Impact of Sodium-Glucose Cotransporter-2 Inhibitors on Post-Transurethral Resection of Bladder Tumor Infection and Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Outcomes
2.3. Statistical Analysis
3. Results
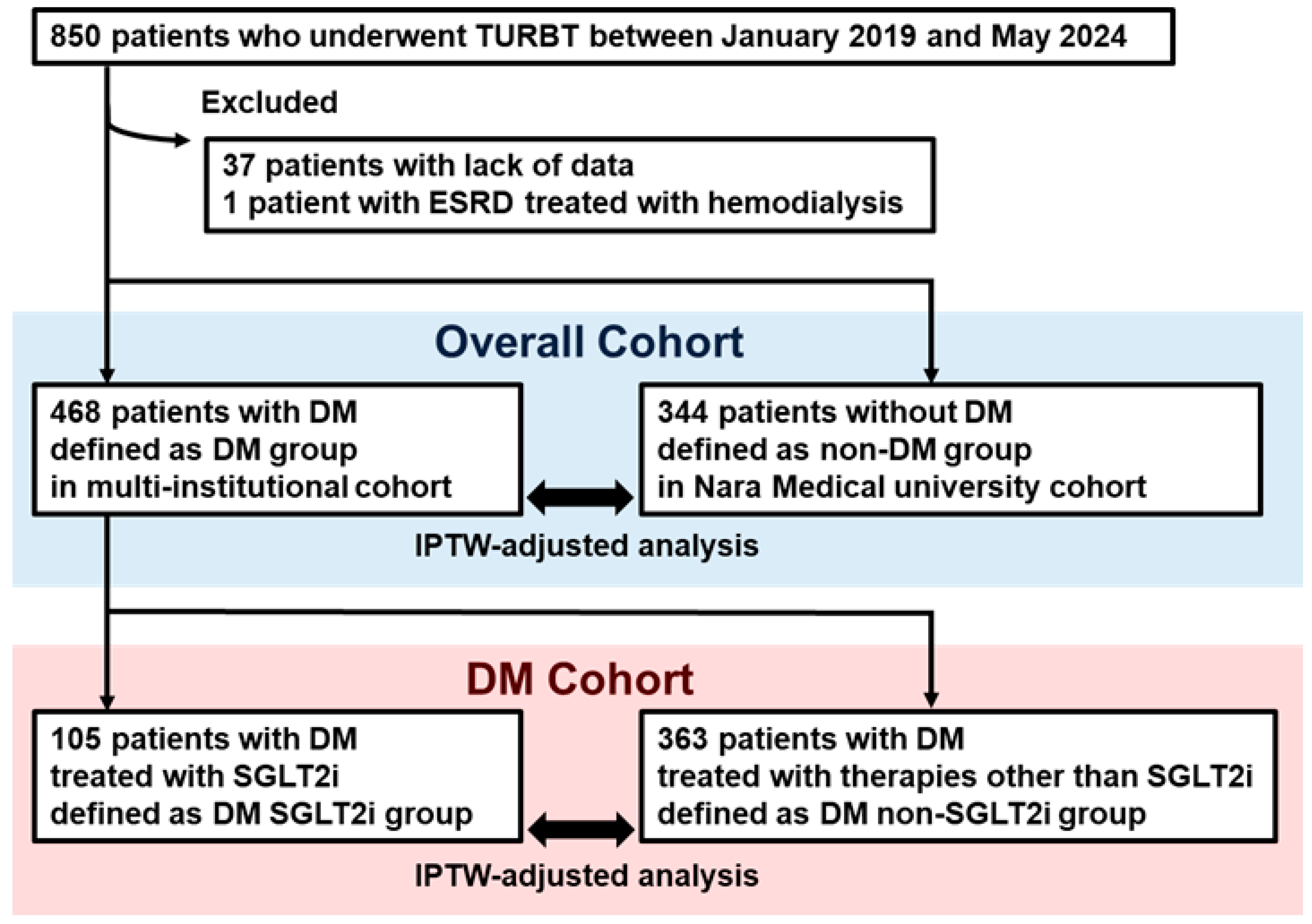
3.1. Patient Selection
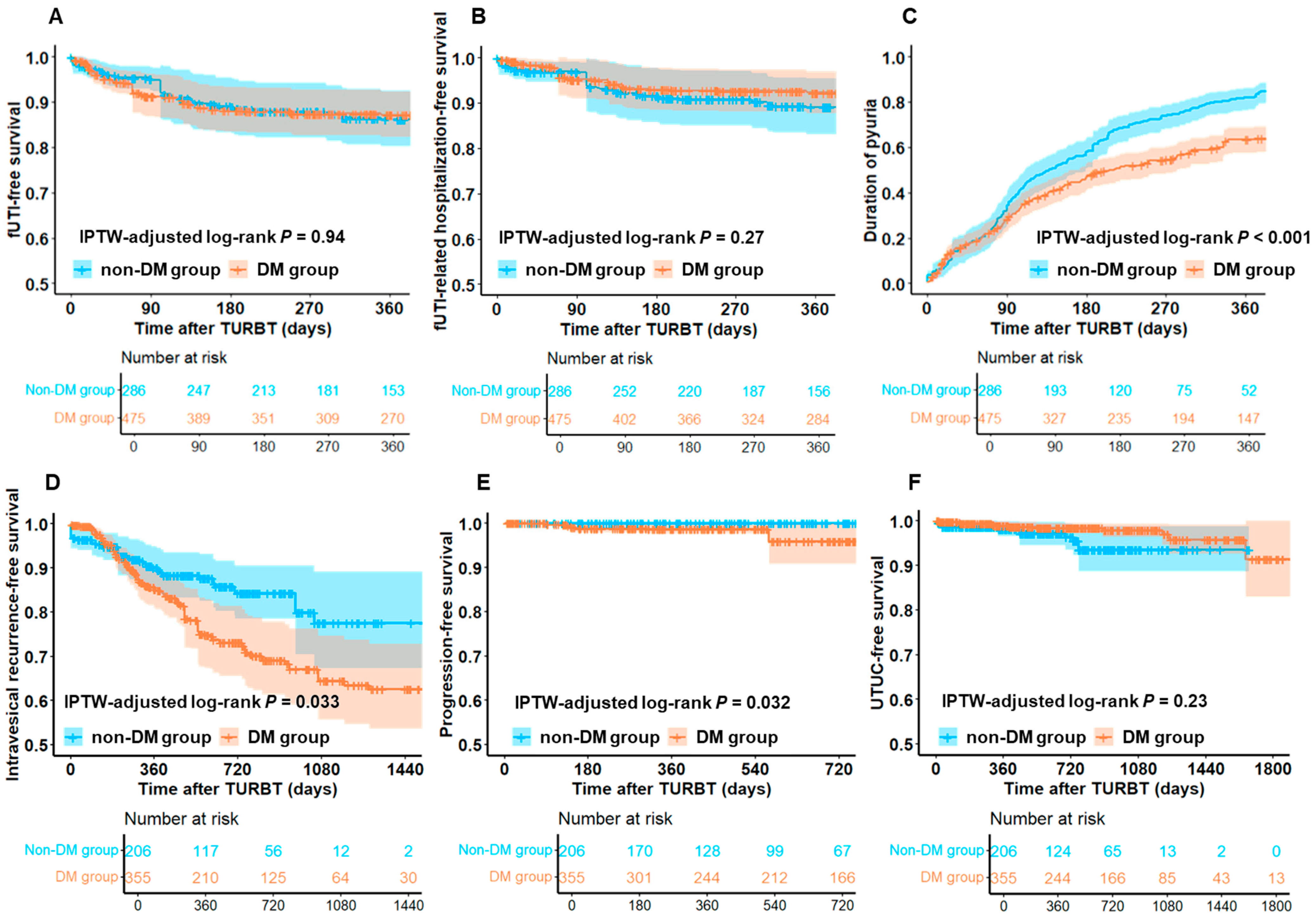
3.2. Comparison of Functional and Oncological Outcomes Between DM and Non-DM Groups
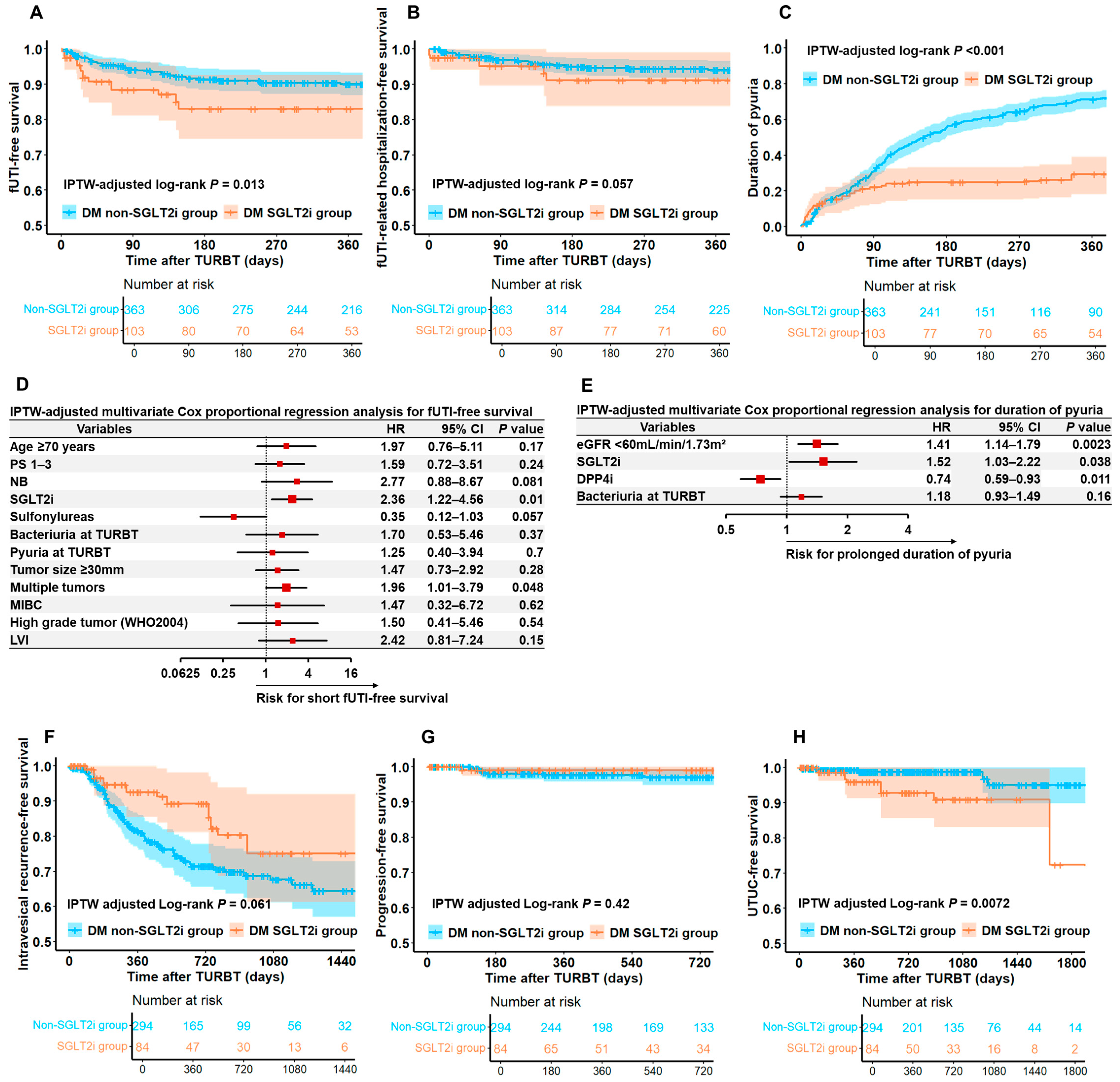
3.3. Comparison of Functional and Oncological Outcomes Between DM SGLT2i and DM Non-SGLT2i Groups
3.4. Functional Outcomes of Differences in Each Type of SGLT2i
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCG | Bacille Calmette–Guérin |
| CI | confidence interval |
| CIS | carcinoma in situ |
| DM | diabetes mellitus |
| ECOG-PS | Eastern Cooperative Oncology Group-performance status |
| fUTI | febrile urinary tract infection |
| HR | hazard ratio |
| IPTW | inverse probability of treatment weighting |
| IVRFS | intravesical recurrence-free survival |
| LVI | lymphovascular invasion |
| MIBC | muscle invasive bladder cancer |
| PFS | progression-free survival |
| SD | standard deviation |
| SGLT2i | sodium-glucose cotransporter-2 inhibitor |
| SMD | standardized mean differences |
| T2DM | type 2 diabetes mellitus |
| TURBT | transurethral resection of bladder tumor |
| UTI | urinary tract infection |
| UTUC | upper urinary tract urothelial carcinoma |
References
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Escrig, J.L.D.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (ta, T1, and carcinoma in situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.; Cerantola, Y.; Fritschi, U.; Hubner, M.; Iglesias, K.; Legris, A.S.; Lucca, I.; Vlamopoulos, Y.; Vaucher, L.; Jichlinski, P. Comorbidity and nutritional indices as predictors of morbidity after transurethral procedures: A prospective cohort study. Can. Urol. Assoc. J. 2014, 8, E600–E604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richards, K.A.; Smith, N.D.; Steinberg, G.D. The importance of transurethral resection of bladder tumor in the management of nonmuscle invasive bladder cancer: A systematic review of novel technologies. J. Urol. 2014, 191, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Kohada, Y.; Goriki, A.; Yukihiro, K.; Ohara, S.; Kajiwara, M. The risk factors of urinary tract infection after transurethral resection of bladder tumors. World J. Urol. 2019, 37, 2715–2719. [Google Scholar] [CrossRef]
- Stalenhoef, J.E.; van Dissel, J.T.; van Nieuwkoop, C. Febrile urinary tract infection in the emergency room. Curr. Opin. Infect. Dis. 2015, 28, 106–111. [Google Scholar] [CrossRef]
- De Nicola, L.D.; Gabbai, F.B.; Liberti, M.E.; Sagliocca, A.; Conte, G.; Minutolo, R. Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: Targeting the renal tubule in diabetes. Am. J. Kidney Dis. 2014, 64, 16–24. [Google Scholar] [CrossRef]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 inhibitors and the diabetic kidney. Diabetes Care 2016, 39 (Suppl. S2), S165–S171. [Google Scholar] [CrossRef]
- Škrtić, M.; Cherney, D.Z.I. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 2015, 24, 96–103. [Google Scholar] [CrossRef]
- van der Aart-van der Beek, A.B.; de Boer, R.A.D.; Heerspink, H.J.L. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat. Rev. Nephrol. 2022, 18, 294–306. [Google Scholar] [CrossRef]
- Jhund, P.S. SGLT2 inhibitors and heart failure with preserved ejection fraction. Heart Fail. Clin. 2022, 18, 579–586. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, S.; Liu, L. Efficacy and safety evaluation of SGLT2i on blood pressure control in patients with type 2 diabetes and hypertension: A new meta-analysis. Diabetol. Metab. Syndr. 2023, 15, 118. [Google Scholar] [CrossRef]
- Pollack, R.; Cahn, A. SGLT2 inhibitors and safety in older patients. Heart Fail. Clin. 2022, 18, 635–643. [Google Scholar] [CrossRef]
- Kittipibul, V.; Cox, Z.L.; Chesdachai, S.; Fiuzat, M.; Lindenfeld, J.; Mentz, R.J. Genitourinary tract infections in patients taking SGLT2 inhibitors: JACC review topic of the week. J. Am. Coll. Cardiol. 2024, 83, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Tanrıverdi, M.; Baştemir, M.; Demirbakan, H.; Ünalan, A.; Türkmen, M.; Tanrıverdi, G.Ö. Association of SGLT-2 inhibitors with bacterial urinary tract infection in type 2 diabetes. BMC Endocr. Disord. 2023, 23, 211. [Google Scholar] [CrossRef] [PubMed]
- Tentolouris, A.; Vlachakis, P.; Tzeravini, E.; Eleftheriadou, I.; Tentolouris, N. SGLT2 inhibitors: A review of their antidiabetic and cardioprotective effects. Int. J. Environ. Res. Public Health 2019, 16, 2965. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Ding, L.L.; Zhang, M.; Zhou, H.R. Safety of four SGLT2 inhibitors in three chronic diseases: A meta-analysis of large randomized trials of SGLT2 inhibitors. Diabetes Vasc. Dis. Res. 2021, 18, 14791641211011016. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Htike, Z.Z.; Youssef, D.; Khunti, K.; Davies, M.J. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: Systematic review and network meta-analysis. Diabetes Obes. Metab. 2016, 18, 783–794. [Google Scholar] [CrossRef]
- Cornu, J.N.; Herrmann, T.; Traxer, O.; Matlaga, B. Prevention and management following complications from endourology procedures. Eur. Urol. Focus. 2016, 2, 49–59. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Joglekar, O.; Alkandari, M.; Joshi, P.M. Management of post TURP strictures. World J. Urol. 2019, 37, 589–594. [Google Scholar] [CrossRef]
- Bausch, K.; Halbeisen, F.S.; Aghlmandi, S.; Sutter, S.U.; Ewald, H.; Appenzeller-Herzog, C.; Roth, J.A.; Widmer, A.F.; Seifert, H.H. Antimicrobial prophylaxis for postoperative urinary tract infections in transurethral resection of bladder tumors: A systematic review and meta-analysis. J. Urol. 2021, 205, 987–998. [Google Scholar] [CrossRef]
- Kim, B.S.; Tae, B.S.; Ku, J.H.; Kwak, C.; Kim, H.H.; Jeong, C.W. Rate and association of lower urinary tract infection with recurrence after transurethral resection of bladder tumor. Investig. Clin. Urol. 2018, 59, 10–17. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hou, C.P.; Juang, H.H.; Chang, P.L.; Chen, T.H.; Chen, C.L.; Tsui, K.H. Association between bladder outlet obstruction and bladder cancer in patients with aging male. J. Clin. Med. 2019, 8, 1550. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, B.; Bogokowsky, B.; Nativ, O.; Sidi, A.A.; Jonas, P.; Many, M. Urinary infections following transurethral resection of bladder tumors—Rate and source. J. Urol. 1983, 129, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.F.; Pareek, G.; Mueller-Leonhard, C.; Zhang, Z.; Amin, A.; Mega, A.; Tucci, C.; Golijanin, D.; Gershman, B. The perioperative morbidity of transurethral resection of bladder tumor: Implications for quality improvement. Urology 2019, 125, 131–137. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 17 July 2025).
- Pishdad, R.; Auwaerter, P.G.; Kalyani, R.R. Diabetes, SGLT-2 inhibitors, and urinary tract infection: A review. Curr. Diab Rep. 2024, 24, 108–117. [Google Scholar] [CrossRef]
- Dave, C.V.; Schneeweiss, S.; Kim, D.; Fralick, M.; Tong, A.; Patorno, E. Sodium-glucose Cotransporter-2 inhibitors and the risk for severe urinary tract infections: A population-based cohort study. Ann. Intern. Med. 2019, 171, 248–256. [Google Scholar] [CrossRef]
- Suzuki, M.; Hiramatsu, M.; Fukazawa, M.; Matsumoto, M.; Honda, K.; Suzuki, Y.; Kawabe, Y. Effect of SGLT2 inhibitors in a murine model of urinary tract infection with Candida albicans. Diabetes Obes. Metab. 2014, 16, 622–627. [Google Scholar] [CrossRef]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J. Pharmacol. Sci. 2016, 130, 159–169. [Google Scholar] [CrossRef]
- Hwang, E.C.; Kim, Y.J.; Hwang, I.S.; Hwang, J.E.; Jung, S.I.; Kwon, D.D.; Park, K.; Ryu, S.B. Impact of diabetes mellitus on recurrence and progression in patients with non-muscle invasive bladder carcinoma: A retrospective cohort study. Int. J. Urol. 2011, 18, 769–776. [Google Scholar] [CrossRef]
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Andersson, I.G.; Liedberg, F.; Mariappan, P.; Mostafid, A.H.; et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2023 update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef]

| Variables | Unweighted Population | IPTW-Adjusted Population | ||||
|---|---|---|---|---|---|---|
| DM Group | Non-DM Group | SMD | DM Group | Non-DM Group | SMD | |
| n (%) | n (%) | % | % | |||
| N | 468 | 344 | 475 | 286 | ||
| Age, years, mean ± SD | 75.8 ± 8.6 | 71.8 ± 11.2 | 0.40 | 74.8 | 73.4 | 0.15 |
| Sex | 0.08 | 0.01 | ||||
| Male | 406 (86.8%) | 289 (84.0%) | 85.3% | 85.6% | ||
| Female | 62 (13.2%) | 55 (16.0%) | 14.7% | 14.4% | ||
| ECOG-PS | 0.42 | 0.22 | ||||
| 0 | 373 (79.7%) | 277 (80.5%) | 78.9% | 77.4% | ||
| 1 | 63 (13.5%) | 29 (8.4%) | 11.7% | 15.5% | ||
| 2 | 21 (4.5%) | 7 (2.0%) | 4.3% | 1.6% | ||
| 3 | 4 (0.9%) | 0 (0.0%) | 0.5% | 0.0% | ||
| Unknown | 7 (1.5%) | 31 (9.0%) | 4.6% | 5.4% | ||
| Smoking history | 0.51 | 0.12 | ||||
| Never | 145 (31.0%) | 53 (15.4%) | 23.7% | 27.4% | ||
| Former | 171 (36.5%) | 133 (38.7%) | 39.4% | 38.2% | ||
| Current | 76 (16.2%) | 42 (12.2%) | 14.3% | 11.2% | ||
| Unknown | 76 (16.2%) | 116 (33.7%) | 22.5% | 23.2% | ||
| BMI, kg/m2, mean ± SD | 23.5 ± 3.9 | 23.2 ± 3.5 | 0.09 | 23.4 | 23.7 | 0.08 |
| Urinary management | 0.19 | 0.14 | ||||
| Normal | 460 (98.3%) | 344 (100.0%) | 99.0% | 100.0% | ||
| Self-catheterization | 3 (0.6%) | 0 (0.0%) | 0.4% | 0.0% | ||
| Urinary catheter placement | 5 (1.1%) | 0 (0.0%) | 0.6% | 0.0% | ||
| Comorbidity | ||||||
| Heart disease | 131 (28.0%) | 27 (7.8%) | 0.54 | 19.9% | 13.5% | 0.17 |
| BPH | 126 (26.9%) | 90 (26.2%) | 0.02 | 25.3% | 21.4% | 0.09 |
| NB | 16 (3.4%) | 7 (2.0%) | 0.09 | 2.4% | 1.8% | 0.04 |
| OAB | 36 (7.7%) | 13 (3.8%) | 0.17 | 6.2% | 4.2% | 0.09 |
| History of pelvic radiation therapy | 0.20 | 0.04 | ||||
| Yes | 15 (3.2%) | 27 (7.8%) | 5.8% | 4.8% | ||
| No | 453 (96.8%) | 317 (92.2%) | 94.2% | 95.2% | ||
| Bacteriuria at TURBT | 0.26 | 0.20 | ||||
| Yes | 112 (23.9%) | 123 (35.8%) | 28.3% | 37.5% | ||
| No | 356 (76.1%) | 221 (64.2%) | 71.7% | 62.5% | ||
| Pyuria at TURBT | 0.28 | 0.01 | ||||
| Yes | 159 (34.0%) | 163 (47.4%) | 38.8% | 38.3% | ||
| No | 309 (66.0%) | 181 (52.6%) | 61.2% | 61.7% | ||
| History of recurrence | 0.13 | 0.08 | ||||
| Primary | 357 (76.3%) | 280 (81.4%) | 78.3% | 74.9% | ||
| Recurrence | 111 (23.7%) | 64 (18.6%) | 21.7% | 25.1% | ||
| Tumor size | 0.09 | 0.03 | ||||
| <30 mm | 384 (82.1%) | 271 (78.8%) | 81.8% | 81.9% | ||
| ≥30 mm | 73 (15.6%) | 62 (18.0%) | 15.3% | 15.6% | ||
| Undefined | 11 (2.4%) | 11 (3.2%) | 2.9% | 2.5% | ||
| Multiplicity | 0.22 | 0.08 | ||||
| Single | 234 (50.0%) | 186 (54.1%) | 51.0% | 53.7% | ||
| Multiple | 212 (45.3%) | 127 (36.9%) | 41.3% | 40.3% | ||
| Undefined | 22 (4.7%) | 31 (9.0%) | 7.7% | 6.0% | ||
| Pathological T stage | 0.33 | 0.14 | ||||
| T0 | 36 (7.7%) | 53 (15.4%) | 11.6% | 11.7% | ||
| Ta | 235 (50.2%) | 131 (38.0%) | 44.7% | 49.7% | ||
| Tis | 43 (9.2%) | 28 (8.1%) | 10.0% | 7.3% | ||
| T1 | 101 (21.6%) | 74 (21.5%) | 20.6% | 17.3% | ||
| MIBC | 53 (11.3%) | 58 (16.9%) | 13.2% | 14.0% | ||
| Grade (WHO2004) | 0.25 | 0.16 | ||||
| Low grade | 190 (40.6%) | 119 (34.6%) | 38.0% | 45.5% | ||
| High grade | 242 (51.7%) | 172 (50.0%) | 50.6% | 43.0% | ||
| No malignancy | 36 (7.7%) | 53 (15.4%) | 11.4% | 11.5% | ||
| Concomitant CIS | 0.04 | 0.05 | ||||
| Yes | 63 (13.5%) | 42 (12.2%) | 12.4% | 10.9% | ||
| No | 405 (86.5%) | 302 (87.8%) | 87.6% | 80.1% | ||
| LVI | 0.12 | 0.04 | ||||
| Yes | 34 (7.3%) | 37 (10.8%) | 8.7% | 7.6% | ||
| No | 434 (92.7%) | 307 (89.2%) | 91.3% | 92.4% | ||
| Second TUR | 0.10 | 0.05 | ||||
| Yes | 62 (13.2%) | 58 (16.9%) | 13.6% | 12.0% | ||
| No | 406 (86.8%) | 286 (83.1%) | 86.4% | 88.0% | ||
| Induction BCG therapy | 0.62 | 0.25 | ||||
| Yes | 76 (16.2%) | 34 (9.9%) | 9.3% | 5.5% | ||
| No | 392 (83.8%) | 310 (90.1%) | 90.7% | 94.5% | ||
| Maintenance BCG therapy | 0.29 | 0.21 | ||||
| Yes | 18 (3.8%) | 0 (0.0%) | 2.2% | 0.0% | ||
| No | 450 (96.2%) | 344 (100.0%) | 97.8% | 100.0% | ||
| Variables | Unweighted Population | IPTW-Adjusted Population | ||||
|---|---|---|---|---|---|---|
| DM SGLT2i Group | DM Non-SGLT2i Group | SMD | DM SGLT2i Group | DM Non-SGLT2i Group | SMD | |
| n (%) | n (%) | % | % | |||
| N | 105 | 363 | 103 | 363 | ||
| Age, years, mean ± SD | 74.3 ± 8.5 | 76.2 ± 8.6 | 0.22 | 76.2 | 75.8 | 0.05 |
| Sex | 0.11 | 0.19 | ||||
| Male | 94 (89.5%) | 312 (86.0%) | 81.5% | 86.5% | ||
| Female | 11 (10.5%) | 51 (14.0%) | 18.5% | 13.5% | ||
| ECOG-PS | 0.23 | 0.23 | ||||
| 0 | 87 (82.9%) | 286 (78.8%) | 75.0% | 79.3% | ||
| 1 | 14 (13.3%) | 49 (13.5%) | 19.2% | 13.7% | ||
| 2 | 3 (2.9%) | 18 (5.0%) | 5.4% | 4.9% | ||
| 3 | 1 (1.0%) | 3 (0.8%) | 0.4% | 0.8% | ||
| Unknown | 0 (0.0%) | 7 (1.9%) | 0.0% | 1.3% | ||
| Smoking history | 0.12 | 0.08 | ||||
| Never | 29 (27.6%) | 116 (32.0%) | 30.8% | 30.3% | ||
| Former | 42 (40.0%) | 129 (35.5%) | 39.8% | 37.5% | ||
| Current | 18 (17.1%) | 58 (16.0%) | 14.0% | 16.4% | ||
| Unknown | 16 (15.2%) | 60 (16.5%) | 15.5% | 15.9% | ||
| BMI, kg/m2, mean ± SD | 23.8 ± 3.8 | 23.5 ± 3.9 | 0.07 | 23.5 | 23.6 | 0.00 |
| Urinary management | 0.21 | 0.19 | ||||
| Normal | 105 (100.0%) | 355 (97.8%) | 100.0% | 98.2% | ||
| Self-catheterization | 0 (0.0%) | 3 (0.8%) | 0.0% | 0.7% | ||
| Urinary catheter placement | 0 (0.0%) | 5 (1.4%) | 0.0% | 1.1% | ||
| Comorbidity | ||||||
| Heart disease | 34 (32.4%) | 97 (26.7%) | 0.12 | 22.8% | 27.0% | 0.10 |
| BPH | 28 (26.7%) | 98 (27.0%) | 0.01 | 22.4% | 26.8% | 0.10 |
| NB | 3 (2.9%) | 13 (3.6%) | 0.04 | 1.4% | 2.7% | 0.09 |
| OAB | 7 (6.7%) | 29 (8.0%) | 0.05 | 4.9% | 7.4% | 0.11 |
| History of pelvic radiation therapy | 0.03 | 0.09 | ||||
| Yes | 3 (2.9%) | 12 (3.3%) | 1.6% | 2.9% | ||
| No | 102 (97.1%) | 351 (96.7%) | 98.4% | 97.1% | ||
| Blood examination at TURBT | ||||||
| eGFR, mL/min/1.73 m2, mean ± SD | 56.7 ± 15.6 | 58.4 ± 17.8 | 0.11 | 57.7 | 58.1 | 0.03 |
| HbA1c, %, mean ± SD | 7.2 ± 0.8 | 7.2 ± 1.1 | 0.03 | 7.23 | 7.24 | 0.01 |
| Antidiabetic drugs | ||||||
| Insulin | 9 (8.6%) | 38 (10.5%) | 0.07 | 8.0% | 9.6% | 0.06 |
| Sulfonylureas | 19 (18.1%) | 81 (22.3%) | 0.11 | 23.7% | 22.1% | 0.04 |
| Biguanides | 35 (33.3%) | 85 (23.4%) | 0.22 | 29.9% | 25.3% | 0.10 |
| DPP4i | 51 (48.6%) | 234 (64.5%) | 0.33 | 62.4% | 61.6% | 0.02 |
| Thiazolidinediones | 6 (5.7%) | 21 (5.8%) | 0.00 | 3.3% | 5.4% | 0.11 |
| GLP-1 receptor agonists | 1 (1.0%) | 4 (1.1%) | 0.02 | 4.0% | 10.0% | 0.07 |
| α-glucosidase inhibitors | 11 (10.5%) | 30 (8.3%) | 0.08 | 12.0% | 8.9% | 0.10 |
| Meglitinides | 4 (3.8%) | 10 (2.8%) | 0.06 | 2.2% | 2.9% | 0.05 |
| Bacteriuria at TURBT | 0.05 | 0.02 | ||||
| Yes | 27 (25.7%) | 85 (23.4%) | 22.0% | 22.9% | ||
| No | 78 (74.3%) | 278 (76.6%) | 78.0% | 77.1% | ||
| Pyuria at TURBT | 0.07 | 0.02 | ||||
| Yes | 33 (31.4%) | 126 (34.7%) | 33.5% | 32.6% | ||
| No | 72 (68.6%) | 237 (65.3%) | 66.5% | 67.4% | ||
| History of recurrence | 0.31 | 0.02 | ||||
| Primary | 90 (85.7%) | 267 (73.6%) | 76.5% | 75.5% | ||
| Recurrence | 15 (14.3%) | 96 (26.4%) | 23.5% | 24.5% | ||
| Tumor size | 0.11 | 0.05 | ||||
| <30 mm | 85 (81.0%) | 299 (82.4%) | 84.3% | 82.8% | ||
| ≥30 mm | 16 (15.2%) | 57 (15.7%) | 14.2% | 15.1% | ||
| Undefined | 4 (3.8%) | 7 (1.9%) | 1.5% | 2.1% | ||
| Multiplicity | 0.21 | 0.03 | ||||
| Single | 52 (49.5%) | 182 (50.1%) | 48.8% | 50.1% | ||
| Multiple | 44 (41.9%) | 168 (46.3%) | 46.5% | 45.1% | ||
| Undefined | 9 (8.6%) | 13 (3.6%) | 4.8% | 4.8% | ||
| Pathological T stage | 0.25 | 0.13 | ||||
| T0 | 8 (7.6%) | 29 (8.0%) | 6.3% | 7.5% | ||
| Ta | 51 (48.6%) | 183 (50.4%) | 55.8% | 51.7% | ||
| Tis | 11 (10.5%) | 32 (8.8%) | 6.0% | 8.9% | ||
| T1 | 28 (26.7%) | 73 (20.1%) | 21.4% | 20.9% | ||
| MIBC | 7 (6.7%) | 46 (12.7%) | 10.6% | 11.0% | ||
| Grade (WHO2004) | 0.17 | 0.10 | ||||
| Low grade | 36 (34.3%) | 153 (42.1%) | 46.7% | 42.0% | ||
| High grade | 61 (58.1%) | 181 (49.9%) | 47.0% | 50.7% | ||
| No malignancy | 8 (7.6%) | 29 (8.0%) | 6.3% | 7.3% | ||
| Concomitant CIS | 0.27 | 0.02 | ||||
| Yes | 22 (21.0%) | 41 (11.3%) | 12.5% | 13.2% | ||
| No | 83 (79.0%) | 322 (88.7%) | 87.5% | 86.8% | ||
| LVI | 0.02 | 0.04 | ||||
| Yes | 8 (7.6%) | 26 (7.2%) | 5.4% | 6.4% | ||
| No | 97 (92.4%) | 337 (92.8%) | 94.6% | 93.6% | ||
| Second TUR | 0.07 | 0.05 | ||||
| Yes | 16 (15.2%) | 46 (12.7%) | 11.4% | 13.0% | ||
| No | 89 (84.8%) | 317 (87.3%) | 88.6% | 87.0% | ||
| Induction BCG therapy | 0.22 | 0.10 | ||||
| Yes | 24 (22.9%) | 52 (14.3%) | 11.9% | 15.4% | ||
| No | 81 (77.1%) | 311 (85.7%) | 88.1% | 84.6% | ||
| Maintenance BCG therapy | 0.06 | 0.07 | ||||
| Yes | 5 (4.8%) | 13 (3.6%) | 2.6% | 3.8% | ||
| No | 100 (95.2%) | 350 (96.4%) | 97.4% | 96.2% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, N.; Miyake, M.; Miyamoto, T.; Ichii, D.; Naoi, M.; Narita, K.; Kohashi, M.; Tomioka, A.; Torimoto, K.; Kawashima, R.; et al. Impact of Sodium-Glucose Cotransporter-2 Inhibitors on Post-Transurethral Resection of Bladder Tumor Infection and Prognosis. Diagnostics 2025, 15, 1824. https://doi.org/10.3390/diagnostics15141824
Nishimura N, Miyake M, Miyamoto T, Ichii D, Naoi M, Narita K, Kohashi M, Tomioka A, Torimoto K, Kawashima R, et al. Impact of Sodium-Glucose Cotransporter-2 Inhibitors on Post-Transurethral Resection of Bladder Tumor Infection and Prognosis. Diagnostics. 2025; 15(14):1824. https://doi.org/10.3390/diagnostics15141824
Chicago/Turabian StyleNishimura, Nobutaka, Makito Miyake, Tatsuki Miyamoto, Daiki Ichii, Makito Naoi, Kosuke Narita, Mikiko Kohashi, Atsushi Tomioka, Kazumasa Torimoto, Ryotaro Kawashima, and et al. 2025. "Impact of Sodium-Glucose Cotransporter-2 Inhibitors on Post-Transurethral Resection of Bladder Tumor Infection and Prognosis" Diagnostics 15, no. 14: 1824. https://doi.org/10.3390/diagnostics15141824
APA StyleNishimura, N., Miyake, M., Miyamoto, T., Ichii, D., Naoi, M., Narita, K., Kohashi, M., Tomioka, A., Torimoto, K., Kawashima, R., Miyazaki, K., Iwao, T., Inoue, K., Matsubara, T., & Fujimoto, K. (2025). Impact of Sodium-Glucose Cotransporter-2 Inhibitors on Post-Transurethral Resection of Bladder Tumor Infection and Prognosis. Diagnostics, 15(14), 1824. https://doi.org/10.3390/diagnostics15141824







