Dynamic URP: Revisiting Urethral Retro-Resistance Pressure for Contemporary Sphincter-Targeted Therapy
Abstract
1. Introduction
2. Functional Anatomy of the External Urethral Sphincter Complex
2.1. Structural Composition
- Sphincter urethrae (rhabdosphincter): A circular muscle embedded within the urethral wall, forming the intrinsic component responsible for tonic closure during bladder filling.
- Compressor urethrae: A strap-like muscle arising bilaterally from the ischiopubic rami and coursing ventrally over the urethra to compress it against the anterior vaginal wall.
- Urethrovaginal sphincter: A sling-like muscle encircling both the urethra and the anterior vaginal wall at the distal urethra, compressing the opening of the vagina and urethra against the pubic bone.
2.2. Histological and Fiber Type Composition
2.3. Neurologic Control of the EUS Complex
3. Clinical and Diagnostic Implications
3.1. The Role of the External Urethral Sphincter (EUS) in Passive Continence and Voiding
3.2. The EUS Dual-Component System Model
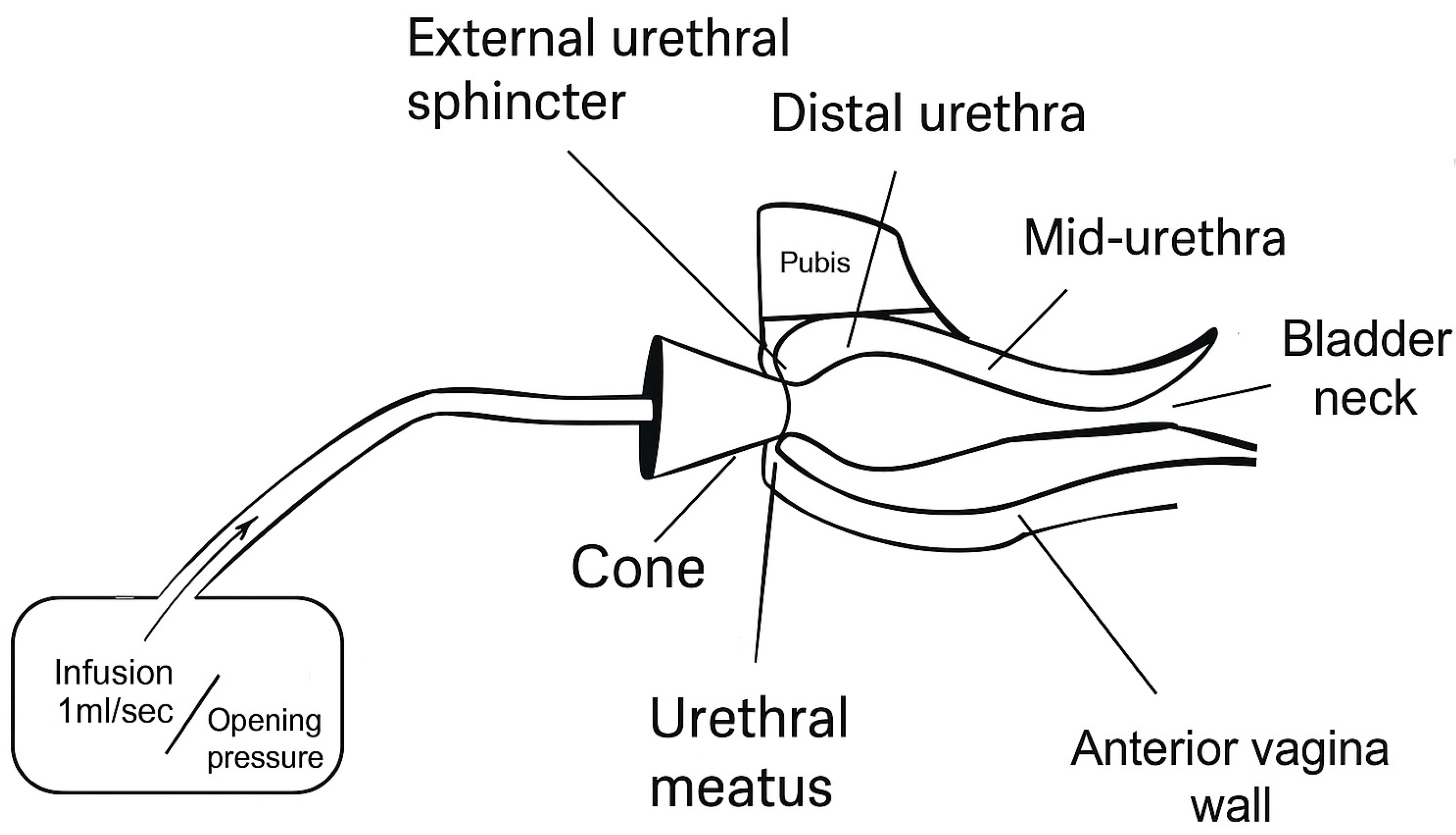
3.3. Quantifying EUS Function
3.4. Therapeutic Applications of Dynamic URP
4. Future Clinical Applications
4.1. Intraoperative Biomarker
4.2. Diagnostic Benchmark
4.3. Artificial Intelligence
4.4. Standardizing Continence Outcomes
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minassian, V.A.; Stewart, W.F.; Wood, G.C. Urinary incontinence in women: Variation in prevalence estimates and risk factors. Obstet. Gynecol. 2008, 111 Pt 1, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Vaughan, C.P.; Goode, P.S.; Redden, D.T.; Burgio, K.L.; Richter, H.E.; Markland, A.D. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet. Gynecol. 2014, 123, 141–148. [Google Scholar] [CrossRef] [PubMed]
- DeLancey, J.O.L. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am. J. Obstet. Gynecol. 1994, 170, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Ahn, H.K.; Huh, Y. Clinical and functional anatomy of the urethral sphincter. Int. Neurourol. J. 2012, 16, 102–106. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, K.D.; Kanai, A.; Panicker, J.N.; Hashitani, H.; Fry, C.H. What do we really know about the external urethral sphincter? Continence 2024, 10, 101223. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, G.; Salciccia, S.; Viscuso, P.; Bevilacqua, G.; Casale, P.; Frisenda, M.; Di Pierro, G.B.; Cattarino, S.; Gentilucci, A.; Rosati, D.; et al. Regenerative medicine-based treatment of stress urinary incontinence with mesenchymal stem cells: A systematic review and meta-analysis. Curr. Stem Cell Res. Ther. 2023, 18, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E.; Williams, K. Cellular regenerative therapy in stress urinary incontinence: New frontiers?—A narrative review. Transl. Androl. Urol. 2024, 13, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, G.; Fu, S.; Hu, Y.; Wang, Q.; Zhai, L.; Yu, R. Three-dimensional measurement and analysis of the compressor urethrae and urethra in postpartum women. Transl. Androl. Urol. 2025, 14, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Oelrich, T.M. The striated urogenital sphincter muscle in the female. Anat. Rec. 1983, 205, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.A.; Pretorius, D.H.; Padda, B.S.; Weinstein, M.M.; Nager, C.W.; den Boer, D.J.; Mittal, R.K. Vaginal high-pressure zone assessed by dynamic 3-dimensional ultrasound images of the pelvic floor. Am. J. Obstet. Gynecol. 2007, 197, 52.e1–52.e7. [Google Scholar] [CrossRef] [PubMed]
- Gosling, J.A.; Dixon, J.S.; Critchley, H.O.; Thompson, S.A. A comparative study of the human external sphincter and periurethral levator ani muscles. Br. J. Urol. 1981, 53, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Wallner, C.; Dabhoiwala, N.F.; DeRuiter, M.C.; Lamers, W.H. The anatomical components of urinary continence. Eur. Urol. 2009, 55, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Thor, K.B.; Morgan, C.; Nadelhaft, I.; Houston, M.; de Groat, W.C. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J. Comp. Neurol. 1989, 288, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M. Membrane properties of external urethral and external anal sphincter motoneurones in the cat. J Physiol. 1991, 440, 345–366. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Griffiths, D.; de Groat, W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hokanson, J.A.; Keast, J.R. Advancing our understanding of the neural control of the female human urethra. Neurourol. Urodyn. 2022, 41, 35. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.J.; Constantinou, C.E.; van Mastrigt, R. Urinary bladder function and its control in healthy females. Am. J. Physiol. 1986, 251 Pt 2, R225–R230. [Google Scholar] [CrossRef] [PubMed]
- Kenton, K.; Fitzgerald, M.P.; Brubaker, L. Striated urethral sphincter activity does not alter urethral pressure during filling cystometry. Am. J. Obstet. Gynecol. 2005, 192, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Panicker, J.N.; Seth, J.H.; Khan, S.; Roosen, A.; Glover, L.; Lucas, C.; Fowler, C.J. Open-label study evaluating outpatient urethral sphincter injections of onabotulinumtoxinA to treat women with urinary retention due to a primary disorder of sphincter relaxation (Fowler’s Syndrome). BJU Int. 2016, 117, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, M.B.; Yoshimura, N. Neurophysiology of stress urinary incontinence. Rev. Urol. 2004, 6 (Suppl. S3), S19–S28. [Google Scholar] [PubMed]
- Petros, P.E. New ambulatory surgical methods using an anatomical classification of urinary dysfunction improve stress, urge and abnormal emptying. Int. Urogynecol. J. Pelvic Floor Dysfunct. 1997, 8, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Thor, K.B.; Donatucci, C. Central nervous system control of the lower urinary tract: New pharmacological approaches to stress urinary incontinence in women. J. Urol. 2004, 172, 27–33. [Google Scholar] [CrossRef] [PubMed]
- de Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327–396. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Takahashi, S.; Ukimura, O. Urethra actively opens from the very beginning of micturition: A new concept of urethral function. Int. J. Urol. 2014, 21, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Deindl, F.M.; Vodusek, D.B.; Bischoff, C.; Hofmann, R.; Hartung, R. Dysfunctional voiding in women: Which muscles are responsible? Br. J. Urol. 1998, 82, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Rud, T.; Andersson, K.E.; Asmussen, M.; Ulmsten, U. Factors maintaining the intraurethral pressure in women. Investig. Urol. 1980, 17, 343–347. [Google Scholar]
- Ashton-Miller, J.A.; DeLancey, J.O. Functional anatomy of the female pelvic floor. Ann. N. Y. Acad. Sci. 2007, 1101, 266–296. [Google Scholar] [CrossRef] [PubMed]
- Venema, P.L.; Kramer, G.; van Koeveringe, G.A.; Heesakkers, J.P.F.A. The maximal urethral pressure at rest and during normal bladder filling is only determined by the activity of the urethral smooth musculature in the female. J. Clin. Med. 2023, 12, 2575. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.C.; O′Leary, D.E.; Quiroz, L.H.; Shobeiri, S.A. Is there a correlation between levator ani and urethral sphincter complex status on 3D ultrasonography? Int. Urogynecol. J. 2015, 26, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Hokanson, J.A.; DeLancey, J.O.L. Urethral failure is a critical factor in female urinary incontinence. Now what? Neurourol. Urodyn. 2022, 41, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Wasenda, E.J.; Kirby, A.C.; Lukacz, E.S.; Nager, C.W. The female continence mechanism measured by high resolution manometry: Urethral bulking versus midurethral sling. Neurourol. Urodyn. 2018, 37, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.M. Is urethral pressure profilometry a useful diagnostic test for stress urinary incontinence? Obstet. Gynecol. Surv. 2001, 56, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Saaby, M.-L. The urethral closure function in continent and stress urinary incontinent women assessed by Urethral Pressure Reflectometry. Dan. Med. J. 2014, 61, B4795. [Google Scholar] [PubMed]
- Slack, M.; Culligan, P.; Tracey, M.; Hunsicker, K.; Patel, B.; Sumeray, M. Urethral retro-resistance pressure: A new clinical measure of urethral function. Neurourol. Urodyn. 2004, 23, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Digesu, G.A.; Athanasiou, S.; Chaliha, C.; Khullar, V. Urethral retro-resistance pressure and urodynamic diagnoses in women with lower urinary tract symptoms. BJOG 2005, 112, 334–338. [Google Scholar]
- Kuhn, A.; Kuhn, P.; Dreher, E. The correlation of urethral resistance pressure with maximum urethral closure pressure and stress incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Thind, P.; Lose, G.; Colstrup, H.; Andersson, K.E. The urethral resistance to rapid dilatation: An analysis of the effect of autonomic receptor stimulation and blockade and of pudendal nerve blockade in healthy females. Scand. J. Urol. Nephrol. 1995, 29, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chaliha, C.; Digesu, G.A.; Salvatore, S.; Khullar, V.; Athanasiou, S. Changes in urethral resistance in the presence of detrusor activity. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2006, 17, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Klarskov, N.; Lose, G. Urethral pressure reflectometry; a novel technique for simultaneous recording of pressure and cross-sectional area in the female urethra. Neurourol. Urodyn. 2007, 26, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Roderick, T.; Paul, M.; Christopher, M.; Douglas, T. Urethral retro-resistance pressure: Association with established measures of incontinence severity and change after midurethral tape insertion. Neurourol. Urodyn. 2009, 28, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Petros, P.E. Re: Slack M. Culligan P, Tracey M, Hunsicker K, Patel B, Sumeray M. 2004. Relationship of urethral retro-resistance pressure to urodynamic measurements and incontinence severity. Neurourol. Urodyn. 2005, 24, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Clearwater, W.L.; Panushka, K.; Najor, A.; Laudano, M.; Fleischmann, N. Reconstruction of Urethral Sphincter with Polyacrylamide Hydrogel. Urogynecology 2024, 30, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Yeh, Y.H.; Tsai, L.H.; Chou, E.C. The Efficacy of Transvaginal Ultrasound-Guided BoNT-A External Sphincter Injection in Female Patients with Underactive Bladder. Toxins 2023, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.A.; Williams, J.K.; Kessler, T.M.; Stenzl, A.; Aicher, W.K.; Andersson, K.-E.; Eberli, D. Treatment of stress urinary incontinence with muscle stem cells and stem cell components: Chances, challenges and future prospects. Int. J. Mol. Sci. 2021, 22, 3981. [Google Scholar] [CrossRef] [PubMed]
- Attari, A.; DeLancey, J.O.; Ashton-Miller, J.A. On Structure-Function Relationships in the Female Human Urethra: A Finite Element Model Approach. Ann. Biomed. Eng. 2021, 49, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Chermansky, C.J.; Ockrim, J.L.; Kheir, G.B.; Chapple, C.R.; Kearney, R.; Toia, B.; Dmochowski, R.R.; Wein, A.J.; Abrams, P. Do We Need to Re-Focus on Functional Female Urethral Disorders in Lower Urinary Tract Dysfunction? ICI-RS 2024. Neurourol. Urodyn. 2025, 44, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Abid, R.; Hussein, A.A.; Guru, K.A. Artificial Intelligence in Urology: Current Status and Future Perspectives. Urol. Clin. N. Am. 2024, 51, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Seely, A.J.E.; Newman, K.; Ramchandani, R.; Herry, C.; Scales, N.; Hudek, N.; Brehaut, J.; Jones, D.; Ramsay, T.; Barnaby, D.; et al. Roadmap for the evolution of monitoring: Developing and evaluating waveform-based variability-derived artificial intelligence-powered predictive clinical decision support software tools. Crit. Care 2024, 28, 404. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An overview of clinical decision support systems: Benefits, risks, and strategies for success. NPJ Digit. Med. 2020, 3, 17. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleischmann, N. Dynamic URP: Revisiting Urethral Retro-Resistance Pressure for Contemporary Sphincter-Targeted Therapy. Diagnostics 2025, 15, 1855. https://doi.org/10.3390/diagnostics15151855
Fleischmann N. Dynamic URP: Revisiting Urethral Retro-Resistance Pressure for Contemporary Sphincter-Targeted Therapy. Diagnostics. 2025; 15(15):1855. https://doi.org/10.3390/diagnostics15151855
Chicago/Turabian StyleFleischmann, Nicole. 2025. "Dynamic URP: Revisiting Urethral Retro-Resistance Pressure for Contemporary Sphincter-Targeted Therapy" Diagnostics 15, no. 15: 1855. https://doi.org/10.3390/diagnostics15151855
APA StyleFleischmann, N. (2025). Dynamic URP: Revisiting Urethral Retro-Resistance Pressure for Contemporary Sphincter-Targeted Therapy. Diagnostics, 15(15), 1855. https://doi.org/10.3390/diagnostics15151855




