Ocular Structural and Vascular Changes in Patients with Severe Asymptomatic Carotid Disease After Undergoing Carotid Endarterectomy (CEA) and Carotid Artery Stenting (CAS)
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, R.W.; Tucker, L.Y.; Rothenberg, K.A.; Lancaster, E.; Faruqi, R.M.; Kuang, H.C.; Flint, A.C.; Avins, A.L.; Nguyen-Huynh, M.N. Incidence of Ischemic Stroke in Patients With Asymptomatic Severe Carotid Stenosis Without Surgical Intervention. JAMA 2022, 327, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Wabnitz, A.M.; Turan, T.N. Symptomatic Carotid Artery Stenosis: Surgery, Stenting, or Medical Therapy? Curr. Treat Options Cardiovasc. Med. 2017, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Halliday, A.; Harrison, M.; Hayter, E.; Kong, X.; Mansfield, A.; Marro, J.; Pan, H.; Peto, R.; Potter, J.; Rahimi, K.; et al. Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): A multicentre randomised trial. Lancet 2010, 376, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Biousse, V. Carotid disease and the eye. Curr. Opin. Ophthalmol. 1997, 8, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Biousse, V.; Newman, N.J. Eye syndromes and the neuro-ophthalmology of stroke. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 595–611. [Google Scholar]
- Wong, T.Y.; Klein, R. Retinal arteriolar emboli: Epidemiology and risk of stroke. Curr. Opin. Ophthalmol. 2002, 13, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Sun, R.; Chen, Y.F.; Ma, Y.; Wang, Y.B.; Jiao, L.Q.; Ling, F. Retinal embolization after carotid endarterectomy and stenting for carotid artery stenosis. J. Clin. Neurosci. 2015, 22, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, C.; Eldrup, N. Asymptomatic carotid stenosis and concomitant silent brain infarctions. Vascular 2020, 28, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Laíns, I.; Wang, J.C.; Cui, Y.; Katz, R.; Vingopoulos, F.; Staurenghi, G.; Vavvas, D.G.; Miller, J.W.; Miller, J.B. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog. Retin. Eye Res. 2021, 84, 100951. [Google Scholar] [CrossRef] [PubMed]
- Phuljhele, S.; Sharma, S.; Chawla, R.; Saxena, R.; Sharma, P. Evaluation of optical coherence tomography angiography changes in nonarteritic anterior ischemic optic neuropathy. Indian. J. Ophthalmol. 2023, 71, 2020–2026. [Google Scholar] [CrossRef] [PubMed]
- Alvernia, J.E.; Hidalgo, J.; Sindou, M.P.; Washington, C.; Luzardo, G.; Perkins, E.; Nader, R.; Mertens, P. The maxillary artery and its variants: An anatomical study with neurosurgical applications. Acta Neurochir. 2017, 159, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Michalinos, A.; Zogana, S.; Kotsiomitis, E.; Mazarakis, A.; Troupis, T. Anatomy of the Ophthalmic Artery: A Review concerning Its Modern Surgical and Clinical Applications. Anat. Res. Int. 2015, 2015, 591961. [Google Scholar] [CrossRef] [PubMed]
- Sadato, A.; Maeda, S.; Hayakawa, M.; Adachi, K.; Toyama, H.; Nakahara, I.; Hirose, Y. Carotid stenting for unilateral stenosis can increase contralateral hemispheric cerebral blood flow. J. Neurointerv Surg. 2018, 10, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Lahme, L.; Marchiori, E.; Panuccio, G.; Nelis, P.; Schubert, F.; Mihailovic, N.; Torsello, G.; Eter, N.; Alnawaiseh, M. Changes in retinal flow density measured by optical coherence tomography angiography in patients with carotid artery stenosis after carotid endarterectomy. Sci. Rep. 2018, 8, 17161. [Google Scholar] [CrossRef] [PubMed]
- Akca Bayar, S.; Kayaarası Öztürker, Z.; Pınarcı, E.Y.; Ercan, Z.E.; Akay, H.T.; Yılmaz, G. Structural Analysis of the Retina and Choroid before and after Carotid Artery Surgery. Curr. Eye Res. 2020, 45, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Patton, N.; Aslam, T.; Macgillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Akçay, B.İ.; Kardeş, E.; Maçin, S.; Ünlü, C.; Özgürhan, E.B.; Maçin, A.; Bozkurt, T.K.; Ergin, A.; Surmeli, R. Evaluation of Subfoveal Choroidal Thickness in Internal Carotid Artery Stenosis. J. Ophthalmol. 2016, 2016, 5296048. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sayin, N.; Kara, N.; Uzun, F.; Akturk, I.F. A quantitative evaluation of the posterior segment of the eye using spectral-domain optical coherence tomography in carotid artery stenosis: A pilot study. Ophthalmic Surg. Lasers Imaging Retina 2015, 46, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Dagdelen, K.; Muz, O.E. Investigation of macular and optic nerve head structural changes using spectral domain optical coherence tomography in internal carotid artery stenosis. Int. Ophthalmol. 2021, 41, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Lareyre, F.; Nguyen, E.; Raffort, J.; Carboni, J.; Doyen, J.; Hassen-Khodja, R.; Gastaud, P.; Chofflet, J.; Jean-Baptiste, E. Changes in Ocular Subfoveal Choroidal Thickness After Carotid Endarterectomy Using Enhanced Depth Imaging Optical Coherence Tomography: A Pilot Study. Angiology 2018, 69, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Biberoglu, E.; Eraslan, M.; Midi, I.; Baltacioglu, F.; Bitargil, M. Ocular blood flow and choroidal thickness changes after carotid artery stenting. Arq. Bras. Oftalmol. 2020, 83, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Cheng, H.C.; Chang, F.C.; Wang, A.G. Optical Coherence Tomography Angiography Evaluation of Retinal Microvasculature Before and After Carotid Angioplasty and Stenting. Sci. Rep. 2019, 9, 14755. [Google Scholar] [CrossRef] [PubMed]
- Ala-Kauhaluoma, M.; Koskinen, S.M.; Silvennoinen, H.; Vikatmaa, P.; Nuotio, K.; Ijäs, P.; Relander, K.; Lindsberg, P.J.; Soinne, L.; Summanen, P.A. Subfoveal choroidal thickness in ipsi- and contralateral eyes of patients with carotid stenosis before and after carotid endarterectomy: A prospective study. Acta Ophthalmol. 2021, 99, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Lee, J.T.; Cheng, C.A.; Liu, M.T.; Chen, C.Y.; Hu, H.H.; Peng, G.S. Reversal of ophthalmic artery flow as a predictor of intracranial hemodynamic compromise: Implication for prognosis of severe carotid stenosis. Eur. J. Neurol. 2013, 20, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Hayashi, M.; Yagi, F.; Sato, K.; Tomita, G.; Iwabuchi, S. Relationship between the Direction of Ophthalmic Artery Blood Flow and Ocular Microcirculation before and after Carotid Artery Stenting. J. Ophthalmol. 2016, 2016, 2530914. [Google Scholar] [CrossRef] [PubMed]
- Heßler, H.; Zimmermann, H.; Oberwahrenbrock, T.; Kadas, E.M.; Mikolajczak, J.; Brandt, A.U.; Kauert, A.; Paul, F.; Schreiber, S.J. No Evidence for Retinal Damage Evolving from Reduced Retinal Blood Flow in Carotid Artery Disease. Biomed. Res. Int. 2015, 2015, 604028. [Google Scholar] [CrossRef] [PubMed]
- Schappe, L.; Klein, C.; Stögbauer, J.; Federspiel, J.; Lochner, P. Die Rolle der Duplexsonographie der Arteria ophthalmica bei Stenosen der Arteria carotis interna [Role of duplex ultrasonography of the ophthalmic artery in internal carotid artery stenosis]. Radiologie 2024, 64, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Sun, H.; Yi, Q. Association Between Retinal Microvascular Metrics Using Optical Coherence Tomography Angiography and Carotid Artery Stenosis in a Chinese Cohort. Front. Physiol. 2022, 13, 824646. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wu, J.; Wang, H.; Kwapong, W.R.; Yan, Y.; Wan, J.; Wang, P.; Liu, G.; Wang, R.; Hu, F.; et al. Influence of Carotid Artery Stenting on the Retina and Choroid. Transl Vis Sci Technol. 2024, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Durusoy, G.K.; Gumus, G.; Onay, M.; Altay, C.M.; Binboga, A.B. Early choroidal structure and choroidal vascularity index change after carotid stenting. Photodiagnosis Photodyn. Ther. 2022, 38, 102748. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Larsen, M.; Munch, I.C. Subfoveal choroidal thickness in relation to sex and axial length in 93 Danish university students. Invest. Ophthalmol. Vis. Sci. 2011, 52, 8438–8441. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.B.; Xu, L.; Jonas, J.B.; Shao, L.; Du, K.F.; Wang, S.; Chen, C.X.; Xu, J.; Wang, Y.X.; Zhou, J.Q.; et al. Subfoveal choroidal thickness: The Beijing Eye Study. Ophthalmology 2013, 120, 175–180. [Google Scholar] [CrossRef] [PubMed]
- István, L.; Czakó, C.; Élő, Á.; Mihály, Z.; Sótonyi, P.; Varga, A.; Ungvári, Z.; Csiszár, A.; Yabluchanskiy, A.; Conley, S.; et al. Imaging retinal microvascular manifestations of carotid artery disease in older adults: From diagnosis of ocular complications to understanding microvascular contributions to cognitive impairment. Geroscience 2021, 43, 1703–1723. [Google Scholar] [CrossRef] [PubMed]
- Lamb, T.D.; Collin, S.P.; Pugh, E.N., Jr. Evolution of the vertebrate eye: Opsins, photoreceptors, retina and eye cup. Nat. Rev. Neurosci. 2007, 8, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.; Kanagasingam, Y.; Sohrabi, H.; Vignarajan, J.; Bourgeat, P.; Salvado, O.; Villemagne, V.; Rowe, C.C.; Macaulay, S.L.; Szoeke, C.; et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl. Psychiatry 2013, 3, 233. [Google Scholar] [CrossRef] [PubMed]


| Total Sample (N = 52; 100%) | Revascularization | p | |||
|---|---|---|---|---|---|
| CEA (N = 35; 67.3%) | CAS (N = 17; 32.7%) | ||||
| Ν (%) | Ν (%) | Ν (%) | |||
| Gender | |||||
| Male | 43 (82.7) | 28 (80.0) | 15 (88.2) | 0.700 ++ | |
| Female | 9 (17.3) | 7 (20.0) | 2 (11.8) | ||
| Age, mean (SD) | 68.4 (7.9) | 67.9 (8.2) | 69.3 (7.3) | 0.560 ‡ | |
| Coronary heart disease | 22 (42.3) | 13 (37.1) | 9 (52.9) | 0.279 + | |
| Hypertension | 47 (90.4) | 30 (85.7) | 17 (100) | 0.159 ++ | |
| Hypercholesterolemia | 52 (100) | 35 (100) | 17 (100) | - | |
| Diabetes | 17 (32.7) | 13 (37.1) | 4 (23.5) | 0.326 + | |
| Peripheral artery disease | 3 (5.9) | 2 (5.9) | 1 (5.9) | >0.999 ++ | |
| Nicotine use | 29 (56.9) | 20 (58.8) | 9 (52.9) | 0.689 + | |
| Bilateral carotid stenosis | 26 (50) | 17 (48.6) | 9 (52.9) | 0.768 + | |
| Side | |||||
| Left | 22 (42.3) | 12 (34.3) | 10 (58.8) | 0.093 + | |
| Right | 30 (57.7) | 23 (65.7) | 7 (41.2) | ||
| Pre-Revascularization | Post-Revascularization | Change | p (Wilcoxon Signed-Rank Test) | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | ||
| ppCTs | 119.7 (70.9) | 89.5 (67–160) | 123.3 (69.3) | 97 (75.5–154) | 3.6 (15.2) | 0.100 |
| ppCTi | 98.6 (62.5) | 79.5 (57–128.5) | 104.6 (62.3) | 86 (65.5–131) | 6 (16.6) | 0.004 |
| ppCTt | 117.1 (64.7) | 103 (62.5–163.5) | 117 (63.7) | 102 (67.5–159.5) | −0.1 (27) | 0.694 |
| ppCTn | 110.8 (62.7) | 89 (62–129) | 115.4 (65.7) | 95 (65.5–132) | 4.6 (21.6) | 0.117 |
| CMT | 247.8 (23.7) | 248.1 (232.5–259) | 246.9 (24.5) | 247.5 (232.5–258) | −1 (16.3) | 0.070 |
| MTs | 276.8 (18.4) | 277.3 (267.5–289) | 278.1 (24.3) | 276.5 (265–287) | 1.3 (18) | 0.049 |
| MTi | 274.4 (17.4) | 274.5 (263.5–284) | 271.4 (16.1) | 271 (260–283) | −3 (9.5) | 0.066 |
| MTst | 265.4 (16.4) | 267.5 (257.5–275.5) | 264.1 (15.3) | 265.5 (255.5–274) | −1.3 (10.6) | 0.021 |
| MTit | 268.1 (14.8) | 269 (258–279.5) | 265.9 (14.2) | 265.5 (255.5–277) | −2.2 (7.4) | 0.013 |
| MTsn | 288.3 (21.3) | 287.5 (276–300.5) | 286.6 (18.1) | 285.5 (275–298.5) | −1.7 (11.1) | 0.068 |
| MTin | 286.2 (17.8) | 286 (276.5–296.5) | 286 (19.4) | 285.5 (275.5–296) | −0.2 (7.3) | 0.783 |
| SFCT | 212 (93.7) | 201.4 (131–281.5) | 220.9 (92.2) | 204 (138.5–292.5) | 8.9 (30.2) | 0.011 |
| CTs | 225.8 (68.5) | 230.7 (190.5–240.5) | 220.4 (66.7) | 228.6 (176–235.5) | −5.4 (27.2) | 0.383 |
| CTi | 227.8 (89.6) | 234.6 (178.5–254) | 226.7 (93.1) | 237.2 (164–256) | −1.1 (24.3) | 0.201 |
| CTst | 216.5 (60.3) | 215.2 (200.5–239.5) | 209.8 (60.5) | 210.8 (176–230) | −6.7 (31.6) | 0.062 |
| Ctit | 219 (75.6) | 221.5 (181.5–268.5) | 219.9 (81) | 221.6 (160.5–283.5) | 0.9 (30.3) | 0.474 |
| CTsn | 187.3 (78.5) | 193 (146.5–198.1) | 187 (77.2) | 197.5 (135–204.5) | −0.3 (24.7) | 0.498 |
| CTin | 186.6 (84.1) | 191.4 (139–196.2) | 186.2 (84.6) | 192.6 (130–205) | −0.4 (19.2) | 0.206 |
| rVDc | 18.3 (5) | 18 (15.4–21.5) | 18.4 (5.1) | 17.6 (15.1–21.2) | 0.1 (4.6) | 0.848 |
| rVDs | 42.2 (5.1) | 42.1 (41–44.1) | 43.2 (4.6) | 43.6 (41.5–44.7) | 1 (4.6) | 0.140 |
| rVDi | 42 (4.7) | 42.3 (40.8–44.6) | 42.5 (4.4) | 42.7 (40.6–44.4) | 0.5 (4.6) | 0.358 |
| rVDt | 42 (3.7) | 42 (40.9–43.5) | 42.6 (3.5) | 42.7 (40–43.3) | 0.6 (3.6) | 0.343 |
| rVDn | 40.6 (5.9) | 40.4 (39–41.8) | 41.7 (6) | 41.7 (39.7–43.4) | 1.2 (5.2) | 0.033 |
| rVDtot | 37 (3.6) | 36.8 (35.8–38.3) | 37.7 (3.3) | 37.8 (35.8–39.2) | 0.7 (2.7) | 0.018 |
| ccVDc | 50.2 (6.8) | 49.6 (46.3–52.5) | 49.8 (5.7) | 49.8 (48.2–52.7) | −0.4 (5.5) | 0.891 |
| ccVDs | 49.3 (4.9) | 49.2 (48.7–51) | 50.1 (3.4) | 50.2 (49.2–51.3) | 0.8 (3) | 0.092 |
| ccVDi | 49.8 (5.3) | 50.8 (49.2–52.2) | 50.2 (3.2) | 50.3 (48.6–51.8) | 0.3 (4.4) | 0.967 |
| ccVDt | 51.1 (3.8) | 51.3 (50.4–52.5) | 52.2 (2.9) | 52.2 (51–53) | 1.1 (2.7) | 0.001 |
| ccVDn | 51 (5.1) | 51.2 (50.7–53.1) | 52.1 (4) | 52.4 (50.9–53.6) | 1.1 (3.6) | 0.049 |
| ccVDtot | 50.3 (3) | 50.4 (49.7–51.6) | 50.9 (2.1) | 50.7 (50.4–51.8) | 0.6 (1.7) | 0.015 |
| Pre-Revascularization | Post-Revascularization | Change | p (Wilcoxon Signed-Rank Test) | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | ||
| ppCTs | 123.1 (59.5) | 108 (80–152) | 123.1 (59.5) | 111 (75.5–155.5) | 0 (15.4) | 0.535 |
| ppCTi | 93.4 (58.9) | 84.5 (54–106) | 93.9 (58.5) | 84 (54–105) | 0.5 (14.3) | 0.963 |
| ppCTt | 111.5 (58.2) | 103.5 (70–132) | 115.4 (64.5) | 103.5 (70.5–127) | 3.9 (22.6) | 0.265 |
| ppCTn | 115.8 (56.1) | 105.5 (70.5–146.5) | 112.5 (57.1) | 100.5 (69–138.5) | −3.3 (26.2) | 0.622 |
| CMT | 246.6 (25.2) | 249 (234.5–262.5) | 245.8 (25.2) | 247 (229.5–262.5) | −0.8 (13) | 0.177 |
| MTs | 271.8 (24.8) | 273.5 (267–284.5) | 277 (17.7) | 277.5 (268–286.5) | 5.2 (19.6) | 0.161 |
| MTi | 271 (18.2) | 271.5 (260.5–280) | 269.4 (18.5) | 272 (258.5–279) | −1.6 (8) | 0.136 |
| MTst | 260.5 (23.1) | 263.5 (255.5–274.5) | 265.4 (15.7) | 265 (258.5–275.5) | 4.9 (18.5) | 0.152 |
| MTit | 266.3 (15.8) | 270 (258.5–274.5) | 266.3 (16.5) | 269 (257.5–275) | 0 (4.9) | 0.895 |
| MTsn | 285.4 (21) | 285 (276.5–296) | 286.6 (20.8) | 287.5 (276.5–297.2) | 1.2 (8.2) | 0.042 |
| MTin | 283.6 (21.9) | 285.5 (272–294.5) | 283.4 (21.2) | 284 (272.5–292.8) | −0.2 (6.6) | 0.975 |
| SFCT | 223.8 (85) | 217 (160.5–265) | 230.3 (83.7) | 220 (166–272) | 6.5 (20) | 0.050 |
| CTs | 221.2 (62.9) | 228.7 (197.5–237) | 224.4 (65.8) | 235.1 (193.5–238) | 3.2 (16.7) | 0.091 |
| CTi | 216.9 (80.1) | 224.3 (181.5–249.5) | 212.9 (78.8) | 225.4 (169–248.5) | −4 (20) | 0.359 |
| CTst | 211.4 (63) | 218.3 (184.5–229.5) | 215 (65) | 221.4 (186.5–229.5) | 3.6 (18.7) | 0.076 |
| CTit | 215.6 (68) | 219.1 (175.5–235.5) | 213.6 (68.4) | 219.8 (163.5–231) | −2 (15.9) | 0.547 |
| CTsn | 187.8 (76.7) | 202.7 (142.5–205.4) | 186.8 (77.1) | 200.1 (134–205) | −1.1 (22.1) | 0.359 |
| CTin | 178.8 (70.7) | 187.5 (139–195.2) | 181.1 (80.8) | 195.5 (124–196) | 2.3 (25.9) | 0.355 |
| rVDc | 18.2 (4.3) | 17.4 (15.9–19.8) | 18.5 (4.5) | 17.7 (16.9–20.6) | 0.3 (2.8) | 0.268 |
| rVDs | 40.3 (4.9) | 40.3 (39.5–42.6) | 41.8 (3.5) | 41.9 (41.1–44) | 1.6 (4.5) | 0.022 |
| rVDi | 40.7 (4.3) | 40.7 (38.4–42.9) | 42.3 (8.9) | 42.2 (40.5–44) | 1.6 (9.8) | 0.003 |
| rVDt | 40.7 (3.6) | 40.7 (39.5–43) | 41.7 (3.4) | 42.2 (40.2–43.8) | 1 (3.8) | 0.043 |
| rVDn | 40 (4.7) | 40.3 (38.1–42.3) | 40.6 (3.5) | 41.1 (39.7–42.5) | 0.7 (4.1) | 0.133 |
| rVDtot | 36 (2.8) | 36.2 (35.2–37.5) | 37 (2.6) | 37 (35.5–38.1) | 1 (2.2) | 0.001 |
| ccVDc | 49.3 (4.8) | 49.4 (46.9–51.5) | 49.9 (4.3) | 49.5 (47.6–52.2) | 0.6 (4.7) | 0.530 |
| ccVDs | 48.7 (3.2) | 48.9 (48.1–50.7) | 49.7 (3.7) | 50 (49.5–51.3) | 1 (3.6) | 0.005 |
| ccVDi | 49.7 (3.3) | 50.3 (48.3–51.3) | 49.7 (3.4) | 50.2 (48.7–51.7) | 0.1 (3.8) | 0.330 |
| ccVDt | 51.4 (2.5) | 51.6 (50.8–52.8) | 52 (2.7) | 52.2 (51.2–52.7) | 0.6 (2.3) | 0.018 |
| ccVDn | 51.1 (3.7) | 51.4 (49–53.4) | 51.8 (3.6) | 51.8 (51–53.4) | 0.7 (2.7) | 0.038 |
| ccVDtot | 50 (2.3) | 50.3 (49.4–51.3) | 50.6 (2) | 50.7 (49.8–51.5) | 0.6 (2.1) | 0.077 |
| Revascularization | Pre-Revascularization | Post-Revascularization | Change | p 1 | p 2 | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | ||||
| RNFLtot | CEA | 104.9 (15.6) | 104 (97–116) | 104.3 (14.6) | 104 (93–115) | −0.6 (3.9) | 0.647 | 0.356 |
| CAS | 102.9 (8.9) | 103 (102–107) | 103.5 (9.6) | 103 (101–109) | 0.5 (3.6) | 0.698 | ||
| RNFLs | CEA | 130 (41.4) | 130 (106–144) | 124.6 (23.5) | 125 (106–142) | −5.4 (29.9) | 0.575 | 0.511 |
| CAS | 124.7 (17.6) | 124 (112–140) | 124.2 (17.9) | 125 (110–134) | −0.5 (6.3) | 0.637 | ||
| RNFLi | CEA | 140.4 (41.3) | 139 (121–149) | 135 (20.2) | 140 (121–152) | −5.4 (35.3) | 0.967 | 0.260 |
| CAS | 129.1 (14.1) | 130 (128–135) | 132.2 (15) | 131 (128–135) | 3.1 (8.4) | 0.452 | ||
| RNFLt | CEA | 79.8 (42.7) | 72 (65–83) | 74.7 (17.3) | 72 (65–81) | −5.1 (29.3) | 0.212 | 0.497 |
| CAS | 71.3 (8.6) | 71.3 (64–79) | 71.5 (9.6) | 72 (63–81) | 0.2 (3.6) | 0.469 | ||
| RNFLn | CEA | 90.3 (40) | 85 (76–93) | 84 (15.1) | 83 (73–94) | −6.3 (31.3) | 0.157 | 0.505 |
| CAS | 87.4 (14.7) | 86 (81–96) | 86.5 (15.8) | 87 (76–93) | −0.9 (4.5) | 0.420 | ||
| ppCTs | CEA | 119.9 (77.4) | 87 (67–161) | 125.1 (73.1) | 93 (77–155) | 5.2 (16) | 0.030 | 0.090 |
| CAS | 119.3 (57.1) | 106 (67–160) | 119.6 (62.8) | 98 (70–153) | 0.4 (13.1) | 0.925 | ||
| ppCTi | CEA | 100.7 (64.4) | 79 (61–133) | 109.4 (61.5) | 96 (70–139) | 8.8 (17) | 0.002 | 0.127 |
| CAS | 94.5 (60) | 81 (44–118) | 94.8 (64.6) | 75 (48–118) | 0.3 (14.6) | 0.460 | ||
| ppCTt | CEA | 115.3 (63.2) | 101 (57–165) | 116.9 (65.8) | 102 (68–165) | 1.6 (27.7) | 0.674 | 0.526 |
| CAS | 120.8 (69.4) | 120.8 (69–148) | 117.1 (61) | 109 (63–154) | −3.7 (26) | 0.924 | ||
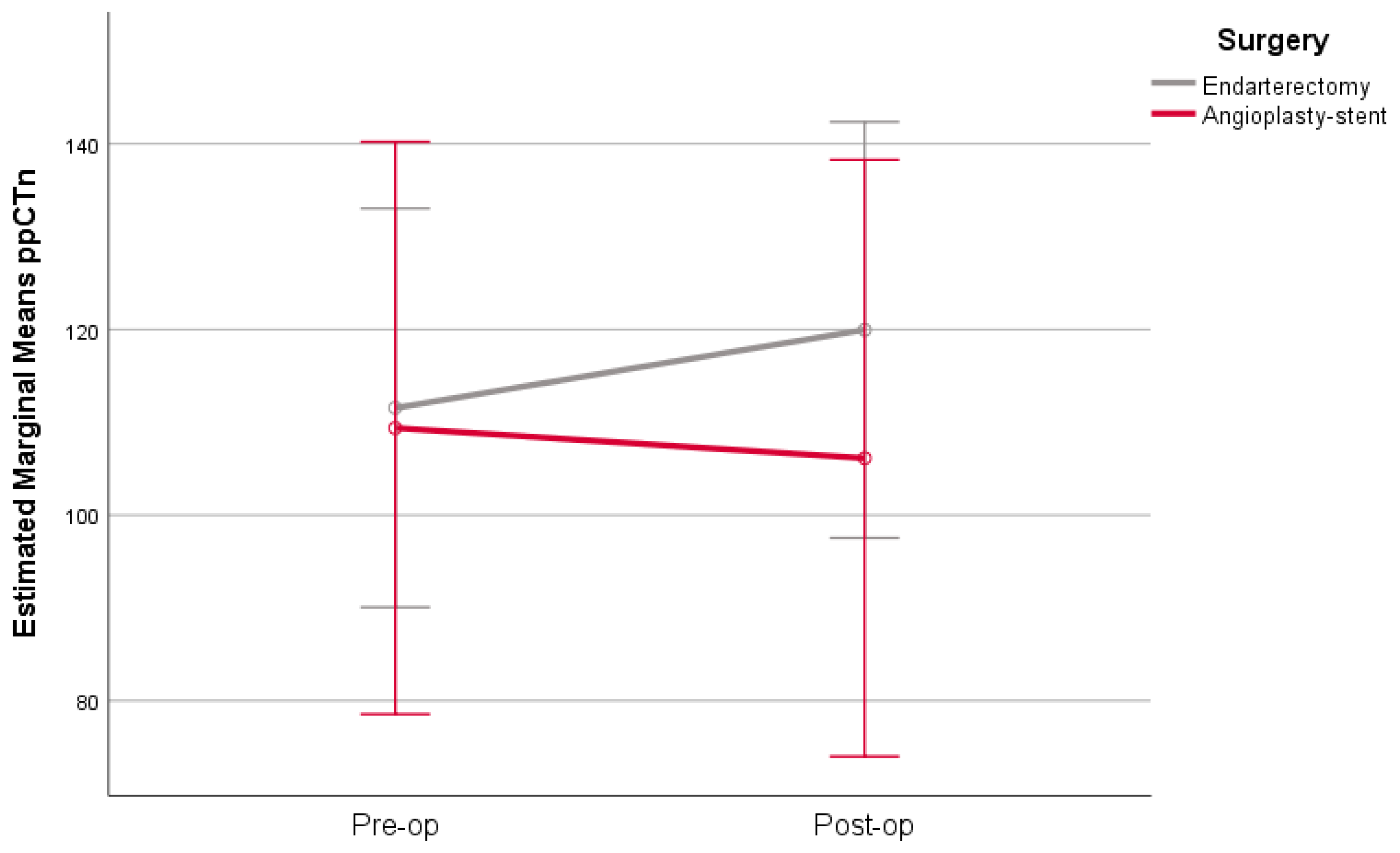
| ppCTn | CEA | 111.5 (63.9) | 104 (61–125) | 119.9 (65.2) | 112 (70–134) | 8.4 (22.3) | 0.034 | 0.038 |
| CAS | 109.4 (62) | 85 (63–133) | 106.1 (67.7) | 79 (63–113) | −3.3 (18.3) | 0.649 | ||
| CMT | CEA | 247.6 (26.6) | 248 (228–261) | 247 (27.8) | 248 (232–261) | −0.6 (19.9) | 0.398 | 0.856 |
| CAS | 248.3 (16.9) | 248.3 (240–254) | 246.5 (16.3) | 247 (239–250) | −1.7 (3.1) | 0.037 | ||
| MTs | CEA | 276.5 (17) | 277 (265–290) | 278 (27.6) | 276 (260–288) | 1.4 (20.4) | 0.111 | 0.892 |
| CAS | 277.3 (21.5) | 277.3 (270–286) | 278.4 (16.2) | 279 (266–281) | 1 (12.5) | 0.255 | ||
| MTi | CEA | 275 (19.3) | 276 (264–284) | 272.5 (17) | 273 (260–284) | −2.6 (9.8) | 0.289 | 0.536 |
| CAS | 273.2 (13) | 273.2 (263–283) | 269.2 (14.3) | 266 (257–274) | −4 (8.9) | 0.100 | ||
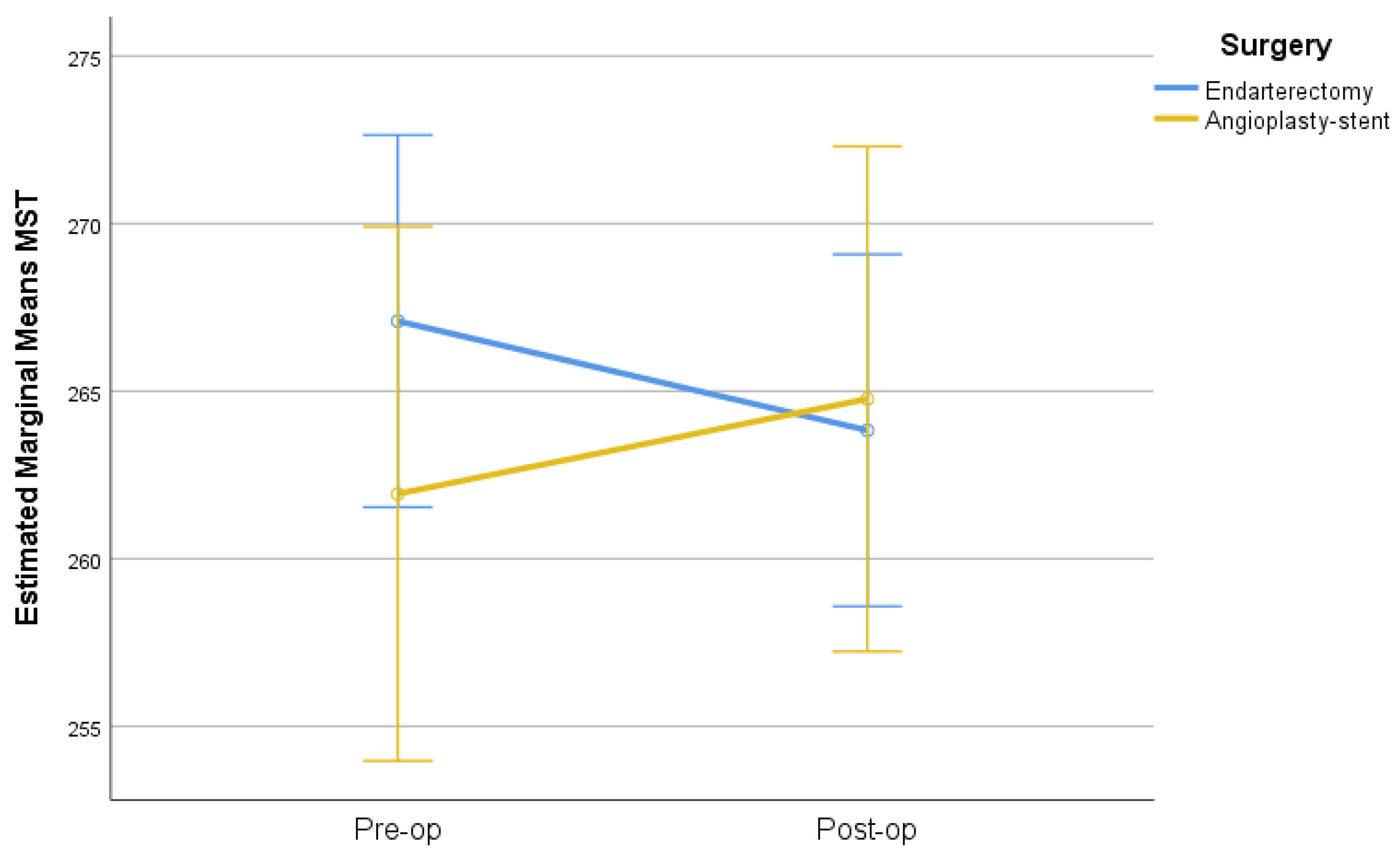
| MTst | CEA | 267.1 (14.6) | 268 (258–277) | 263.8 (16.1) | 266 (252–275) | −3.3 (8.7) | 0.008 | 0.047 |
| CAS | 261.9 (19.6) | 263 (255–274) | 264.8 (14.1) | 264 (256–272) | 2.8 (13.1) | 0.756 | ||
| MTit | CEA | 269.5 (15.1) | 269 (259–280) | 266.5 (15) | 267 (254–278) | −3 (7.7) | 0.010 | 0.280 |
| CAS | 265.4 (14.1) | 267 (256–272) | 264.8 (12.7) | 265 (256–271) | −0.6 (6.5) | 0.488 | ||
| MTsn | CEA | 287.2 (22.6) | 287 (275–302) | 285 (18.6) | 286 (272–300) | −2.2 (11.4) | 0.122 | 0.655 |
| CAS | 290.6 (18.8) | 290.6 (282–300) | 289.9 (17.1) | 285 (279–297) | −0.7 (10.7) | 0.343 | ||
| MTin | CEA | 284.5 (18.2) | 284 (276–295) | 285.6 (20.1) | 289 (275–296) | 1.1 (6.4) | 0.329 | 0.075 |
| CAS | 289.7 (16.9) | 289.7 (277–298) | 286.9 (18.2) | 283 (276–296) | −2.8 (8.4) | 0.344 | ||
| Pre | Post | Change | p 1 | p 2 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | ||||
| SFCT | CEA | 213.5 (95.8) | 194 (130–284) | 223.1 (95) | 206 (134–300) | 9.6 (28.7) | 0.011 | 0.928 |
| CAS | 208.9 (92.1) | 208.9 (134–254) | 216.5 (88.8) | 202 (163–266) | 7.6 (34.1) | 0.407 | ||
| CTs | CEA | 230.7 (62.6) | 230.7 (198–239) | 228.5 (60.9) | 228.6 (203–230) | −2.2 (19.3) | 0.723 | 0.168 |
| CAS | 215.5 (80.3) | 215.5 (168–242) | 203.6 (76.6) | 203.6 (147–245) | −11.9 (38.7) | 0.246 | ||
| Cti | CEA | 234.6 (82.2) | 234.6 (206–242) | 237.2 (82.6) | 237.2 (186–249) | 2.6 (17.2) | 0.001 | 0.075 |
| CAS | 213.7 (104.6) | 213.7 (143–262) | 205.1 (111.4) | 191 (135–261) | −8.6 (34.1) | 0.276 | ||
| CTst | CEA | 215.2 (51.6) | 215.2 (203–228) | 210.8 (52.5) | 210.8 (189–223) | −4.5 (20.1) | 0.171 | 0.470 |
| CAS | 219.2 (77) | 219.2 (165–263) | 207.8 (76.2) | 207.8 (163–231) | −11.4 (47.9) | 0.237 | ||
| CTit | CEA | 221.5 (67.9) | 221.5 (190–260) | 221.6 (64) | 221.6 (176–281) | 0.1 (17.6) | 0.056 | 0.529 |
| CAS | 213.8 (91.6) | 213.8 (165–299) | 216.3 (110.3) | 204 (144–298) | 2.5 (47.6) | 0.538 | ||
| CTsn | CEA | 198.1 (74.7) | 198.1 (157–214) | 198.1 (76.1) | 198.1 (153–211) | 0 (17.2) | 0.617 | 0.963 |
| CAS | 165 (83.7) | 155 (123–165) | 164.1 (76.6) | 151 (128–197) | −0.9 (36.3) | 0.776 | ||
| CTin | CEA | 191.4 (80) | 191.4 (143–201) | 192.5 (80.1) | 192.6 (142–206) | 1.1 (15.2) | 0.045 | 0.383 |
| CAS | 176.5 (93.7) | 176.5 (125–182) | 173 (94.3) | 170 (116–204) | −3.5 (25.9) | 0.943 | ||
| rVDc | CEA | 18 (4.9) | 18 (15.3–20.8) | 17.6 (4.7) | 17.6 (14.5–21.2) | −0.4 (4.9) | 0.646 | 0.321 |
| CAS | 19 (5.2) | 19 (15.4–22.1) | 20 (5.5) | 20 (16.6–22.5) | 1 (3.9) | 0.554 | ||
| rVDs | CEA | 41.6 (5.9) | 41.6 (39.3–44.3) | 43.9 (4.8) | 43.9 (41.2–45.9) | 2.3 (4.6) | 0.004 | 0.007 |
| CAS | 43.5 (2.8) | 43.5 (42.3–43.9) | 41.9 (3.9) | 43.2 (41.9–44.4) | −1.7 (3.5) | 0.102 | ||
| rVDi | CEA | 41.4 (5.5) | 41.4 (38.1–44.5) | 42.3 (4.9) | 42.3 (40.8–44.2) | 0.9 (4.8) | 0.094 | 0.326 |
| CAS | 43.4 (1.9) | 43.2 (41.8–44.7) | 43 (3.3) | 43 (40.5–44.9) | −0.4 (4.1) | 0.434 | ||
| rVDt | CEA | 42 (4) | 42 (40.8–43.6) | 43 (3.5) | 43 (40–44.3) | 1 (3.4) | 0.159 | 0.254 |
| CAS | 41.9 (3.2) | 41.9 (41.3–43.2) | 41.8 (3.5) | 41.8 (39.7–42.9) | −0.1 (3.9) | 0.653 | ||
| rVDn | CEA | 41.2 (6.5) | 41.2 (38.9–42.7) | 42.1 (6.5) | 42.1 (39.8–43.5) | 0.9 (5.1) | 0.225 | 0.590 |
| CAS | 39.3 (4.5) | 39.3 (39–40.5) | 40.9 (4.7) | 40.9 (38.7–41.8) | 1.6 (5.4) | 0.049 | ||
| rVDtot | CEA | 36.8 (4.1) | 36.8 (35.5–38.3) | 37.8 (3.7) | 37.8 (35.7–39.3) | 0.9 (2.7) | 0.018 | 0.261 |
| CAS | 37.4 (2.2) | 37.4 (36.8–38.3) | 37.5 (2.6) | 37.7 (37–38.5) | 0.1 (2.6) | 0.356 | ||
| ccVDc | CEA | 49.2 (6.9) | 49.2 (45.8–52.2) | 48.8 (5.7) | 49.5 (47.1–52.7) | −0.4 (6.1) | 0.909 | 0.999 |
| CAS | 52.3 (6.4) | 51.6 (49.3–53.3) | 51.8 (5.1) | 51.6 (48.3–52.8) | −0.5 (4.3) | 0.687 | ||
| ccVDs | CEA | 49.2 (5.8) | 49.2 (48.7–51.1) | 50.2 (3.8) | 50.2 (49.2–51.4) | 1 (3.3) | 0.088 | 0.341 |
| CAS | 49.6 (2.1) | 49.5 (48.7–50.2) | 49.8 (2.3) | 49.8 (49.3–50.1) | 0.2 (2.4) | 0.507 | ||
| ccVDi | CEA | 49.4 (6.3) | 49.4 (49.1–52.3) | 49.7 (3.3) | 49.7 (48.4–51.8) | 0.3 (5.2) | 0.961 | 0.853 |
| CAS | 50.8 (1.6) | 51 (50.1–51.7) | 51.2 (2.7) | 51.2 (49.4–52.3) | 0.5 (2.5) | 0.868 | ||
| ccVDt | CEA | 51.3 (4.5) | 51.3 (50.7–53.2) | 52.4 (3.2) | 52.3 (51.1–53) | 1.1 (3.1) | 0.032 | 0.797 |
| CAS | 50.8 (1.7) | 50.8 (49.7–51.5) | 51.8 (2.3) | 51.8 (50.7–53.1) | 1.1 (1.7) | 0.015 | ||
| ccVDn | CEA | 51.1 (6) | 51.4 (51–54.3) | 52.4 (4.4) | 52.4 (51.3–53.7) | 1.3 (4) | 0.127 | 0.562 |
| CAS | 50.8 (2.4) | 50.8 (49.9–52.6) | 51.6 (3) | 51.6 (50.5–53.4) | 0.8 (2.9) | 0.209 | ||
| ccVDtot | CEA | 50 (3.4) | 50.2 (49.8–51.6) | 50.7 (2.4) | 50.7 (50.3–51.5) | 0.7 (1.8) | 0.011 | 0.553 |
| CAS | 50.9 (1.5) | 50.9 (49.7–51.9) | 51.3 (1.4) | 51.3 (50.6–52.2) | 0.4 (1.5) | 0.381 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xanthou, F.; Dastiridou, A.; Giannoukas, A.; Matsagkas, M.; Tzavara, C.; Chaidoulis, A.; Androudi, S.; Tsironi, E.E. Ocular Structural and Vascular Changes in Patients with Severe Asymptomatic Carotid Disease After Undergoing Carotid Endarterectomy (CEA) and Carotid Artery Stenting (CAS). Diagnostics 2025, 15, 1826. https://doi.org/10.3390/diagnostics15141826
Xanthou F, Dastiridou A, Giannoukas A, Matsagkas M, Tzavara C, Chaidoulis A, Androudi S, Tsironi EE. Ocular Structural and Vascular Changes in Patients with Severe Asymptomatic Carotid Disease After Undergoing Carotid Endarterectomy (CEA) and Carotid Artery Stenting (CAS). Diagnostics. 2025; 15(14):1826. https://doi.org/10.3390/diagnostics15141826
Chicago/Turabian StyleXanthou, Foteini, Anna Dastiridou, Athanasios Giannoukas, Miltiadis Matsagkas, Chara Tzavara, Athanasios Chaidoulis, Sofia Androudi, and Evangelia E. Tsironi. 2025. "Ocular Structural and Vascular Changes in Patients with Severe Asymptomatic Carotid Disease After Undergoing Carotid Endarterectomy (CEA) and Carotid Artery Stenting (CAS)" Diagnostics 15, no. 14: 1826. https://doi.org/10.3390/diagnostics15141826
APA StyleXanthou, F., Dastiridou, A., Giannoukas, A., Matsagkas, M., Tzavara, C., Chaidoulis, A., Androudi, S., & Tsironi, E. E. (2025). Ocular Structural and Vascular Changes in Patients with Severe Asymptomatic Carotid Disease After Undergoing Carotid Endarterectomy (CEA) and Carotid Artery Stenting (CAS). Diagnostics, 15(14), 1826. https://doi.org/10.3390/diagnostics15141826





