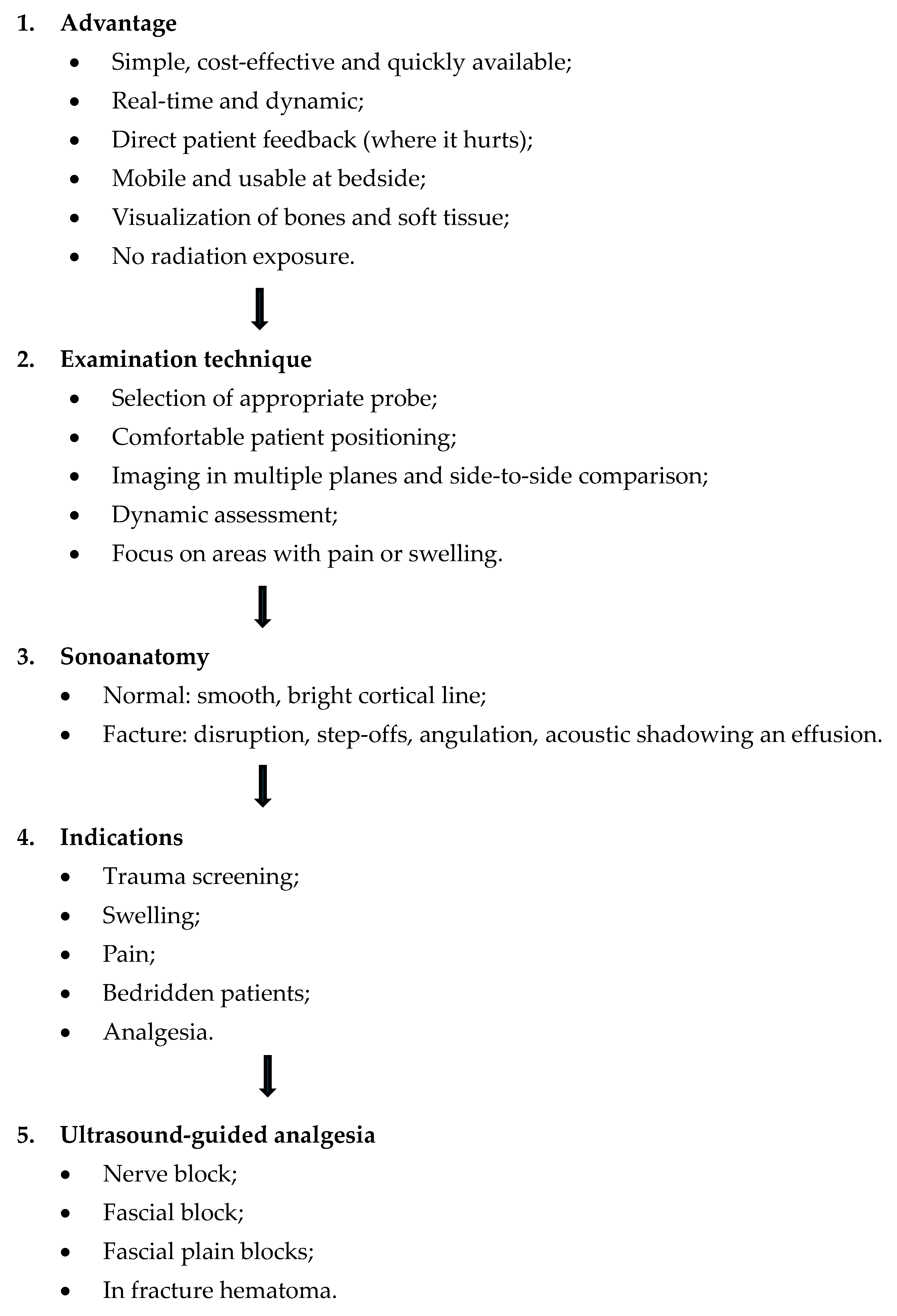
A Plea for a Paradigm Shift from X-Ray to Ultrasound in Adults: An Update for Emergency Physicians, General Practitioners, Orthopedists and Sports Medicine Physicians
Abstract
1. Introduction
- Objectives;
- Indications;
- Sonographic signs of normal anatomy and fractures;
- Examination technique;
- Systematic workflow of ultrasound application in fracture diagnostics and regional pain therapy;
- Limitations and future directions;
- Narrative and visual reviews;
- Clinical case examples—advantages and limitations of ultrasound compared to X-Ray and CT;
- Conclusion.
2. Objectives
- Reduce reliance on X-rays;
- Promote a more rational use of X-rays, CTs and MRIs;
- Reduce ionizing radiation exposure;
- Reduce costs;
- Optimize personnel and infrastructural resources;
- Shorten the length of stay in the emergency department and other waiting areas;
- Improve diagnostic accuracy;
- Increase patient comfort;
- Use in algorithms as, for example, the Ottawa Rule.
3. Indications
- Ultrasound screening in suspected fractures may improve X-ray selection and specificity by integrating ultrasound findings into clinical decision rules like the Ottawa Ankle and Knee Rules—limiting X-ray use to patients with positive ultrasound signs of fracture.
- Evaluation of patients with suspected occult or stress fractures: Ultrasound offers a radiation-free method for early detection, especially when X-rays are negative or inconclusive but clinical suspicion remains high.
- Screening for fractures and hematoma during the primary survey in Advanced Trauma Life Support (ATLS), particularly in polytrauma patients, ultrasound enables rapid identification of fractures that are associated with life-threatening hemorrhage (e.g., pelvic or femoral fractures).
- Detection of instability signs in pelvic and spinal fractures: this is an emerging application where ultrasound can be used to assess dynamic instability or progressive displacement of fractures.
- Rapid exclusion of fractures in shoulder dislocations: ultrasound can be used prior to reduction to quickly rule out associated fractures, facilitating timely and safe management.
- Assessment of bone alignment during and after fracture reduction: ultrasound allows immediate verification of anatomical positioning to confirm adequate reduction.
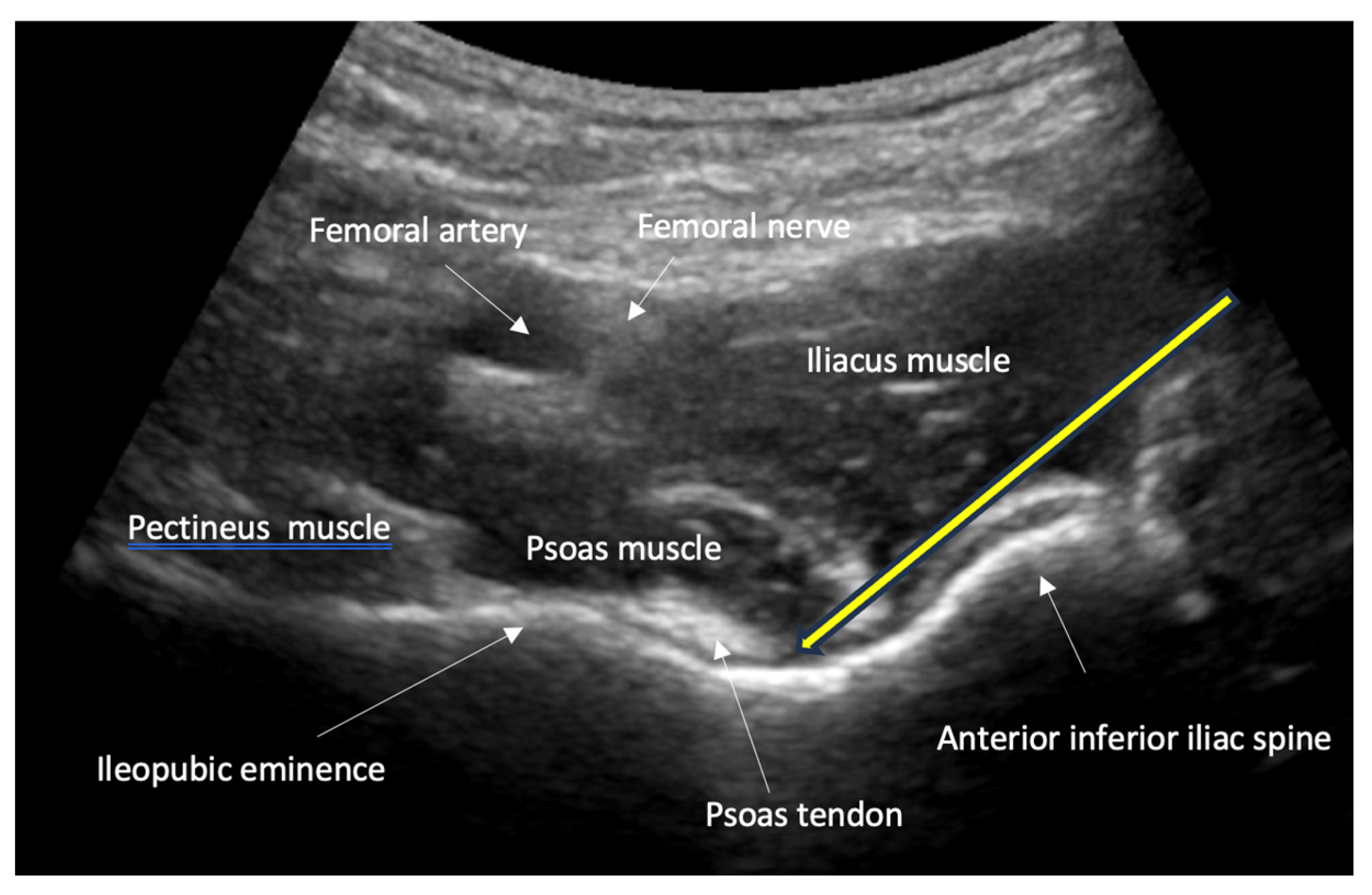
- Guidance of nerve blocks for pain management and regional anesthesia: ultrasound guidance improves the precision of nerve blocks, which are essential for pain control and safe repositioning of fractures.
4. Sonographic Signs of Normal Osseous Anatomy and Fractures
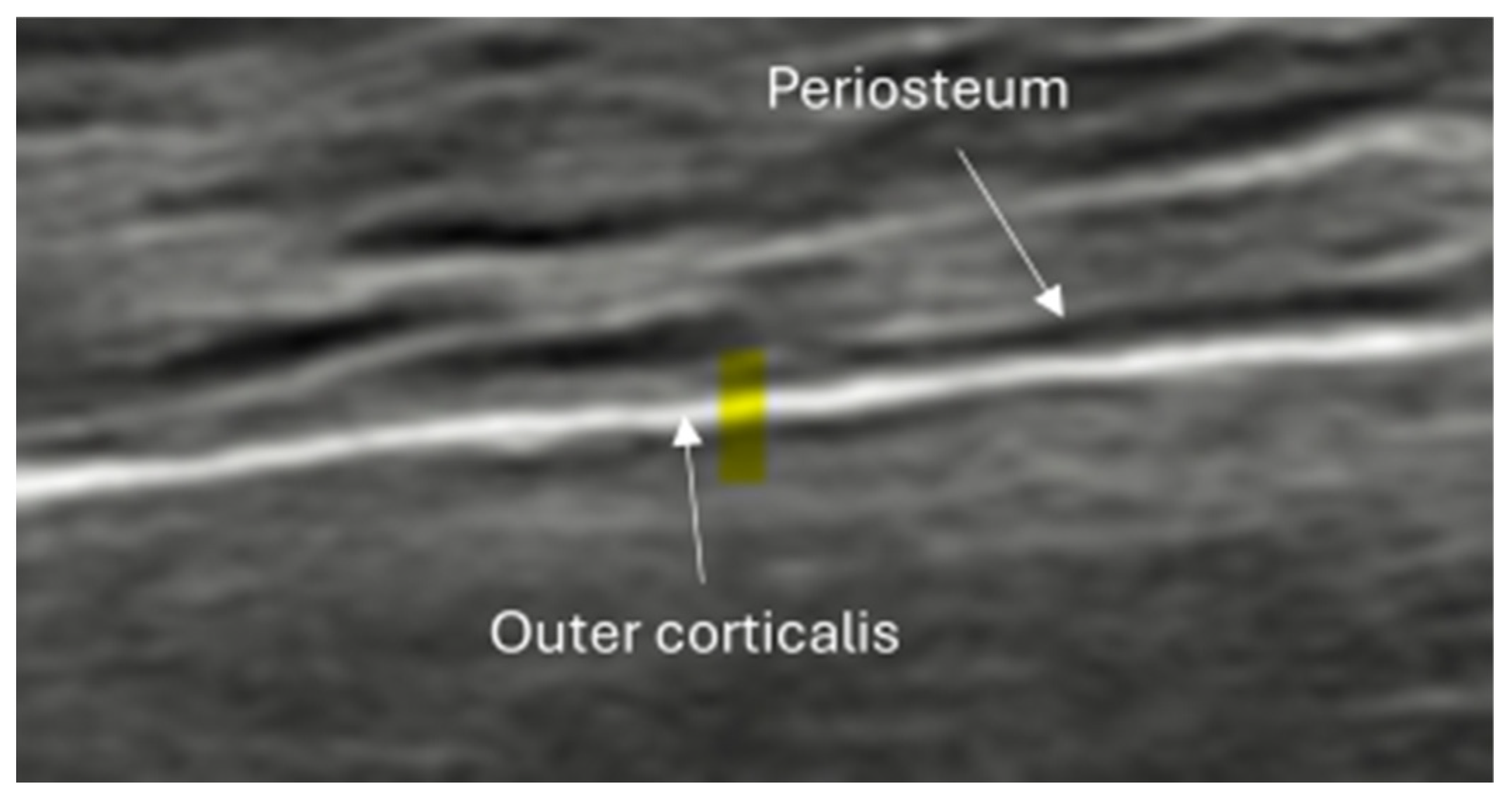
4.1. Basic Structure of Bones and Normal Anatomy
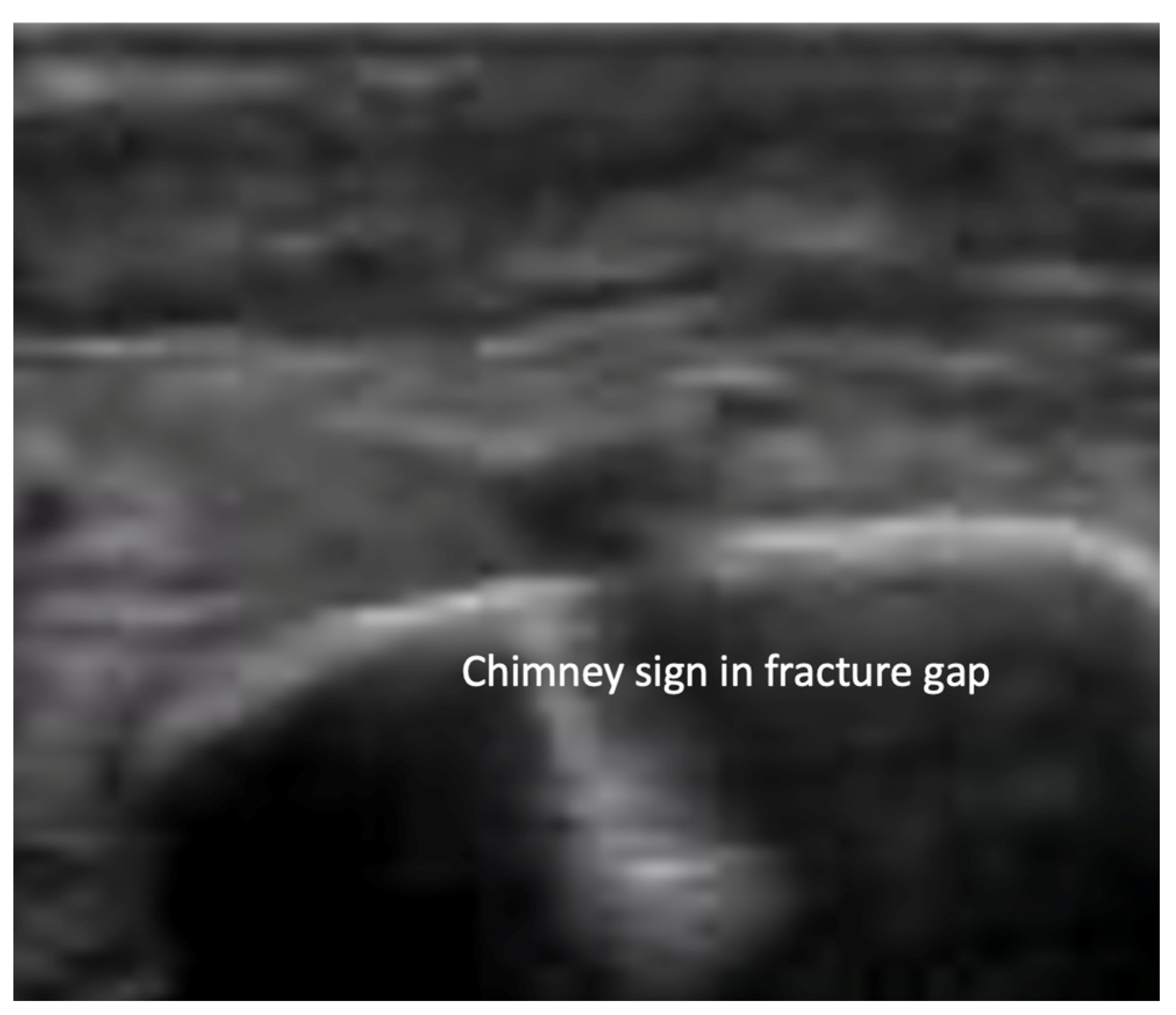
4.2. Sonoanatomy of Fractures [Figure 5, Figure 6, Figure 7 and Figure 8]
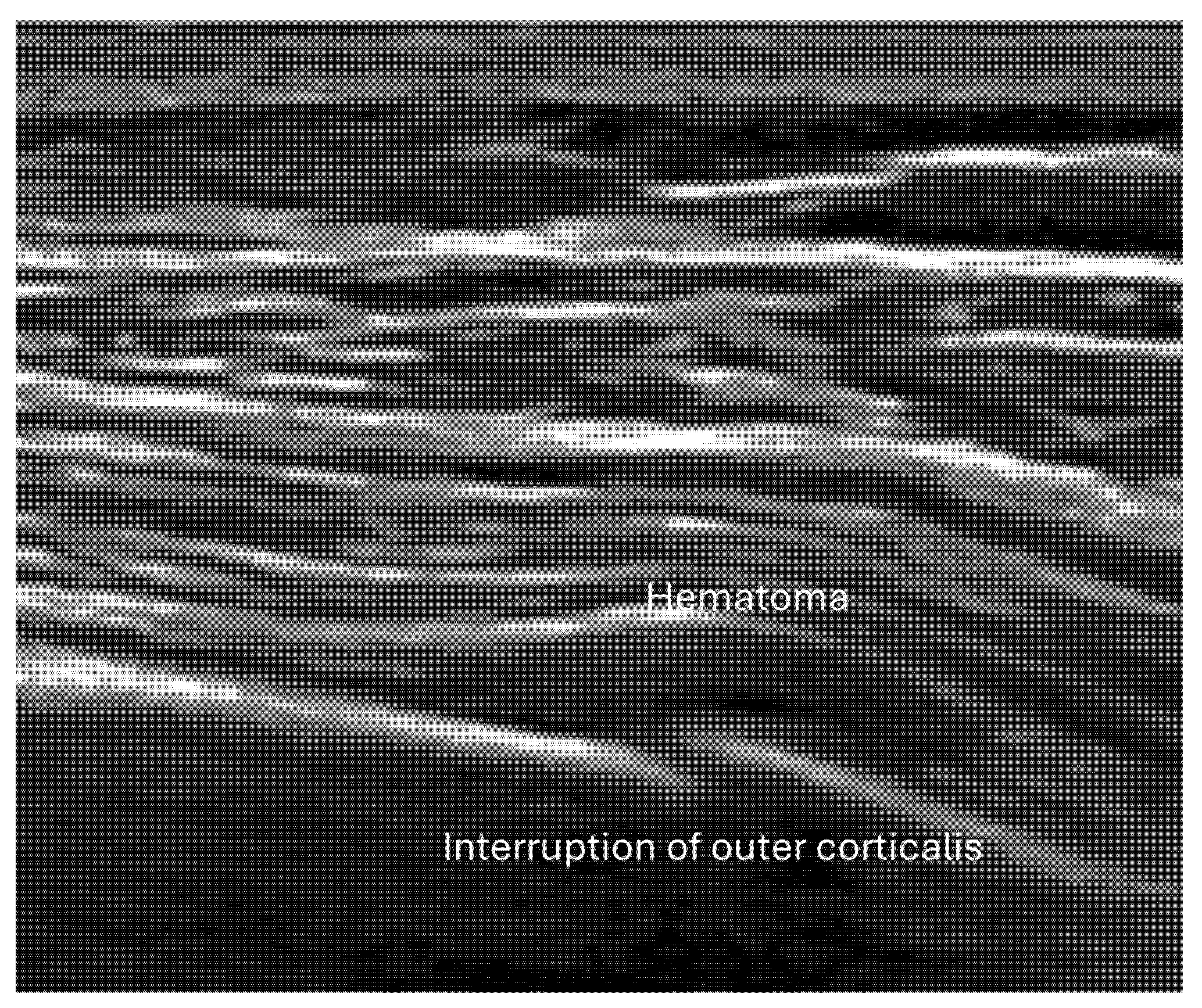

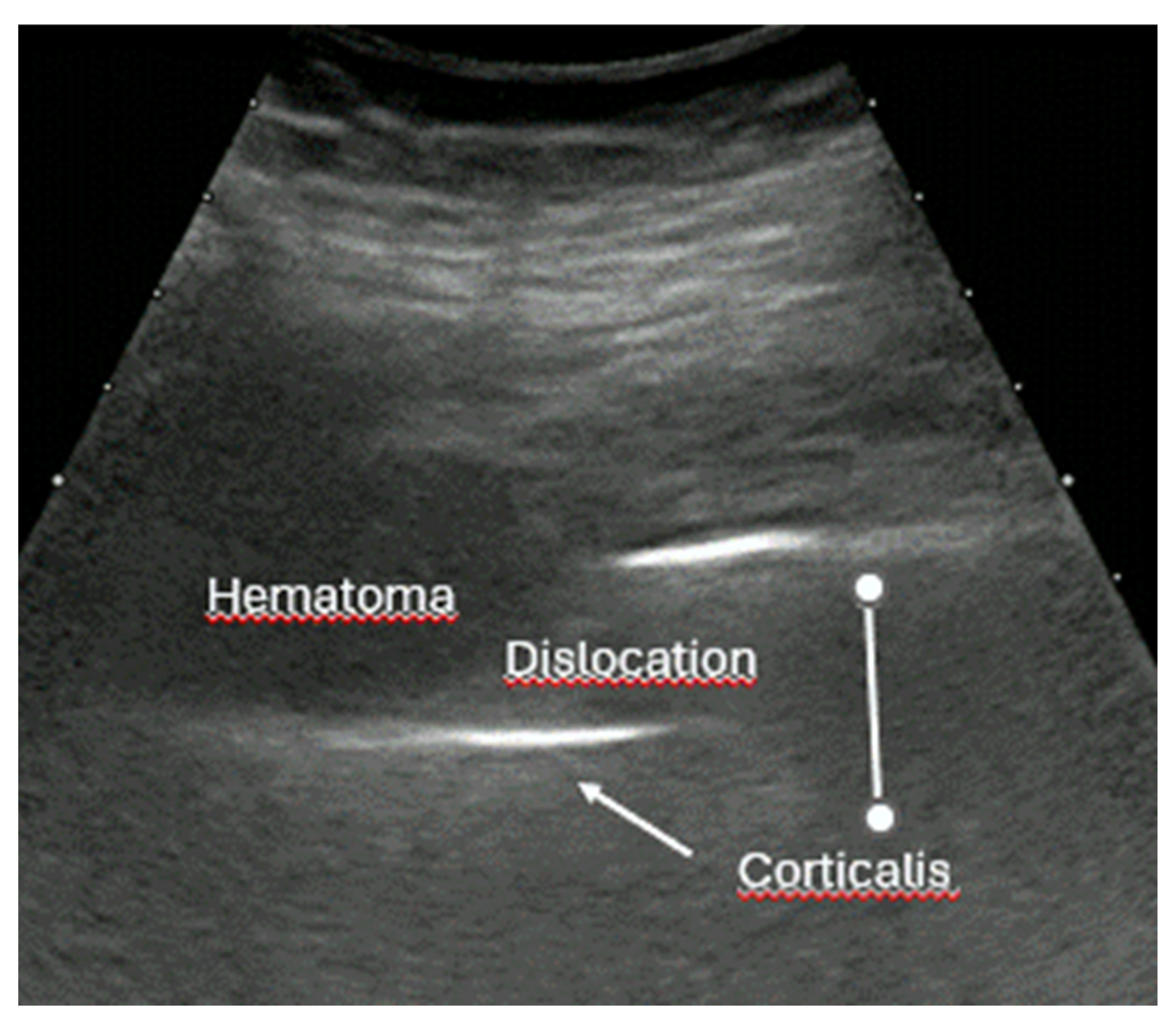
- Irregularity, interruption, or gaps in the cortical line: the cortical bone normally appears as a continuous, bright echogenic line, which is interrupted or irregular in the presence of a fracture.
- Reverberation artifacts within or adjacent to the fracture gap (also known as the “chimney sign”): these repetitive echoes are caused by ultrasound waves reflecting off the fracture surfaces.
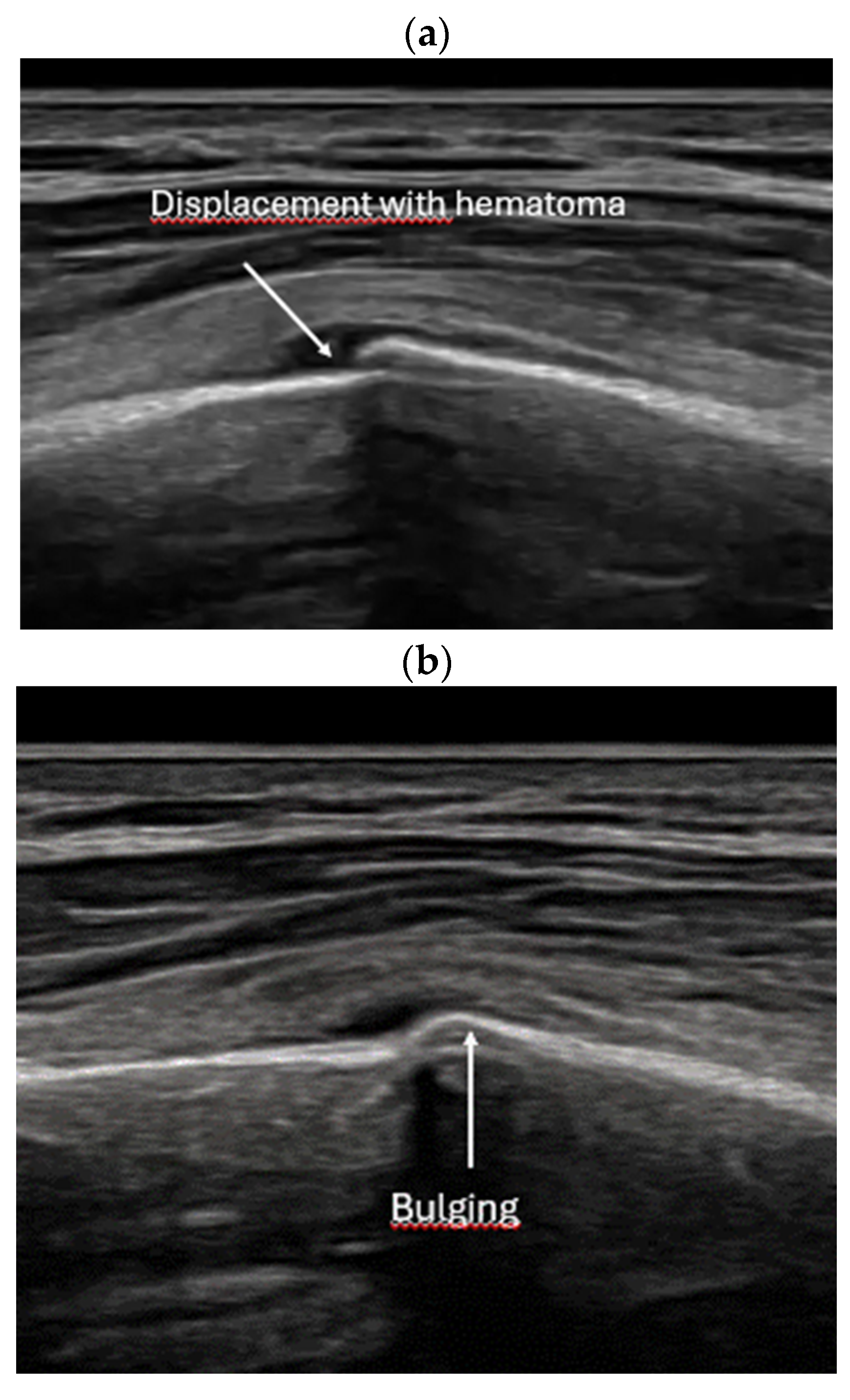
- Bulging or abnormal angulation of the cortical layer: deformities or outpouchings of the normally straight cortical surface indicate displacement or bending at the fracture site.
- Dislocation: misalignment of bone fragments, visible as separation or shift from their normal anatomical position.
- Angulation: an abnormal angle formed between fracture fragments, indicating malalignment.
- Osseous avulsions and small bone fragments: detached bone pieces that appear as discrete, hyperechoic fragments adjacent to the main bone.
- Local hematoma or soft tissue edema: fluid collections or increased echogenicity near the fracture site indicating bleeding and inflammation.
- Periosteal thickening or elevation: the periosteum may appear thickened or lifted due to injury or early callus formation.
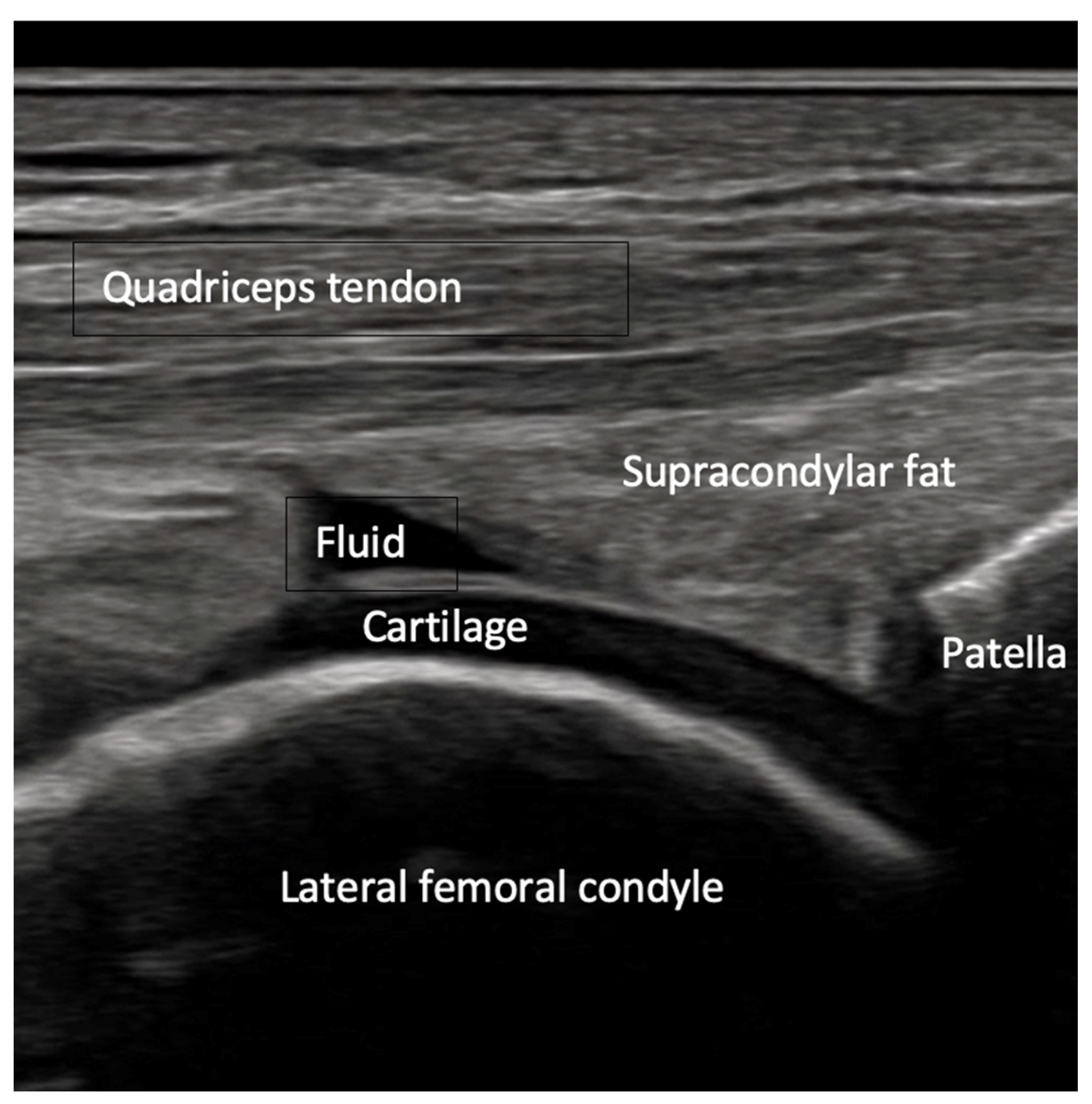
- Joint effusion and liphemarthrosis: fluid accumulation within a joint, sometimes containing fat droplets, which often indicates an intra-articular fracture.
5. Examination Technique
- Use of a silicon pad, generous amounts of ultrasound gel, or a water bath: These methods help minimize patient discomfort caused by transducer pressure on sensitive or injured areas. Additionally, they improve acoustic coupling between the transducer and the skin, which is critical for obtaining high-quality images and reducing artifacts.
- Selection of the appropriate transducer: It is advisable to use the highest possible frequency probe to maximize spatial resolution, particularly when imaging superficial bones. Depending on the clinical application and anatomical region, specialized probes such as linear-convex arrays or small footprint probes (e.g., hockey stick probes) may be preferred to facilitate access to difficult-to-reach areas and improve visualization.
- Adjustment of dynamic range: setting the dynamic range lower than usual enhances contrast resolution, making subtle cortical irregularities and fracture lines more conspicuous on the ultrasound image.
- Focusing and scanning technique: The focal zone should be positioned at the level of the cortical bone to maximize image sharpness. The probe should be oriented perpendicular to the cortical surface and scanned systematically in longitudinal and transverse planes. Scanning in multiple planes—typically three to four different angles—is recommended to avoid missing fracture lines or misinterpreting artifacts.
- Image magnification: excessive zooming or magnification of the image section should be avoided, as this can exaggerate normal cortical irregularities or artifacts, potentially leading to false-positive interpretations.
- Comparison with the contralateral side: whenever possible, imaging the corresponding site on the asymptomatic or unaffected side provides a valuable reference for normal anatomy and helps differentiate pathological findings from anatomical variants.
- Use of color and power Doppler: When employing Doppler techniques to assess vascularity related to inflammation or healing, care must be taken to avoid excessive probe pressure. Over compression of the tissue can collapse small vessels, leading to false-negative findings.
- Role of advanced ultrasound techniques: Currently, there is limited evidence supporting the routine use of elastography or contrast-enhanced ultrasound (CEUS) in fracture diagnosis. Although these modalities offer potential advantages in tissue characterization and perfusion assessment, their clinical utility in fracture imaging remains investigational and is not yet established.
6. Systematic Workflow of Ultrasound Application in Fracture Diagnostics and Regional Pain Therapy
7. Limitations and Future Directions
- Limitations
- Not suitable for all types of fractures: intra-articular fractures often need complimentary imaging: X-ray and/or CT.
- Limited access to deep or anatomically complex fractures: anatomical regions such as hip or spine are difficult to evaluate using ultrasound.
- Intrinsic limitations due to bone structure: Only the bone surface is visible. The internal architecture remains inaccessible.
- Operator dependency: detecting fractures of small bones and small fractures require considerable expertise.
- Lack of standardization: there are few standardized protocols for ultrasound-based fracture assessment in routine clinical practice.
- Future directions
- Fracture healing monitoring: Ultrasound facilitates early callus formation. This can be useful for radiation-free monitoring of healing progression.
- Portable use in prehospital medicine: handheld ultrasound devices permit rapid fracture assessment at the site of injury, including sports, military and emergency rescue service environment.
- Integration of artificial intelligence for image analysis: machine learning algorithms can assist in the automated identification of bone discontinuities or callus formation, thereby enhancing diagnostic accuracy.
- Dynamic real-time imaging during movement: Ultrasound allows for functional assessment under motion. This is helpful for detecting joint instability and diagnosing complications such as pseudarthroses.
- Multimodal imaging fusion (e.g., MRI-ultrasound fusion): combining ultrasound with other imaging techniques can augment diagnostic capabilities, especially for complex fractures and for assessing surrounding soft tissue injuries.
8. Narrative and Illustrative Review
8.1. Screening of Patients with Suspicion of Fracture to Improve the Specificity of X-Rays [Figure 9 and Figure 10]
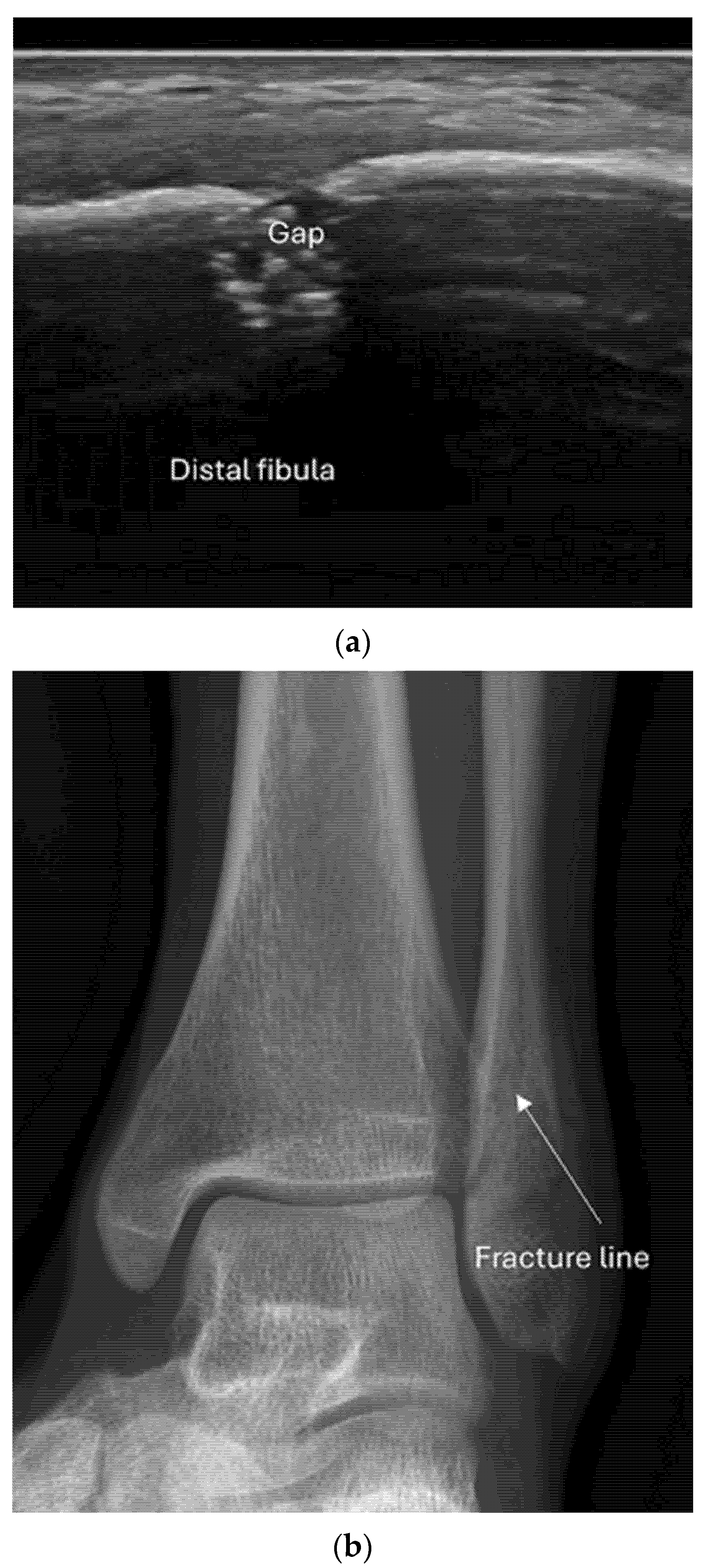
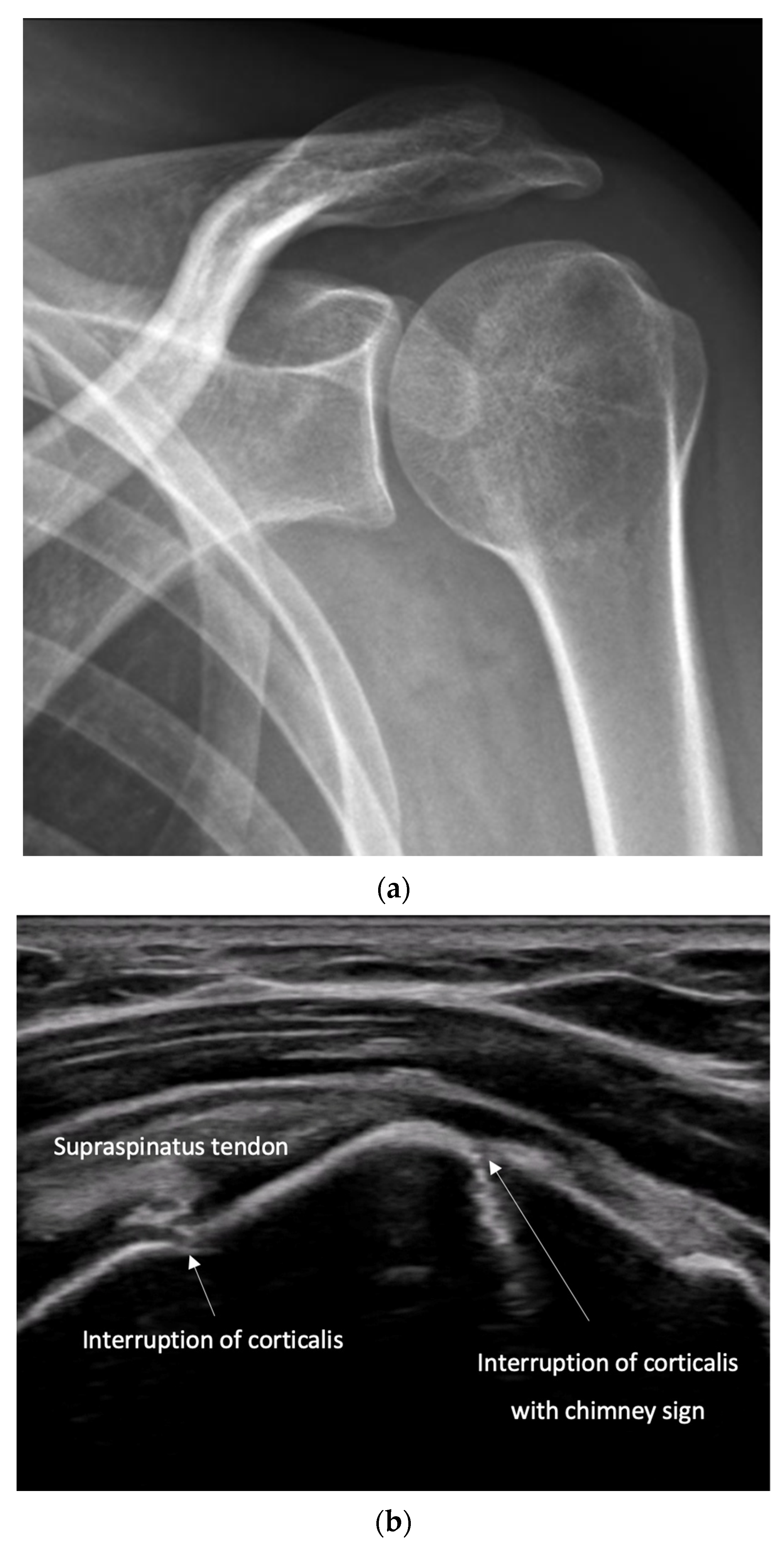
- Recommendation 1: Ultrasound should be used as a screening tool for suspected fractures, followed by further imaging (X-ray or CT) if necessary. In cases of obvious simple fractures, conventional X-ray remains the preferred method.
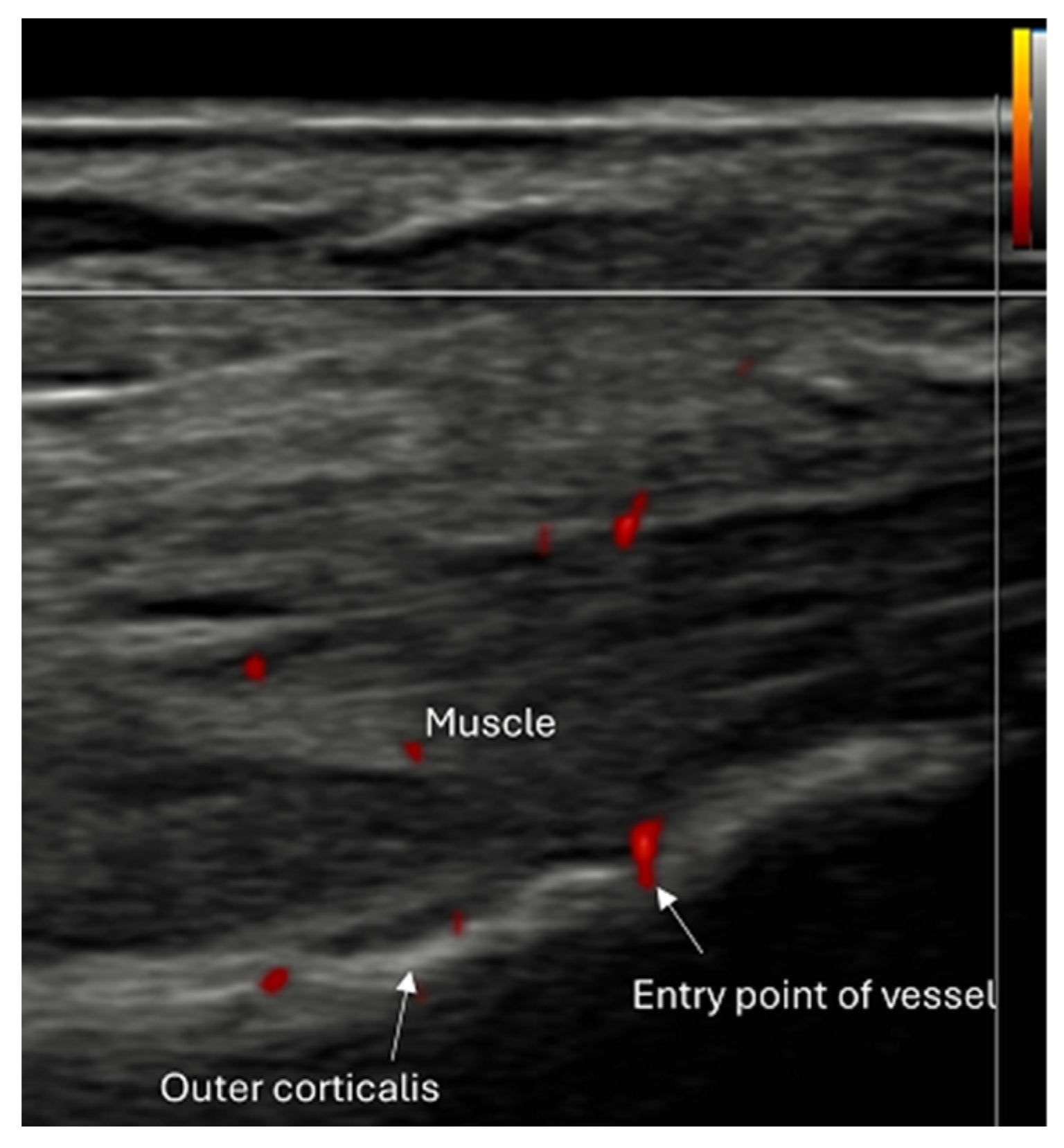
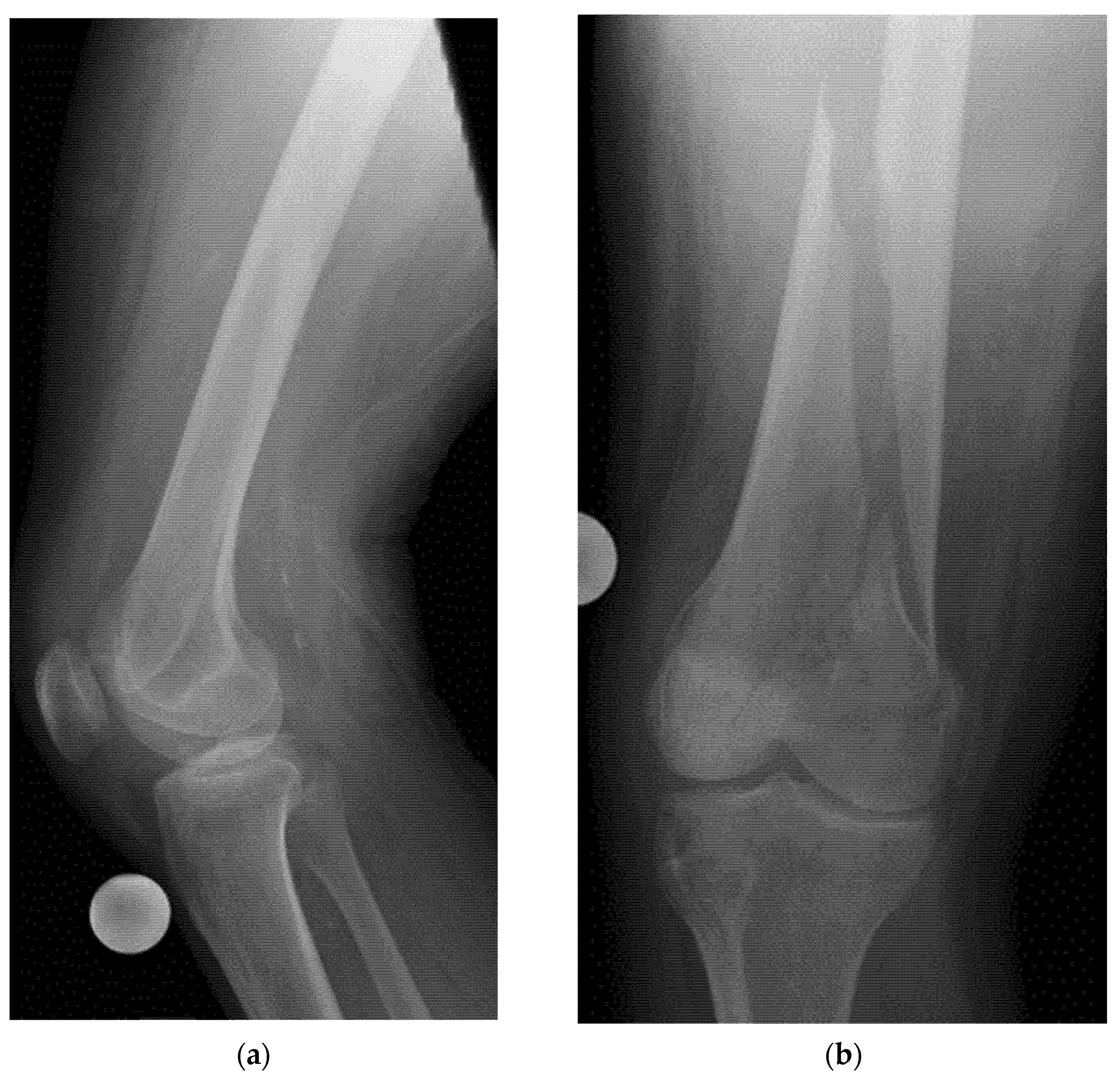
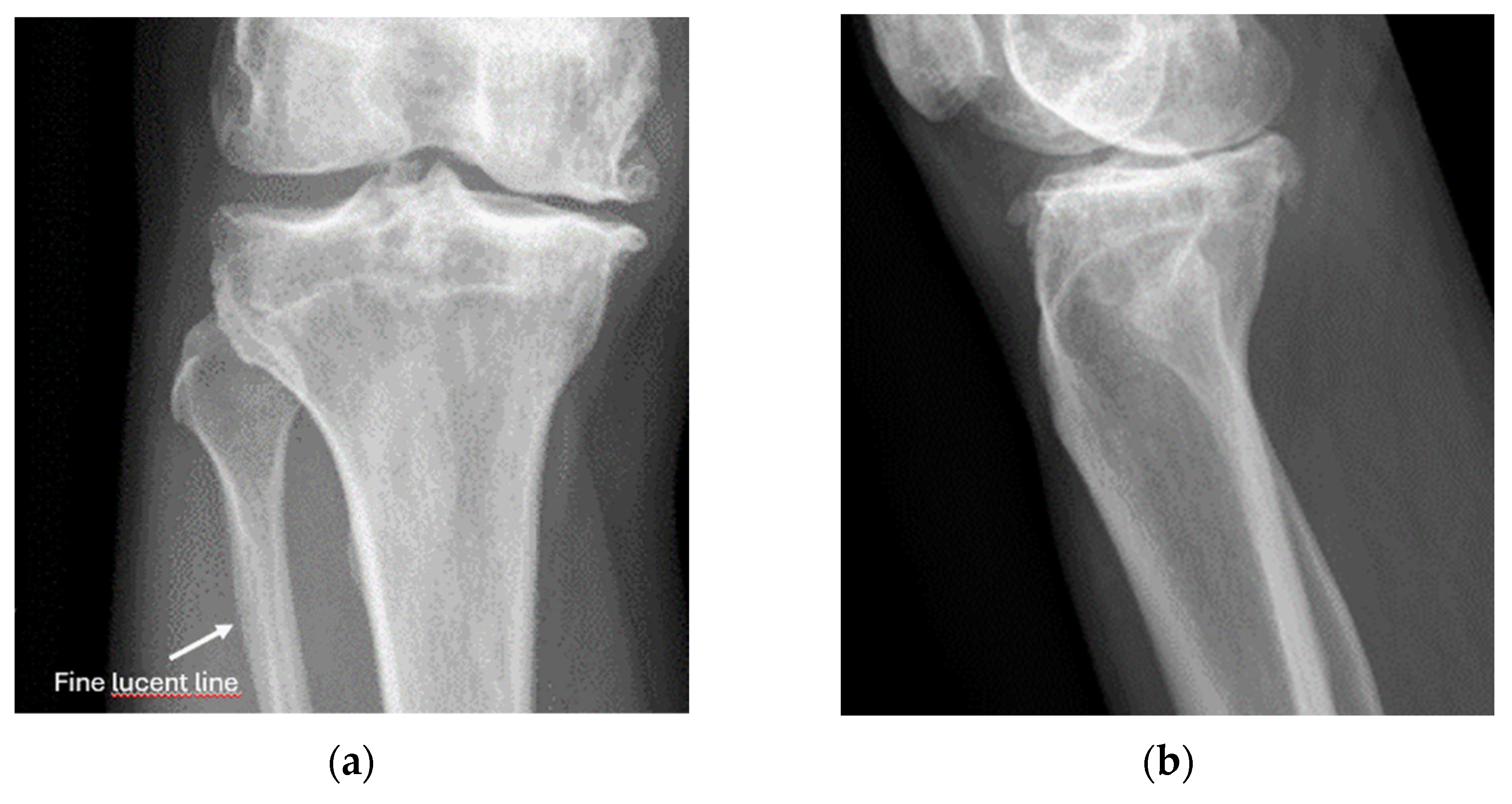
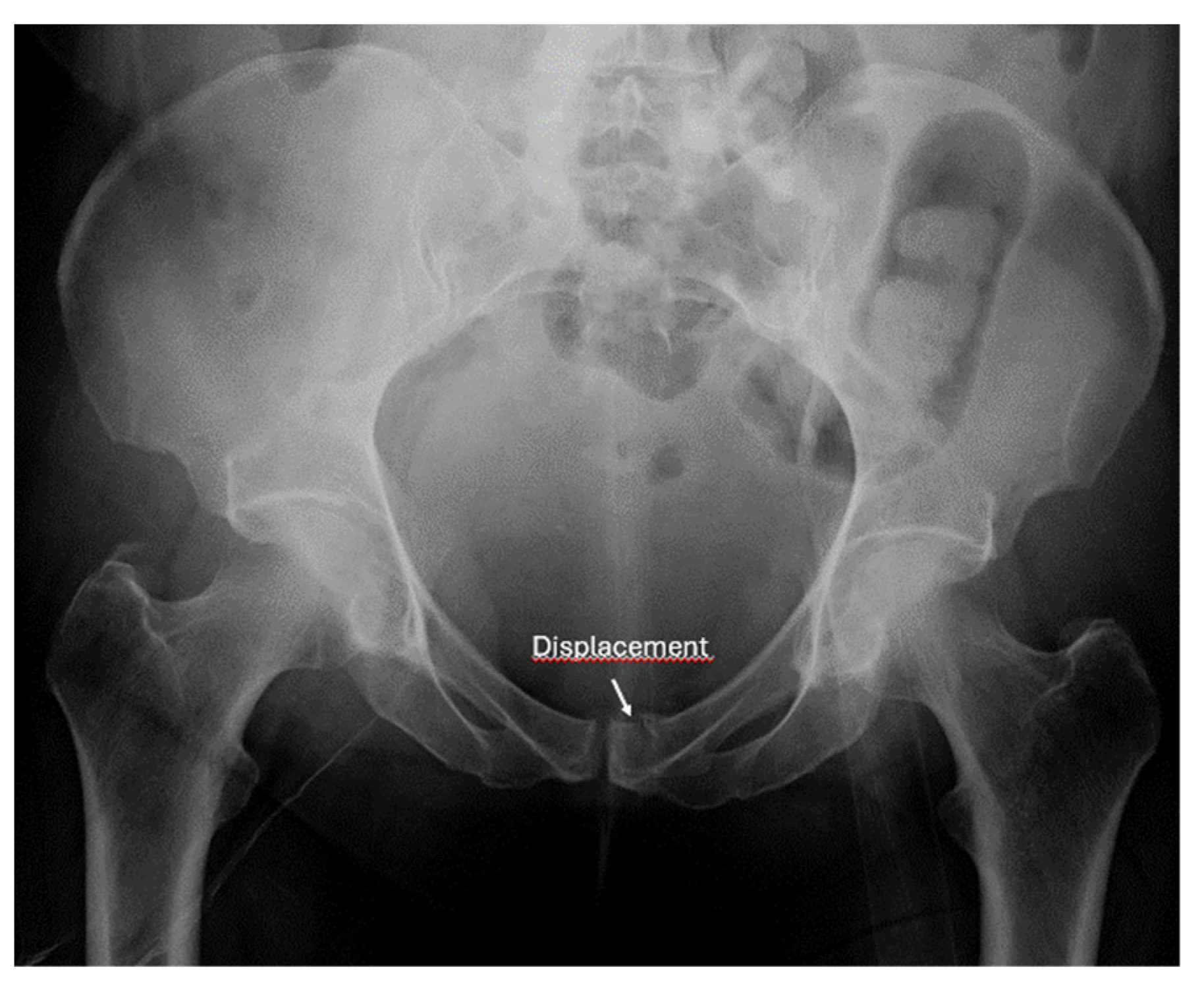
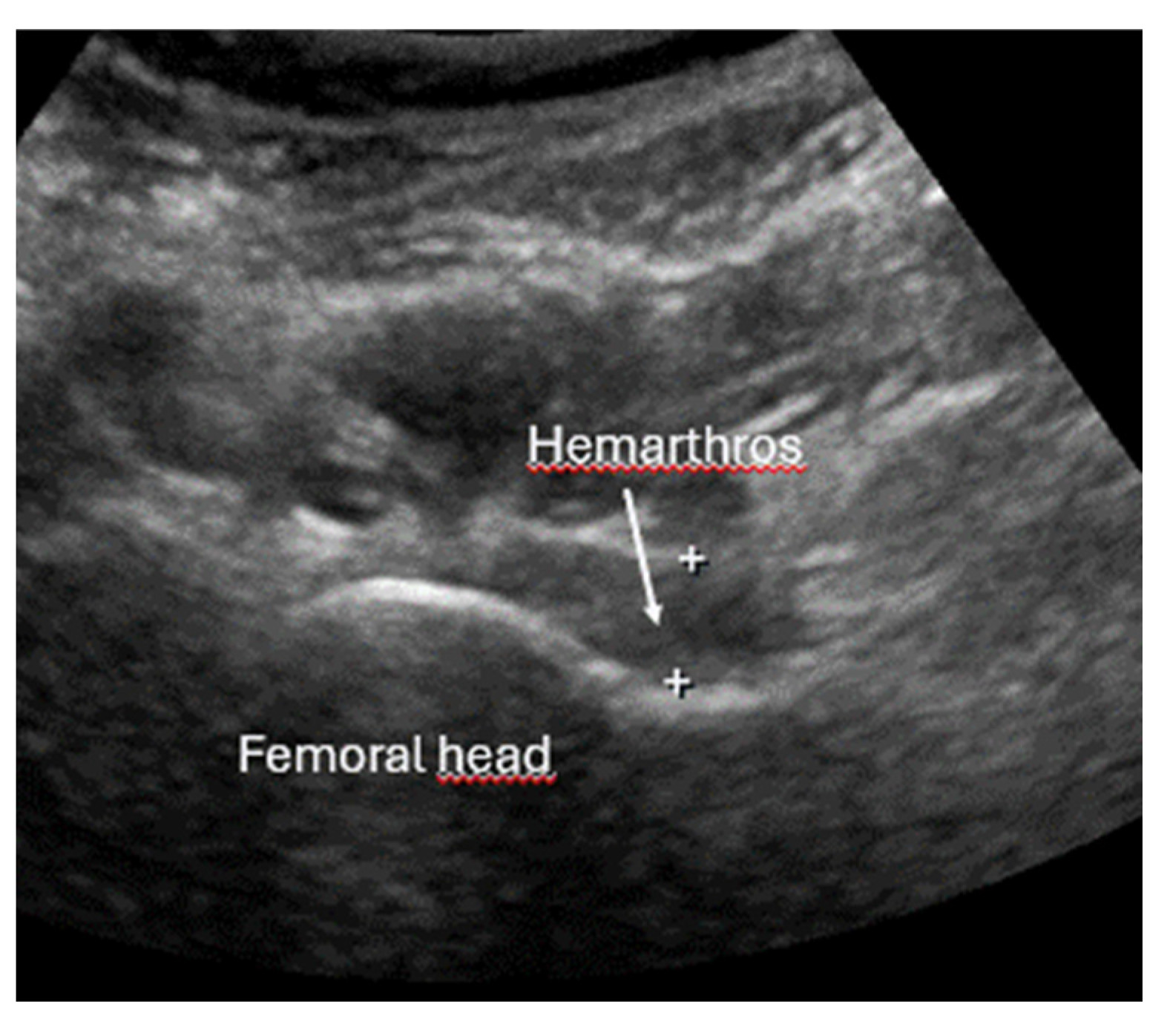
8.2. Screening of Patients with Suspicion of Occult and Stress Fractures (Figure 11 and Figure 12)
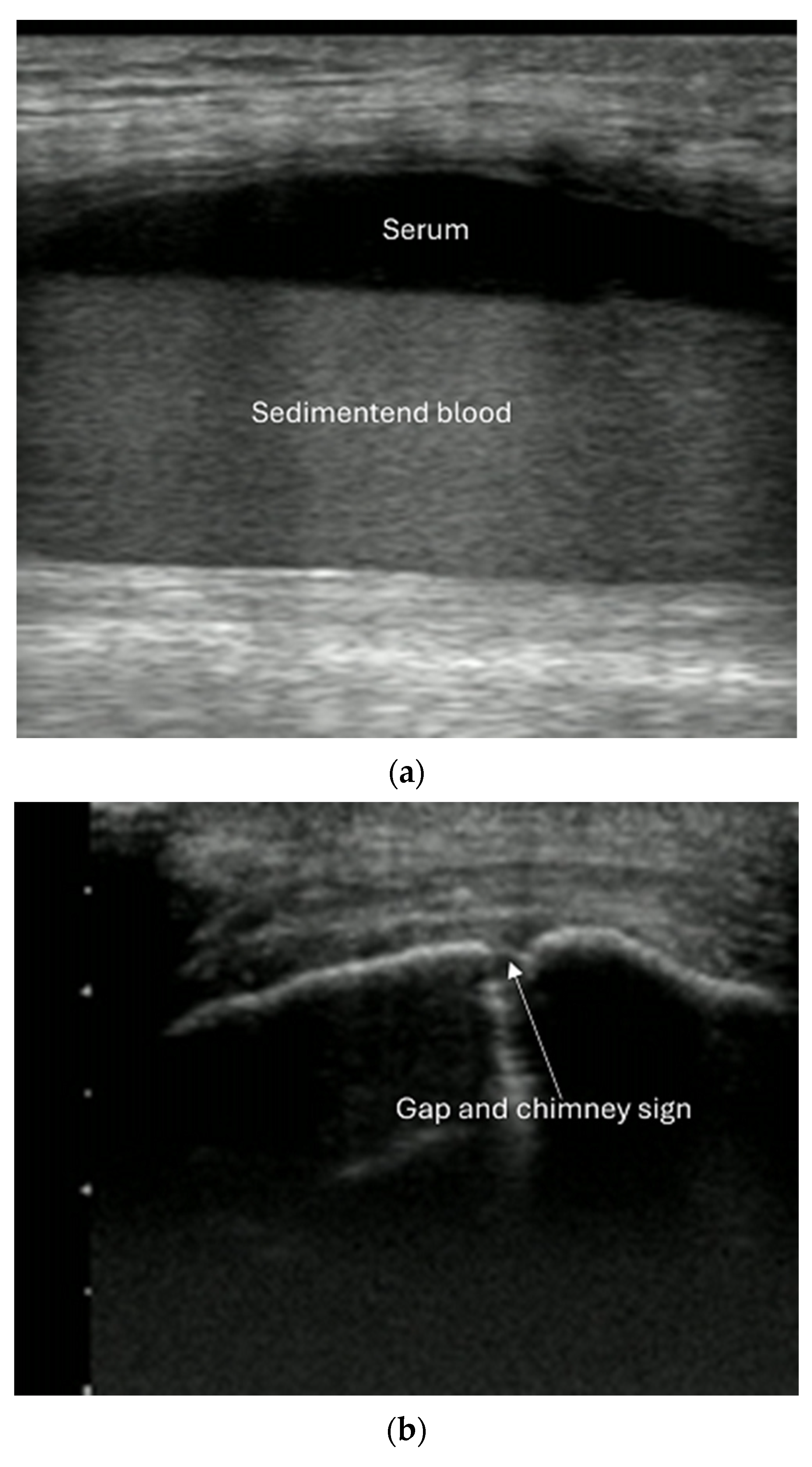

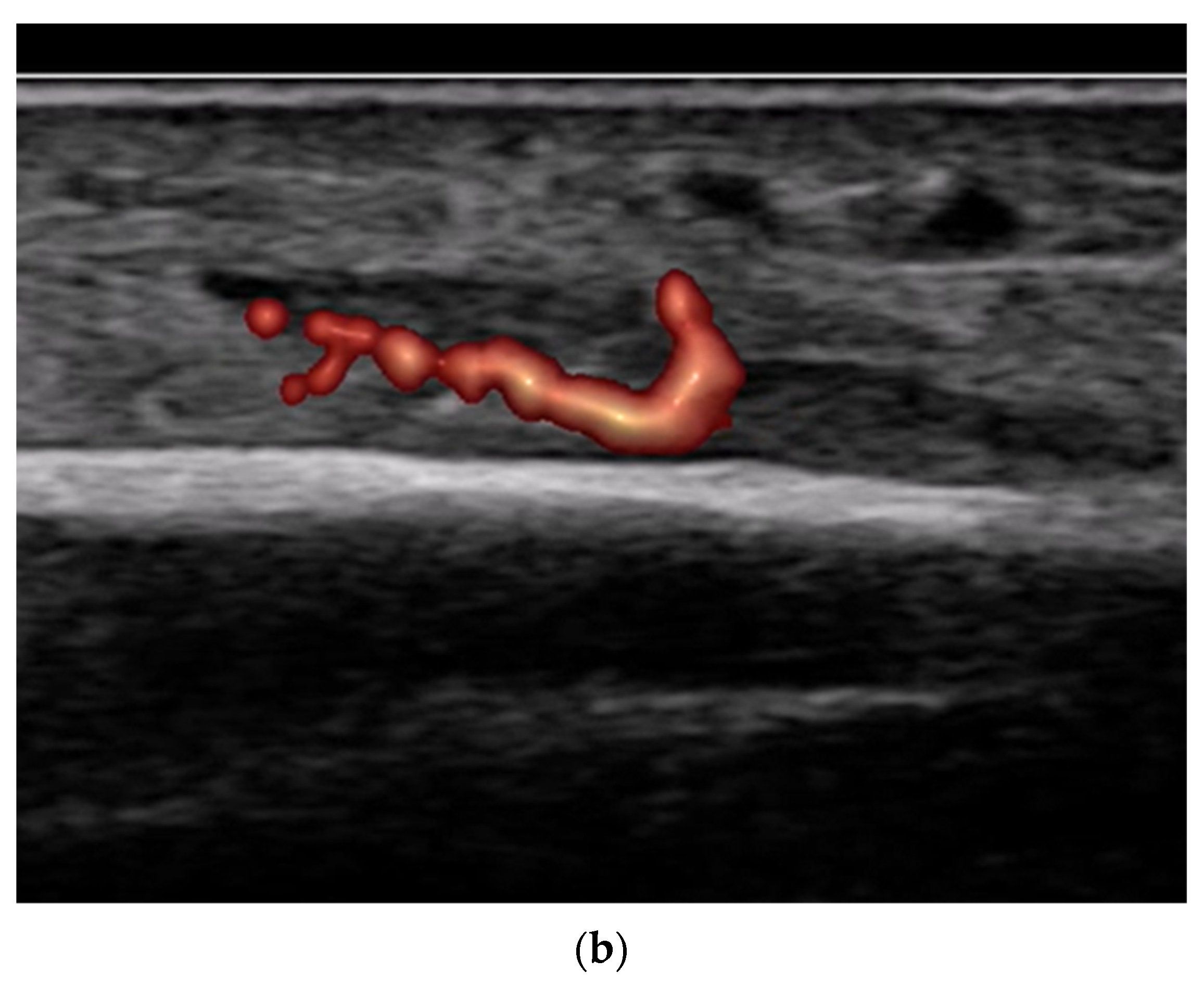
- Recommendation 2: Given the promising but limited evidence, ultrasound should be considered a first-line imaging tool for suspected occult fractures, except in the pelvis and vertebral column.
- Recommendation 3: Ultrasound should be used as first-line imaging for suspected stress fractures, with follow-up X-ray if evidence of a stress fracture is identified. Exceptions include pelvic and vertebral column fractures.
- Screening of fractures in the primary ATLS (Advanced Trauma Life Support) survey that are associated with or may cause life-threatening bleeding in polytrauma
- Recommendation 4: The E-FAST protocol, indicated for severely injured patients in shock, should be expanded to include fracture screening of long tubular bones and open-book fractures when the torso scan is negative for free fluid. This could identify hidden injuries and bleeding.
- 2.
- Screening for instability in pelvic and spine fractures
- Recommendation 5: In settings where CT or MRI is unavailable, ultrasound should be used during the secondary survey to help identify signs of instability in spinal injuries.
- 3.
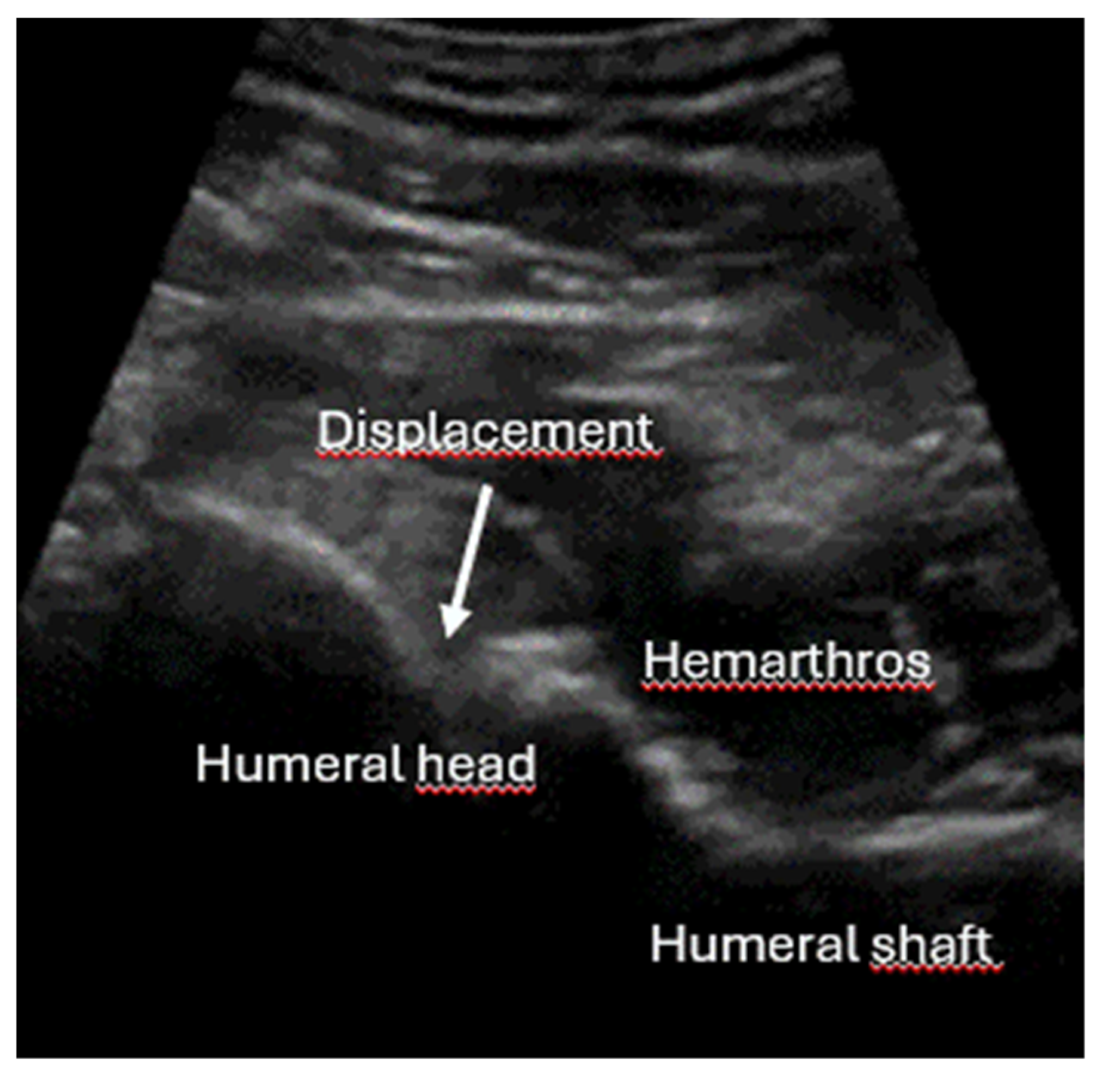
- Quick exclusion of fractures in shoulder dislocations for timely reduction (Figure 13).
- Recommendation 6: Ultrasound should be integrated into the assessment and management of shoulder injuries, including proximal humerus fractures, shoulder dislocations, and post-relocation maneuvers, both prehospitally, in emergency departments, and other acute care settings.
- 4
- Evaluating bone position during and after reduction to avoid considerable radiation exposure (video)
- Recommendation 7: The potential of ultrasound for positional control of fracture during fracture reduction in emergency situations should be explored in emergency departments.
- 5
- Nerve, fascia and fascial plane blocks
- Recommendation 8: Simple fascia and fascial blocks are highly suited for analgesia in patients with rib and femoral neck fractures. Local anesthesia using nerve blocks and fracture hematoma blocks should be preferred over analog sedation for fracture reduction whenever possible, as they are less risky.
9. Clinical Case Examples—Advantages and Limitations of Ultrasound Compared to X-Ray and CTC
- Example: Hemorrhagic Shock Due to Distal Femur Fracture—Rapid Diagnosis via Ultrasound
- Example: Stress Fracture
- Example: Suspected left shoulder dislocation
- Example: Common Case of “occult” rib Fractures
10. Conclusions
- The sonoanatomy of fractures is simple and can be learned quickly. It does not place high demands on the technical equipment of the devices or the skill of the examiner.
- The examination technique is simple. If available, high-frequency linear probes with a right-angled position on the cortical bone and, if necessary, silicone pads for coupling should be used. Important knobology parameters, such as a low dynamic range, non-excessive magnification, and focus on the cortical bone, should be set. The bone should be scanned longitudinally, compared with the healthy side, and not pressed too hard when using the color/power Doppler.
- Seven indications are recommended. Ultrasound should be used:
- To screen for suspected fracture, and, if positive, follow-up by further imaging (X-ray or CT if indicated).
- As first-line imaging for suspected occult or stress fractures.
- In E-FAST, which is indicated for moderately to severely injured patients with shock, it can be expanded to fracture screening of long tubular bones and open-book fractures if the result of the torso scan is negative for free fluid.
- In the secondary survey to help identify signs of spinal injury instability, whenever CT and MRI are not available.
- Integrated into the assessment and management of shoulder injuries, including proximal humerus fracture, shoulder dislocation, and post-relocation maneuver, both pre-hospital and in emergency department settings.
- Positional control of fractures during and after reduction.
- For simple fascia and fascial blocks, such as rib and femoral neck fractures. Local anesthesia using nerve blocks is preferred to analog sedation for fracture reduction.
- The consistent use of ultrasound for a given indication could save millions of X-ray examinations worldwide.
- This growing body of evidence supports a broader role for ultrasound as a frontline imaging modality in fracture diagnosis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matschiner, E.; Serban, O.; Fodor, D.; Blaivas, M.; Horn, R.; Koch, J.; Jakobi, M.-L.; Grevelding, L.; Osterwalder, J.; Srivastava, D.; et al. Ultrasound in bone fracture diagnosis—A comparative meta-analysis and systematic review. Med. Ultrason. 2025, 27, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, O.; Fischer, C.A.; Grosser, K.; Hauenstein, C.; Kluge, S.; Moritz, J.D.; Berthold, L.; Kaisenberg, C.V.; Tesch, C. Fracture sonography-review of literature and current recommendations. Arch. Orthop. Trauma. Surg. 2024, 144, 3025–3043. [Google Scholar] [CrossRef]
- Schaper, M.; Harcus, J. Preliminary image findings of lower limb stress fractures to aid ultrasonographic diagnoses: A sys-tematic review and narrative synthesis. Ultrasound 2021, 29, 208–217. [Google Scholar] [CrossRef]
- Stiell, I.G.; McDowell, I.; Nair, R.C.; Aeta, H.; Greenberg, G.; McKnight, R.D.; Ahuja, J. Use of radiography in acute ankle injuries: Physicians’ attitudes and practice. CMAJ 1992, 147, 1671–1678. [Google Scholar]
- Baratloo, A.; Ahmadzadeh, K.; Forouzanfar, M.M.; Yousefifard, M.; Ranjbar, M.F.; Hashemi, B.; Aghili, S.H. NEXUS vs. Canadian C-Spine Rule (CCR) in Predicting Cervical Spine Injuries; a Systematic Review and Meta-analysis. Arch. Acad. Emerg. Med. 2023, 11, e66. [Google Scholar]
- Gomes, Y.E.; Chau, M.; Banwell, H.A.; Causby, R.S. Diagnostic accuracy of the Ottawa ankle rule to exclude fractures in acute ankle injuries in adults: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2022, 23, 885. [Google Scholar] [CrossRef]
- Sims, J.I.; Chau, M.T.; Davies, J.R. Diagnostic accuracy of the Ottawa Knee Rule in adult acute knee injuries: A systematic review and meta-analysis. Eur. Radiol. 2020, 30, 4438–4446. [Google Scholar] [CrossRef]
- Hedelin, H.; Goksör, L.; Karlsson, J.; Stjernström, S. Ultrasound-assisted triage of ankle trauma can decrease the need for radiographic imaging. Am. J. Emerg. Med. 2013, 31, 1686–1689. [Google Scholar] [CrossRef]
- Yousefifard, M.; Baikpour, M.; Ghelichkhani, P.; Asady, H.; Darafarin, A.; Esfahani, M.R.A.; Hosseini, M.; Yaseri, M.; Safari, S. Comparison of Ultrasonography and Radiography in Detection of Thoracic Bone Fractures; a Systematic Review and Meta-Analysis. Emergy 2016, 4, 55–64. [Google Scholar]
- Malghem, J.; Berg, B.C.V.; Lecouvet, F.E.; Maldague, B.E. Costal cartilage fractures as revealed on CT and sonography. Am. J. Roentgenol. 2001, 176, 429–432. [Google Scholar] [CrossRef]
- Bianchi, S. Ultrasound and bone: A pictorial review. J. Ultrasound 2020, 23, 227–257. [Google Scholar] [CrossRef]
- Gyftopoulos, S.; Chitkara, M.; Bencardino, J.T. Misses and errors in upper extremity trauma radiographs. Am. J. Roentgenol. 2014, 203, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Ha, A.S.; Porrino, J.A.; Chew, F.S. Radiographic pitfalls in lower extremity trauma. Am. J. Roentgenol. 2014, 203, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Kwee, R.M.; Kwee, T.C. Ultrasound for diagnosing radiographically occult scaphoid fracture. Skelet. Radiol. 2018, 47, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Safran, O.; Goldman, V.; Applbaum, Y.; Milgrom, C. Posttraumatic painful hip: Sonography as a screening test for occult hip fractures. J. Ultrasound Med. 2009, 28, 1447–1452. [Google Scholar] [CrossRef]
- Bonnefoy, O.; Diris, B.; Moinard, M.; Aunoble, S.; Diard, F.; Hauger, O. Acute knee trauma: Role of ultrasound. Eur. Radiol. 2006, 16, 2542–2548. [Google Scholar] [CrossRef]
- Pavić, R.; Margetić, P.; Hnatešen, D. Diagnosis of occult radial head and neck fracture in adults. Injury 2015, 46, S119–S124. [Google Scholar] [CrossRef]
- Demetriades, D.; Karaiskakis, M.; Toutouzas, K.; Alo, K.; Velmahos, G.; Chan, L. Pelvic fractures: Epidemiology and predictors of associated abdominal injuries and outcomes. J. Am. Coll. Surg. 2002, 195, 1–10. [Google Scholar] [CrossRef]
- Ianniello, S.; Conte, P.; Di Serafino, M.; Miele, V.; Trinci, M.; Vallone, G.; Galluzzo, M. Diagnostic accuracy of pubic symphysis ultrasound in the detection of un-stable pelvis in polytrauma patients during e-FAST: The value of FAST-PLUS protocol. A preliminary experience. J. Ultrasound 2021, 24, 423–428. [Google Scholar] [CrossRef]
- Zhang, B.-F.; Lei, J.-L.; Zhang, H.; Wang, P.-F.; Wang, H.; Cong, Y.-X.; Huang, H.; Zhuang, Y. Use of ultrasonography for evaluation of stability of lateral compression type 1 (LC-1) pelvic fractures to assist determination of treatment strategy. J. Orthop. Surg. Res. 2019, 14, 7. [Google Scholar] [CrossRef]
- Zhang, B.-F.; Zhang, H.; Wang, P.-F.; Wang, H.; Lei, J.-L.; Fu, Y.-H.; Cong, Y.-X.; Huang, H.; Huo, X.-M.; Zhuang, Y.; et al. The role of ultrasonography in examination of the stability of Tile-B2 pelvic fractures: 7 case reports and a literature review. Med. (Baltim.) 2017, 96, e8100. [Google Scholar] [CrossRef]
- Vk, V.; Bhoi, S.; Aggarwal, P.; Murmu, L.; Agrawal, D.; Kumar, A.; Sinha, T.P.; Galwankar, S. Diagnostic utility of point of care ultrasound in identifying cervical spine injury in emergency settings. Australas. J. Ultrasound Med. 2021, 24, 208–216. [Google Scholar] [CrossRef]
- Ravikanth, R. Diagnostic Accuracy and Prognostic Significance of Point-of-Care Ultrasound (POCUS) for Traumatic Cervical Spine in Emergency care setting: A Comparison of clinical outcomes between POCUS and Computed Tomography on a Cohort of 284 Cases and Review of Literature. J. Craniovertebr Junction Spine 2021, 12, 257–262. [Google Scholar]
- Gabriel, A.-C.; Ángel, J.-C.; Juan, J.-P.; Luis, R.-S.; Hernando, R.-M.; Rubén, S.-B. Diagnostic accuracy of ultrasound for detecting posterior ligamentous complex injuries of the thoracic and lumbar spine: A systematic review and meta-analysis. J. Craniovertebral Junction Spine 2013, 4, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Patel, D.; Marks, A.; Peksa, G.D. Ultrasound for the diagnosis of shoulder dislocation and reduction: A systematic review and meta-analysis. Acad. Emerg. Med. 2022, 29, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, M.A.A.; Jarman, R.D.; Cardona, T. Diagnostic accuracy of point-of-care ultrasound (PoCUS) for shoulder dislocations and reductions in the emergency department: A diagnostic randomised control trial (RCT). Emerg. Med. J. 2022, 39, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.; Appelboam, A.; Nunns, M. Ultrasound-directed reduction of distal radius fractures in adults: A systematic review. Emerg. Med. J. 2021, 38, 537–542. [Google Scholar] [CrossRef]
- McManus, J.G.; Morton, M.J.; Crystal, C.S.; McArthur, T.J.; Helphenstine, J.S.; Masneri, D.A.; Young, S.E.; Miller, M.A. Use of ultrasound to assess acute fracture reduction in emergency care settings. Am. J. Disaster Med. 2008, 3, 241–247. [Google Scholar]
- Kim, D.-H.; Kim, K.-S. Usefulness of Ultrasonography-Assisted Closed Reduction for Nasal Fracture under Local Anesthesia. Arch. Craniofac. Surg. 2015, 16, 151–153. [Google Scholar] [CrossRef]
- Shigemura, Y.; Akamatsu, J.; Sugita, N.; Nuri, T.; Ueda, K. Water can make the clearest ultrasonographic image during reduction of nasal fracture. Plast. Reconstr. Surg. Glob. Open 2014, 2, e203. [Google Scholar] [CrossRef]
- Hayashi, M.; Yamamoto, N.; Kuroda, N.; Kano, K.; Miura, T.; Kamimura, Y.; Shiroshita, A. Peripheral Nerve Blocks in the Preoperative Management of Hip Fractures: A Systematic Review and Network Meta-Analysis. Ann. Emerg. Med. 2024, 83, 522–538. [Google Scholar] [CrossRef]
- Kolli, S.; Nimma, S.R.; Kukreja, P.; Peng, P. How I Do It: PEricapsular Nerve Group (PENG) Block; ASRA Pain Medicine: Pittsburgh, PA, USA, 2023. [Google Scholar]
- Koushik, S.S.; Bui, A.; Slinchenkova, K.; Badwal, A.; Lee, C.; Noss, B.O.; Raghavan, J.; Viswanath, O.; Shaparin, N. Analgesic Techniques for Rib Fractures-A Comprehensive Review Article. Curr. Pain. Headache Rep. 2023, 27, 747–755. [Google Scholar] [CrossRef]
- Xie, C.; Ran, G.; Chen, D.; Lu, Y. A narrative review of ultrasound-guided serratus anterior plane block. Ann. Palliat. Med. 2021, 10, 700–706. [Google Scholar] [CrossRef]
- Kuypers, M.I.; Veldhuis, L.I.; Mencl, F.; van Riel, A.; Thijssen, W.A.; Tromp, E.; Goslings, J.C.; Plötz, F.B. Procedural sedation and analgesia versus nerve blocks for reduction of frac-tures and dislocations in the emergency department: A systematic review and meta-analysis. J. Am. Coll. Emerg. Physicians Open 2023, 4, e12886. [Google Scholar] [CrossRef]



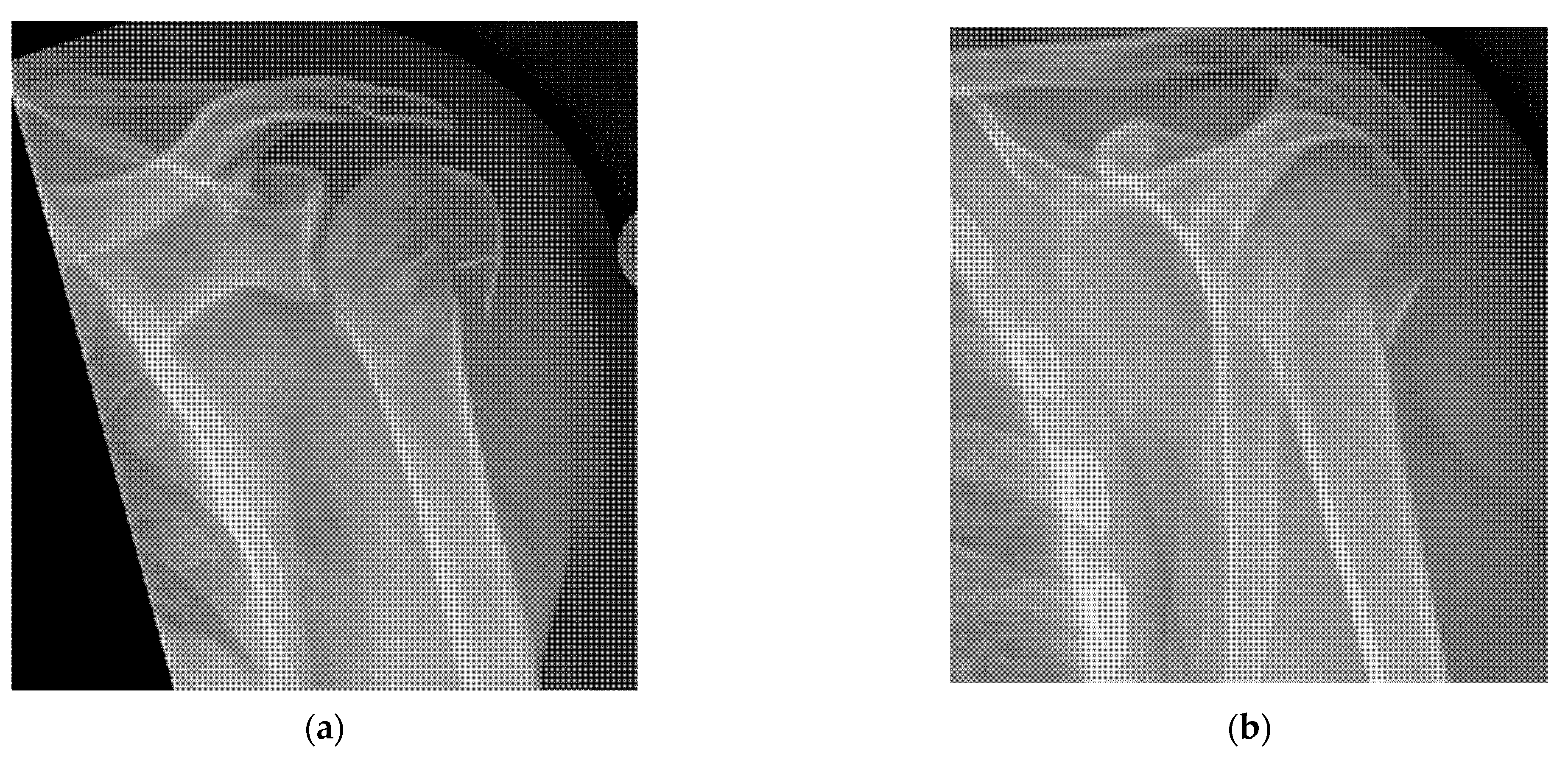

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osterwalder, J.; Hoffmann, B.; Blaivas, M.; Horn, R.; Matchiner, E.; Dietrich, C.F. A Plea for a Paradigm Shift from X-Ray to Ultrasound in Adults: An Update for Emergency Physicians, General Practitioners, Orthopedists and Sports Medicine Physicians. Diagnostics 2025, 15, 1827. https://doi.org/10.3390/diagnostics15141827
Osterwalder J, Hoffmann B, Blaivas M, Horn R, Matchiner E, Dietrich CF. A Plea for a Paradigm Shift from X-Ray to Ultrasound in Adults: An Update for Emergency Physicians, General Practitioners, Orthopedists and Sports Medicine Physicians. Diagnostics. 2025; 15(14):1827. https://doi.org/10.3390/diagnostics15141827
Chicago/Turabian StyleOsterwalder, Joseph, Beatrice Hoffmann, Mike Blaivas, Rudolf Horn, Eric Matchiner, and Christoph F. Dietrich. 2025. "A Plea for a Paradigm Shift from X-Ray to Ultrasound in Adults: An Update for Emergency Physicians, General Practitioners, Orthopedists and Sports Medicine Physicians" Diagnostics 15, no. 14: 1827. https://doi.org/10.3390/diagnostics15141827
APA StyleOsterwalder, J., Hoffmann, B., Blaivas, M., Horn, R., Matchiner, E., & Dietrich, C. F. (2025). A Plea for a Paradigm Shift from X-Ray to Ultrasound in Adults: An Update for Emergency Physicians, General Practitioners, Orthopedists and Sports Medicine Physicians. Diagnostics, 15(14), 1827. https://doi.org/10.3390/diagnostics15141827





