Differential Visual Outcomes in Neovascular AMD Based on Ellipsoid Zone Integrity and Fluid Presence: Insights from a Phase III Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. HAWK Study Design
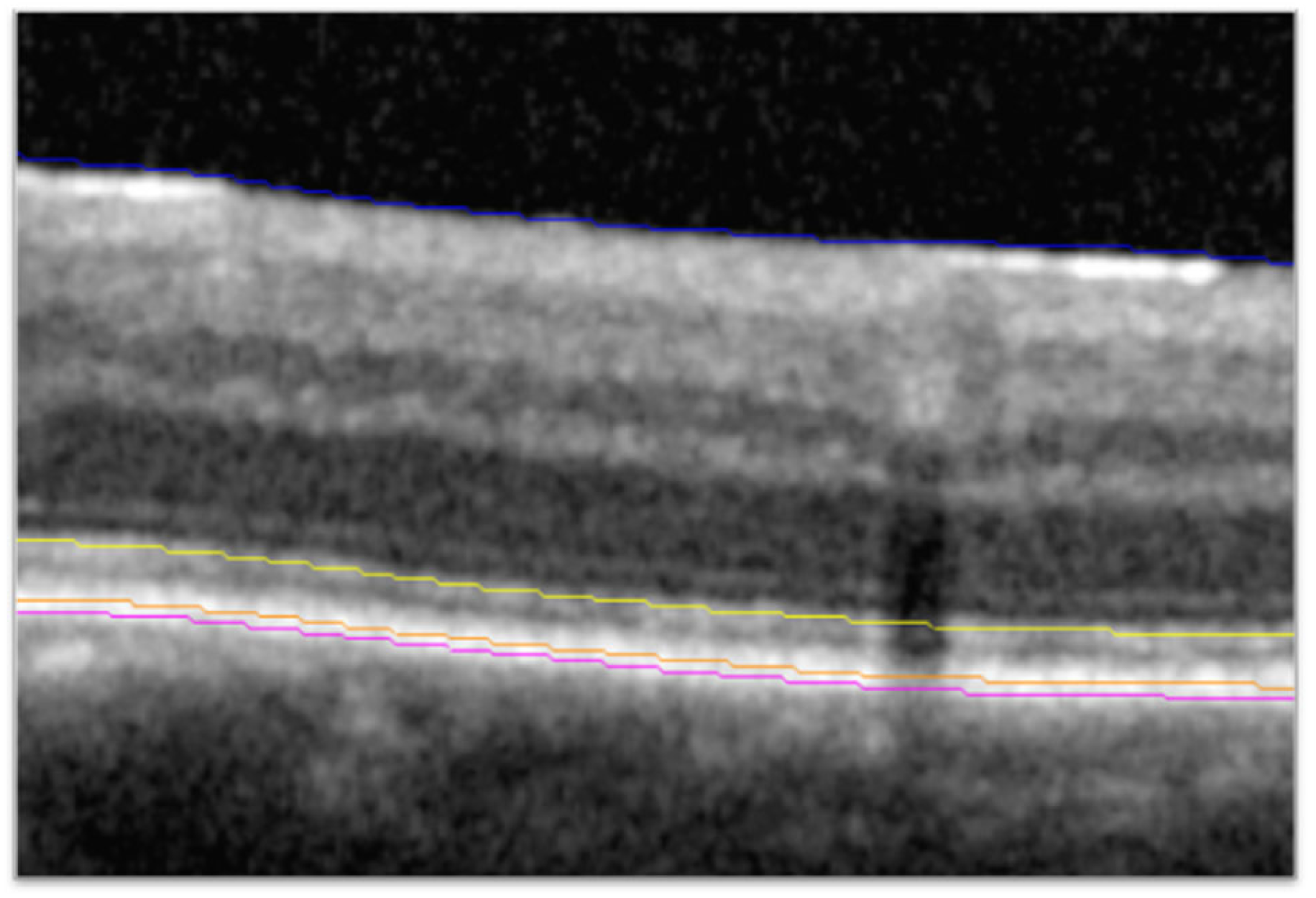
2.2. Higher-Order Optical Coherence Tomography Analysis
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics
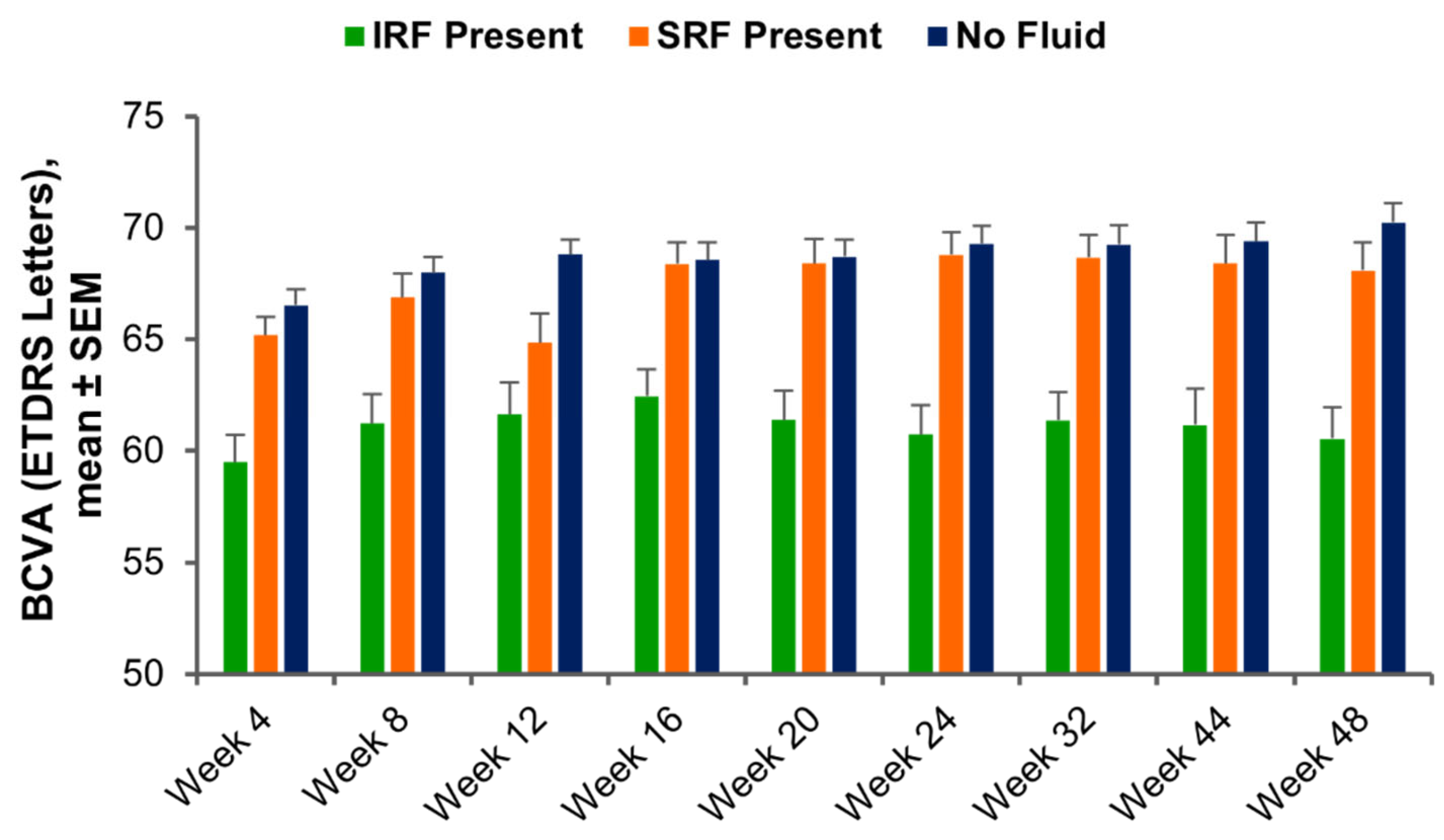
3.2. Visit-by-Visit Assessment of BCVA Based on Fluid Status and Ellipsoid Zone Integrity
3.3. Functional Outcomes Based on Fluid Dynamics and Ellipsoid Zone Integrity
3.3.1. Eyes That Achieved and Maintained No Fluid
3.3.2. Eyes with Any SRF or IRF at All Time Points
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keane, P.A.; de Salvo, G.; Sim, D.A.; Goverdhan, S.; Agrawal, R.; Tufail, A. Strategies for improving early detection and diagnosis of neovascular age-related macular degeneration. Clin. Ophthalmol. 2015, 9, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef] [PubMed]
- CATT Research Group; Martin, D.F.; Maguire, M.G.; Ying, G.-S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [PubMed]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Martin, D.F.; Toth, C.A.; Daniel, E.; Maguire, M.G.; Ying, G.-S.; Grunwald, J.E.; Huang, J. Macular morphology and visual acuity in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology 2013, 120, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Toth, C.A.; Daniel, E.; Grunwald, J.E.; Maguire, M.G.; Ying, G.-S.; Huang, J.; Martin, D.F.; Jaffe, G.J. Macular morphology and visual acuity in the second year of the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology 2016, 123, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N.M.; Sadda, S.; Sarraf, D.; Guymer, R.; Hill, L.; Blotner, S.; Spicer, G.; Gune, S. Effect of residual retinal fluid on visual function in ranibizumab-treated neovascular age-related macular degeneration. Am. J. Ophthalmol. 2022, 233, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.; Cooney, L.; Grechenig, C.; Sadeghipour, A.; Pablik, E.; Seaman, J.W.; Waldstein, S.M.; Schmidt-Erfurth, U. Topographic analysis of photoreceptor loss correlated with disease morphology in neovascular age-related macular degeneration. Retina 2020, 40, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Zahid, R.; Kaiser, P.K.; Heier, J.S.; Brown, D.M.; Meng, X.; Reese, J.; Le, T.K.; Lunasco, L.; Hu, M.; et al. Longitudinal assessment of ellipsoid zone integrity, subretinal hyperreflective material, and subretinal pigment epithelium disease in neovascular age-related macular degeneration. Ophthalmol. Retin. 2021, 5, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Lunasco, L.M.; Yordi, S.; Cetin, H.; Le, T.K.; Sarici, K.; Kaiser, P.K.; Khanani, A.M.; Talcott, K.E.; Hu, J.; et al. Compartmental Exudative Dynamics in Neovascular Age-Related Macular Degeneration: Volumetric Outcomes and Impact of Volatility in a Phase III Clinical Trial. Ophthalmol. Retin. 2024, 8, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K.; Wykoff, C.C.; Singh, R.P.; Khanani, A.M.; Do, D.V.; Patel, H.; Patel, N. Retinal fluid and thickness as measures of disease activity in neovascular age-related macular degeneration. Retina 2021, 41, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Yordi, S.; Cakir, Y.; Kalra, G.; Cetin, H.; Hu, M.; Abraham, J.; Reese, J.; Srivastava, S.K.; Ehlers, J.P. Ellipsoid Zone Integrity and Visual Function in Dry Age-Related Macular Degeneration. J. Pers. Med. 2024, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, T.; Dobson, E.T.A.; Wiedenmann, M.; Oden, N.; VanVeldhuisen, P.; Scott, I.U.; Ip, M.S.; Eliceiri, K.W.; Blodi, B.A.; Domalpally, A. Ellipsoid zone defects in retinal vein occlusion correlates with visual acuity prognosis: SCORE2 Report 14. Transl. Vis. Sci. Technol. 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Patel, N.; Kaiser, P.K.; Heier, J.S.; Brown, D.M.; Meng, X.; Reese, J.; Lunasco, L.; Le, T.K.; Hu, M.; et al. The association of fluid volatility with subretinal hyperreflective material and ellipsoid zone integrity in neovascular AMD. Investig. Ophthalmol. Vis. Sci. 2022, 63, 17. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Uchida, A.; Hu, M.; Figueiredo, N.; Kaiser, P.K.; Heier, J.S.; Brown, D.M.; Boyer, D.S.; Do, D.V.; Gibson, A.; et al. Higher-order assessment of OCT in diabetic macular edema from the VISTA study: Ellipsoid zone dynamics and the retinal fluid index. Ophthalmol. Retin. 2019, 3, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Sarici, K.; Abraham, J.R.; Sevgi, D.D.; Lunasco, L.; Srivastava, S.K.; Whitney, J.; Cetin, H.; Hanumanthu, A.; Bell, J.M.; Reese, J.L.; et al. Risk classification for progression to subfoveal geographic atrophy in dry age-related macular degeneration using machine learning-enabled outer retinal feature extraction. Ophthalmic Surg. Lasers Imaging Retina 2022, 53, 31–39. [Google Scholar] [CrossRef]
- Bell, J.; Whitney, J.; Cetin, H.; Le, T.; Cardwell, N.; Srivasatava, S.K.; Ehlers, J.P. Validation of Inter-Reader Agreement/Consistency for Quantification of Ellipsoid Zone Integrity and Sub-RPE Compartmental Features Across Retinal Diseases. Diagnostics 2024, 14, 2395. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehlers, J.P.; Yordi, S.; Cetin, H.; Amine, R.; Matar, K.; Indurkar, A.; Talcott, K.E.; Kaiser, P.K.; Khanani, A.M.; Hu, J.; et al. Differential Visual Outcomes in Neovascular AMD Based on Ellipsoid Zone Integrity and Fluid Presence: Insights from a Phase III Trial. Diagnostics 2025, 15, 1815. https://doi.org/10.3390/diagnostics15141815
Ehlers JP, Yordi S, Cetin H, Amine R, Matar K, Indurkar A, Talcott KE, Kaiser PK, Khanani AM, Hu J, et al. Differential Visual Outcomes in Neovascular AMD Based on Ellipsoid Zone Integrity and Fluid Presence: Insights from a Phase III Trial. Diagnostics. 2025; 15(14):1815. https://doi.org/10.3390/diagnostics15141815
Chicago/Turabian StyleEhlers, Justis P., Sari Yordi, Hasan Cetin, Reem Amine, Karen Matar, Asmita Indurkar, Katherine E. Talcott, Peter K. Kaiser, Arshad M. Khanani, Joanne Hu, and et al. 2025. "Differential Visual Outcomes in Neovascular AMD Based on Ellipsoid Zone Integrity and Fluid Presence: Insights from a Phase III Trial" Diagnostics 15, no. 14: 1815. https://doi.org/10.3390/diagnostics15141815
APA StyleEhlers, J. P., Yordi, S., Cetin, H., Amine, R., Matar, K., Indurkar, A., Talcott, K. E., Kaiser, P. K., Khanani, A. M., Hu, J., & Srivastava, S. K. (2025). Differential Visual Outcomes in Neovascular AMD Based on Ellipsoid Zone Integrity and Fluid Presence: Insights from a Phase III Trial. Diagnostics, 15(14), 1815. https://doi.org/10.3390/diagnostics15141815






