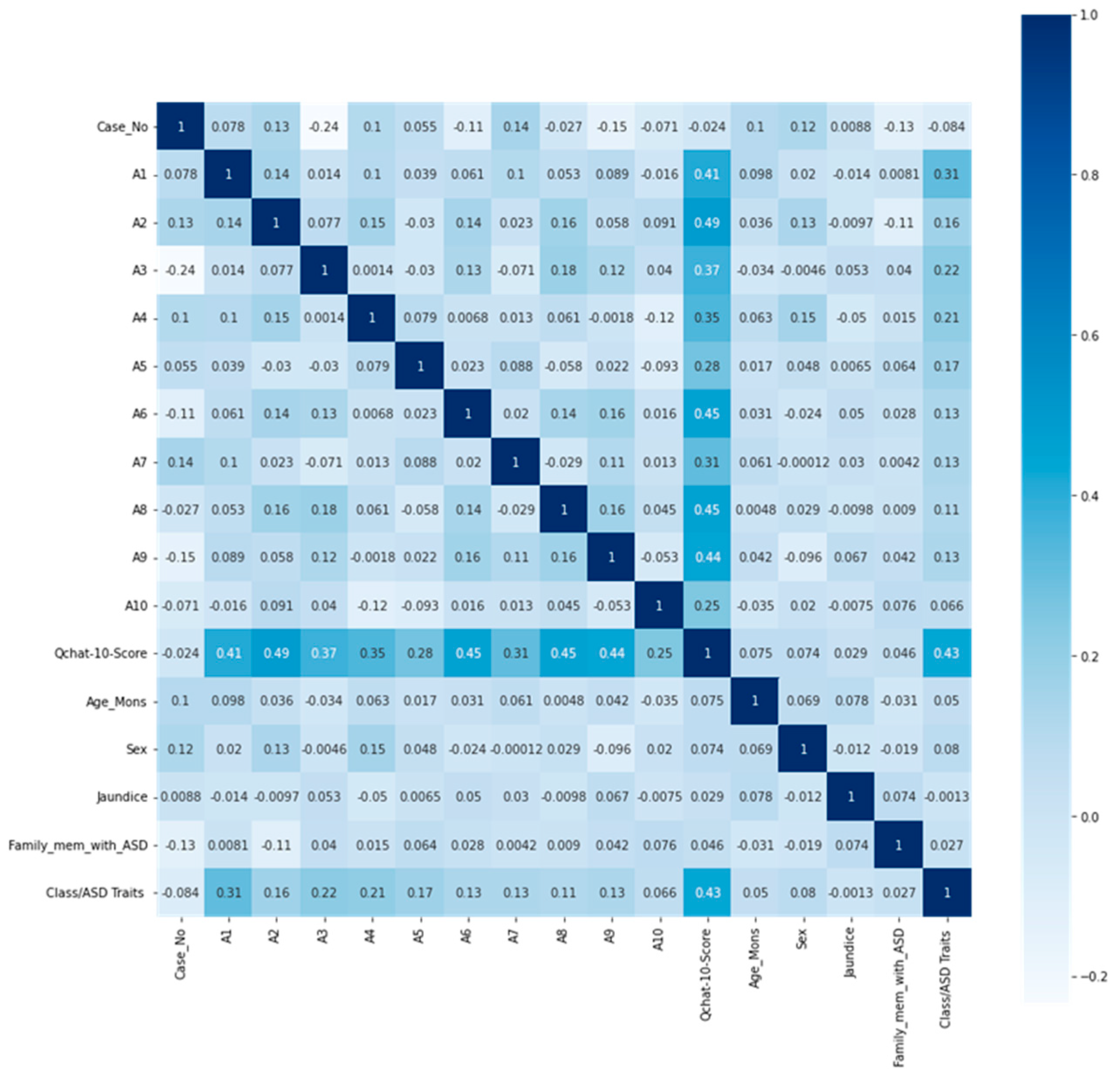
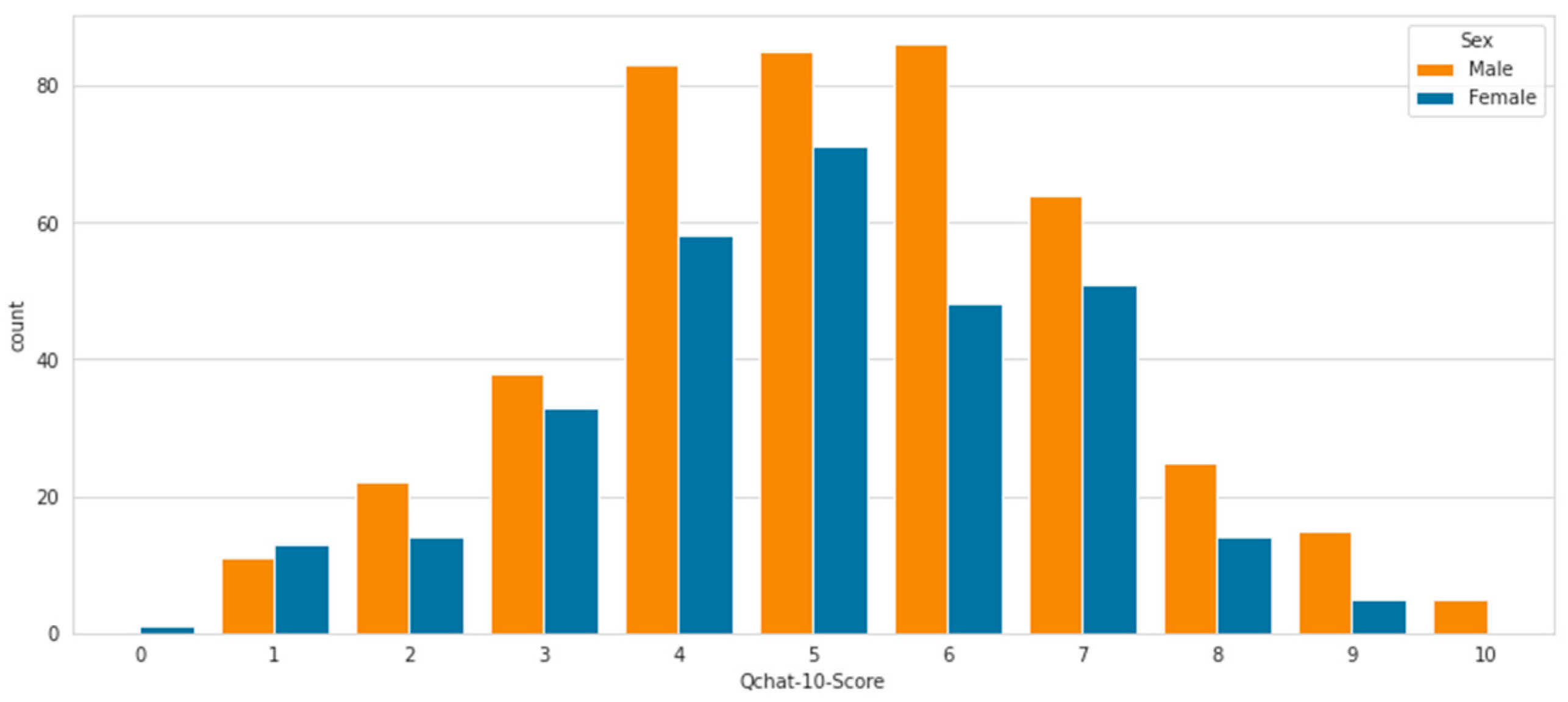
4.1. Data Analysis
4.2. Experimental Results
4.3. Verification of the Experiment
5. Comparative Analysis
6. Discussion
7. Conclusions and Future Detection
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vakadkar, K.; Purkayastha, D.; Krishnan, D. Detection of Autism Spectrum Disorder in Children Using Machine Learning Techniques. SN Comput. Sci. 2021, 2, 386. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tanwar, D.P. Deep Analysis of Autism Spectrum Disorder Detection Techniques. In Proceedings of the 2020 International Conference on Intelligent Engineering and Management (ICIEM), London, UK, 17–19 June 2020. [Google Scholar]
- Teja, A.S.; Abhay, A.S.; Mounika, D.; Pujitha, M.V. Autism Spectrum Disorder Detection Techniques. In Proceedings of the 2022 International Conference on Communication, Computing and Internet of Things, IC3IoT, Chennai, India, 10–11 March 2022; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Zwaigenbaum, L.; Brian, J.A.; Ip, A. Early detection for autism spectrum disorder in young children. Paediatr. Child Health 2019, 24, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Salerno, C.; Bravaccio, C.; Ingenito, A.; Sangianantoni, G.; Cantile, T. Autism spectrum disorders and oral health status: Review of the literature. Eur. J. Paediatr. Dent. 2020, 21, 9–12. [Google Scholar] [PubMed]
- Błażewicz, A.; Grywalska, E.; Macek, P.; Mertowska, P.; Mertowski, S.; Wojnicka, J.; Makarewicz, A. Research into the association of cadmium and manganese excretion with thyroid function and behavioral areas in adolescents with autism spectrum disorders. J. Clin. Med. 2022, 11, 579. [Google Scholar] [CrossRef] [PubMed]
- Erkan, U.; Thanh, D.N.H. Autism Spectrum Disorder Detection with Machine Learning Methods. Curr. Psychiatry Res. Rev. 2019, 15, 297–308. [Google Scholar] [CrossRef]
- Zope, V.; Shetty, T.; Dandekar, M.; Devnani, A.; Meghrajani, P. ML based Approaches for Detection and Development of Autism Spectrum Disorder: A Review. In Proceedings of the International Conference on Sustainable Computing and Data Communication Systems, ICSCDS, Erode, India, 7–9 April 2022; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2022; pp. 79–84. [Google Scholar] [CrossRef]
- Tariq, Q.; Daniels, J.; Schwartz, J.N.; Washington, P.; Kalantarian, H.; Wall, D.P.; Saria, S. Mobile detection of autism through machine learning on home video: A development and prospective validation study. PLoS Med. 2018, 15, e1002705. [Google Scholar] [CrossRef] [PubMed]
- Omar, K.S.; Mondal, P.; Khan, N.S.; Rizvi, R.K.; Islam, N. A Machine Learning Approach to Predict Autism Spectrum Disorder. In Proceedings of the 2019 International Conference on Electrical, Computer and Communication Engineering (ECCE), Cox’s Bazar, Bangladesh, 7–9 February 2019; IEEE: Piscataway, NJ, USA, 2019. [Google Scholar]
- Roman-Urrestarazu, A.; Yáñez, C.; López-Garí, C.; Elgueta, C.; Allison, C.; Brayne, C.; Troncoso, M.; Baron-Cohen, S. Autism screening and conditional cash transfers in Chile: Using the Quantitative Checklist (Q-CHAT) for early autism detection in a low resource setting. Autism 2021, 25, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Damico, S.J.; Müller, N.; Ball, M.J.; Prelock, P.A. The Handbook of Language and Speech Disorders, 2nd ed.; Edited Autism Spectrum Disorders; Wiley-Blackwell: Hoboken, NJ, USA, 2021. [Google Scholar]
- Ahmed, I.A.; Senan, E.M.; Rassem, T.H.; Ali, M.A.H.; Shatnawi, H.S.A.; Alwazer, S.M.; Alshahrani, M. Eye Tracking-Based Diagnosis and Early Detection of Autism Spectrum Disorder Using Machine Learning and Deep Learning Techniques. Electronics 2022, 11, 530. [Google Scholar] [CrossRef]
- Raj, S.; Masood, S. Analysis and Detection of Autism Spectrum Disorder Using Machine Learning Techniques. In Procedia Computer Science; Elsevier B.V.: Amsterdam, The Netherlands, 2020; pp. 994–1004. [Google Scholar] [CrossRef]
- Hemu, A.A.; Mim, R.B.; Ali, M.; Nayer; Ahmed, K.; Bui, F.M. Identification of Significant Risk Factors and Impact for ASD Prediction among Children Using Machine Learning Approach. In Proceedings of the 2022 2nd International Conference on Advances in Electrical, Computing, Communication and Sustainable Technologies, ICAECT 2022, Bhilai, India, 20–21 April 2022; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Nogay, H.S.; Adeli, H. Machine learning (ML) for the diagnosis of autism spectrum disorder (ASD) using brain imaging. Rev. Neurosci. 2020, 31, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Zaman, N.; Ferdus, J.; Sattar, A. Autism Spectrum Disorder Detection Using Machinelearning Approach. In Proceedings of the 2021 12th International Conference on Computing Communication and Networking Technologies, ICCCNT 2021, Kharagpur, India, 6–8 July 2021; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2021. [Google Scholar] [CrossRef]
- McCarty, P.; Frye, R.E. Early Detection and Diagnosis of Autism Spectrum Disorder: Why Is It So Difficult? Semin. Pediatr. Neurol. 2020, 35, 100831. [Google Scholar] [CrossRef] [PubMed]
- Hossain, D.; Kabir, M.A.; Anwar, A.; Islam, Z. Detecting autism spectrum disorder using machine learning techniques: An experimental analysis on toddler, child, adolescent and adult datasets. Health Inf. Sci. Syst. 2021, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.; Ali, M.H.; Satu, S.; Hasan, K.F.; Moni, M.A. Efficient Machine Learning Models for Early Stage Detection of Autism Spectrum Disorder. Algorithms 2022, 15, 166. [Google Scholar] [CrossRef]
- Rahman, M.; Usman, O.L.; Muniyandi, R.C.; Sahran, S.; Mohamed, S.; A Razak, R. A review of machine learning methods of feature selection and classification for autism spectrum disorder. Brain Sci. 2020, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Romero-García, R.; Martínez-Tomás, R.; Pozo, P.; de la Paz, F.; Sarriá, E. Q-CHAT-NAO: A robotic approach to autism screening in toddlers. J. Biomed. Inform. 2021, 118, 103797. [Google Scholar] [CrossRef] [PubMed]
- Alsuliman, M.; Al-Baity, H.H. Efficient Diagnosis of Autism with Optimized Machine Learning Models: An Experimental Analysis on Genetic and Personal Characteristic Datasets. Appl. Sci. 2022, 12, 3812. [Google Scholar] [CrossRef]
- Tejaswi, K.S.; Meghavarshini, K.; Nivedhitha, P. Autism Prediction using ML Algorithms. In Proceedings of the 2022 1st International Conference on Computational Science and Technology (ICCST), Chennai, India, 9–10 November 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Vishal, V.; Singh, A.; Jinila, Y.; C, K.; Shyry, S.; Jabez, J. A Comparative Analysis of Prediction of Autism Spectrum Disorder (ASD) using Machine Learning. In Proceedings of the 2022 6th International Conference on Trends in Electronics and Informatics, ICOEI, Tirunelveli, India, 28–30 April 2022; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2022; pp. 1355–1358. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; text rev.; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Marlow, M.; Servili, C.; Tomlinson, M. A review of screening tools for the identification of autism spectrum disorders and developmental delay in infants and young children: Recommendations for use in low- and middle-income countries. Autism Res. 2019, 12, 176–199. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.A.T.; Jadhav, M.E. A Review of Early Detection of Autism Based on Eye-Tracking and Sensing Technology. In Proceedings of the 5th International Conference on Inventive Computation Technologies, ICICT 2020, Coimbatore, India, 26–28 February 2020; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2020; pp. 160–166. [Google Scholar] [CrossRef]
- Jacob, S.G.; Sulaiman, M.M.B.A.; Bennet, B.; Gastaldo, P. Feature Signature Discovery for Autism Detection: An Automated Machine Learning Based Feature Ranking Framework. Comput. Intell. Neurosci. 2023, 2023, 6330002. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Akter, T.; Zakir, S.; Sabreen, S.; Hossain, M.I. Autism Spectrum Disorder Detection in Toddlers for Early Diagnosis Using Machine Learning. In Proceedings of the 2020 IEEE Asia-Pacific Conference on Computer Science and Data Engineering, CSDE 2020, Gold Coast, Australia, 16–18 December 2020; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Stevanović, D. Quantitative Checklist for Autism in Toddlers (Q-CHAT): A psychometric study with Serbian Toddlers. Res. Autism Spectr. Disord. 2021, 83, 101760. [Google Scholar] [CrossRef]
- Waring, J.; Lindvall, C.; Umeton, R. Automated machine learning: Review of the state-of-the-art and opportunities for healthcare. Artif. Intell. Med. 2020, 104, 101822. [Google Scholar] [CrossRef] [PubMed]
- Hutter, F.; Kotthoff, L.; Vanschoren, J. Automated Machine Learning: Methods, Systems, Challenges; Springer Nature: Berlin/Heidelberg, Germany, 2019; p. 219. [Google Scholar]
- Thabtah, F. An accessible and efficient autism screening method for behavioural data and predictive analyses. Health Inform. J. 2019, 25, 1739–1755. [Google Scholar] [CrossRef] [PubMed]
- Ruta, L.; Chiarotti, F.; Arduino, G.M.; Apicella, F.; Leonardi, E.; Maggio, R.; Carrozza, C.; Chericoni, N.; Costanzo, V.; Turco, N.; et al. Validation of the Quantitative CHecklist for Autism in Toddlers (Q-CHAT) in an Italian clinical sample of young children with Autism and Other Developmental Disorders. Front. Psychiatry 2019, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Tartarisco, G.; Cicceri, G.; Di Pietro, D.; Leonardi, E.; Aiello, S.; Marino, F.; Chiarotti, F.; Gagliano, A.; Arduino, G.M.; Apicella, F.; et al. Use of machine learning to investigate the quantitative checklist for autism in toddlers (Q-CHAT) towards early autism screening. Diagnostics 2021, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiecka, A.; Pisula, E. Symptoms of Autism Spectrum Disorders Measured by the Qualitative Checklist for Autism in Toddlers in a Large Sample of Polish Toddlers. Int. J. Environ. Res. Public Health 2022, 19, 3072. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, N.; Bukhari, F.; Iqbal, W. Predictive Analysis of Autism Spectrum Disorder (ASD) using Machine Learning. In Proceedings of the 2021 International Conference on Frontiers of Information Technology, FIT 2021, Islamabad, Pakistan, 13–14 December 2021; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2021; pp. 305–310. [Google Scholar] [CrossRef]
- Truong, A.; Walters, A.; Goodsitt, J.; Hines, K.; Bruss, C.B.; Farivar, R. Towards automated machine learning: Evaluation and comparison of AutoML approaches and tools. In Proceedings of the 2019 IEEE 31st International Conference on Tools with Artificial Intelligence (ICTAI), Portland, OR, USA, 4–6 November 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1471–1479. [Google Scholar]
- Olson, R.S.; Moore, J.H. TPOT: A tree-based pipeline optimization tool for automating machine learning. In Workshop on Automatic Machine Learning; PMLR: New York, NY, USA, 2016; pp. 66–74. [Google Scholar]
- Sherkatghanad, Z.; Akhondzadeh, M.; Salari, S.; Zomorodi-Moghadam, M.; Abdar, M.; Acharya, U.R.; Khosrowabadi, R.; Salari, V. Automated detection of autism spectrum disorder using a convolutional neural network. Front. Neurosci. 2020, 13, 1325. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.G.; Sulaiman, M.M.B.A.; Bennet, B. Algorithmic approaches to classify autism spectrum disorders: A research perspective. Procedia Comput. Sci. 2022, 201, 470–477. [Google Scholar] [CrossRef]
- Elshoky, B.R.G.; Younis, E.M.G.; Ali, A.A.; Ibrahim, O.A.S. Comparing automated and non-automated machine learning for autism spectrum disorders classification using facial images. ETRI J. 2022, 44, 613–623. [Google Scholar] [CrossRef]
- Dafflon, J.; Pinaya, W.H.L.; Turkheimer, F.; Cole, J.H.; Leech, R.; Harris, M.A.; Cox, S.R.; Whalley, H.C.; McIntosh, A.M.; Hellyer, P.J. An automated machine learning approach to predict brain age from cortical anatomical measures. Hum. Brain Mapp. 2020, 41, 3555–3566. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.T.; Sultan, K.; Sheraz, M.; Chuah, T.C. A Comparative Analysis of Automated Machine Learning Tools: A Use Case for Autism Spectrum Disorder Detection. Information 2024, 15, 625. [Google Scholar] [CrossRef]
- Allison, C.; Baron-Cohen, S.; Wheelwright, S.; Charman, T.; Richler, J.; Pasco, G.; Brayne, C. The Q-CHAT (Quantitative CHecklist for Autism in Toddlers): A normally distributed quantitative measure of autistic traits at 18–24 months of age: Preliminary report. J. Autism Dev. Disord. 2008, 38, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Allison, C.; Auyeung, B.; Baron-Cohen, S. Toward brief “red flags” for autism screening: The short Autism Spectrum Quotient and the short Quantitative Checklist in 1000 cases and 3000 controls. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 202–212.e7. [Google Scholar] [CrossRef] [PubMed]



| Ref. Year | Key Findings | Limitations |
|---|---|---|
| [1] 2021 | An automated model that supports medical professionals identify ASD was presented in this study. | The limitations are the unavailability of open-source datasets coupled to ASD. |
| [7] 2019 | Three datasets for youngsters, teenagers, and grown-ups with ASD were employed to classify ASD using the SVM, KNN, and RF algorithms. | Unavailability of a complete dataset related to ASD. |
| [35] 2019 | In this research, the ASDTests app was proposed for data collection and assisting health professionals in ASD detection. | It was not possible to conduct feature analysis using the app. |
| [36] 2019 | An ML approach was used on a dataset collected by authors Q-CHAT with 25 items was evaluated. | The sample size was relatively small and not open-source. |
| [37] 2021 | The study assessed multiple machine learning methods on four datasets to classify autism spectrum disorder. SVM and KNN emerged as the most effective techniques for accurate classification | The study did not investigate the influence of demographic factors (age, gender, race/ethnicity) on machine learning accuracy |
| [42] 2020 | In this paper, the automated detection system using a convolutional neural network (CNN) achieved high accuracy in detecting ASD in children based on their functional magnetic resonance imaging (fMRI) data. | This study was conducted on a relatively small sample size of 40 participants (20 with ASD and 20 typically developing controls). Further, the study only used dormant-state fMRI data. |
| [43] 2022 | In this study, various works on algorithmic approaches to classify ASD were presented, e.g., one study showed that the multichannel deep attention neural network (DANN) performed better than support vector machines (SVMs). | This study did not explore the detailed ethical implications of using algorithmic approaches in the diagnosis and treatment of ASD. |
| [44] 2022 | This study showed automated methods. This study outperformed non-automated ones in accuracy, sensitivity, and specificity. Combining machine learning techniques enhanced ASD diagnosis accuracy. | This study only focused on facial images as a diagnostic tool and did not consider other potential sources of information |
| [45] 2020 | The research revealed that employing automated machine learning techniques enables precise forecasting of brain age based on cortical anatomical measurements | The study was conducted on a proportionate small sample size |
| [41] 2016 | The study found that the Tree-based Pipeline Optimization Tool (TPOT) autonomously outperforms basic machine learning without human input on benchmarks. | The study used supervised classification benchmarks; further research is needed to assess TPOT’s performance on different ML problems |
| [46] 2024 | The study compares the AUTOML tools TPOT and KNIME for ASD detection. | The study did not compare other available AUTOML tools. |
| Dataset Variables | Description of Q-CHAT-10 DATASET FEATURES |
|---|---|
| α1 | Does your child show attention by turning toward you when you call their name? |
| α2 | How easily can you make direct eye contact with your child? |
| α3 | Does your child use gestures to ask for things he wants, such as a toy that is out of his reach? |
| α4 | Does your child point to objects or events to draw others’ attention or express excitement? |
| α5 | Does your child engage in imaginative activities, such as pretending to feed a doll or talk to a toy telephone? |
| α6 | Does your child follow another person’s gaze or look in the same direction when someone else is looking at something? |
| α7 | When a family member seems upset or distressed, does your child attempt to comfort them, for example by offering physical contact or affection? |
| α8 | How would you characterize your child’s early verbal communication? |
| α9 | Does your child use simple nonverbal gestures, such as waving to say goodbye? |
| α10 | Does your child often stare at objects or into space for extended periods without a clear purpose? |
| Dataset Variables | Data Type | Attribute Description |
|---|---|---|
| Age_Months | Number | Child’s age in months |
| Sex | String | Male/female |
| Jaundice | Boolean (Yes/No) | Whether the child was born with jaundice |
| Family_mem_with_ASD | Boolean (Yes/No) | Any family member diagnosed with ASD |
| Who completed the test | String | Parent, caregiver, medical staff, clinician |
| Qchat-10-Score | Integer | Final results based on the scoring function |
| Class/ASD Traits | Boolean | The class label represents whether ASD-related traits are observed, with a value of ‘0’ indicating no traits present and ‘1’ indicating that such traits are present |
| TPOT Best Pipeline Parameters | Values |
|---|---|
| Alpha | 1.0 |
| fit_prior | False |
| Bootstrap | True |
| Criterion | gini |
| max_features | 0.05 |
| min_samples_leaf | 1 |
| min_samples_split | 4 |
| n_estimators | 100 |
| Experiment | Precision | Recall | F1-Score | Support |
|---|---|---|---|---|
| Non-autistic | 0.68 | 0.53 | 0.60 | 43 |
| Autistic | 0.83 | 0.90 | 0.86 | 106 |
| Macro avg | 0.75 | 0.72 | 0.73 | 149 |
| Weighted avg | 0.78 | 0.79 | 0.78 | 149 |
| Accuracy | - | - | - | 0.83 |
| Verification | Precision | Recall | F1-Score | Support |
|---|---|---|---|---|
| Non-autistic | 0.68 | 0.53 | 0.60 | 43 |
| Autistic | 0.83 | 0.90 | 0.86 | 106 |
| Macro avg | 0.75 | 0.72 | 0.73 | 149 |
| Weighted avg | 0.78 | 0.79 | 0.78 | 149 |
| Accuracy | - | - | - | 0.83 |
| Model | Accuracy | Precision | Recall | F1-Score |
|---|---|---|---|---|
| Support Vector Machine | 0.71 | 0.718 | 1.000 | 0.836 |
| K-Nearest Neighbour | 0.74 | 0.785 | 0.888 | 0.833 |
| Naive Bayes | 0.80 | 0.847 | 0.887 | 0.866 |
| Logistic Regression | 0.757 | 0.805 | 0.875 | 0.838 |
| Decision Tree | 0.70 | 0.804 | 0.769 | 0.786 |
| Random Forest | 0.780 | 0.821 | 0.888 | 0.854 |
| AUTOML | 0.83 | 0.868 | 0.868 | 0.868 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehsan, K.; Sultan, K.; Fatima, A.; Sheraz, M.; Chuah, T.C. Early Detection of Autism Spectrum Disorder Through Automated Machine Learning. Diagnostics 2025, 15, 1859. https://doi.org/10.3390/diagnostics15151859
Ehsan K, Sultan K, Fatima A, Sheraz M, Chuah TC. Early Detection of Autism Spectrum Disorder Through Automated Machine Learning. Diagnostics. 2025; 15(15):1859. https://doi.org/10.3390/diagnostics15151859
Chicago/Turabian StyleEhsan, Khafsa, Kashif Sultan, Abreen Fatima, Muhammad Sheraz, and Teong Chee Chuah. 2025. "Early Detection of Autism Spectrum Disorder Through Automated Machine Learning" Diagnostics 15, no. 15: 1859. https://doi.org/10.3390/diagnostics15151859
APA StyleEhsan, K., Sultan, K., Fatima, A., Sheraz, M., & Chuah, T. C. (2025). Early Detection of Autism Spectrum Disorder Through Automated Machine Learning. Diagnostics, 15(15), 1859. https://doi.org/10.3390/diagnostics15151859








