Feasibility of Observing Glymphatic System Activity During Sleep Using Diffusion Tensor Imaging Analysis Along the Perivascular Space (DTI-ALPS) Index
Abstract
1. Introduction
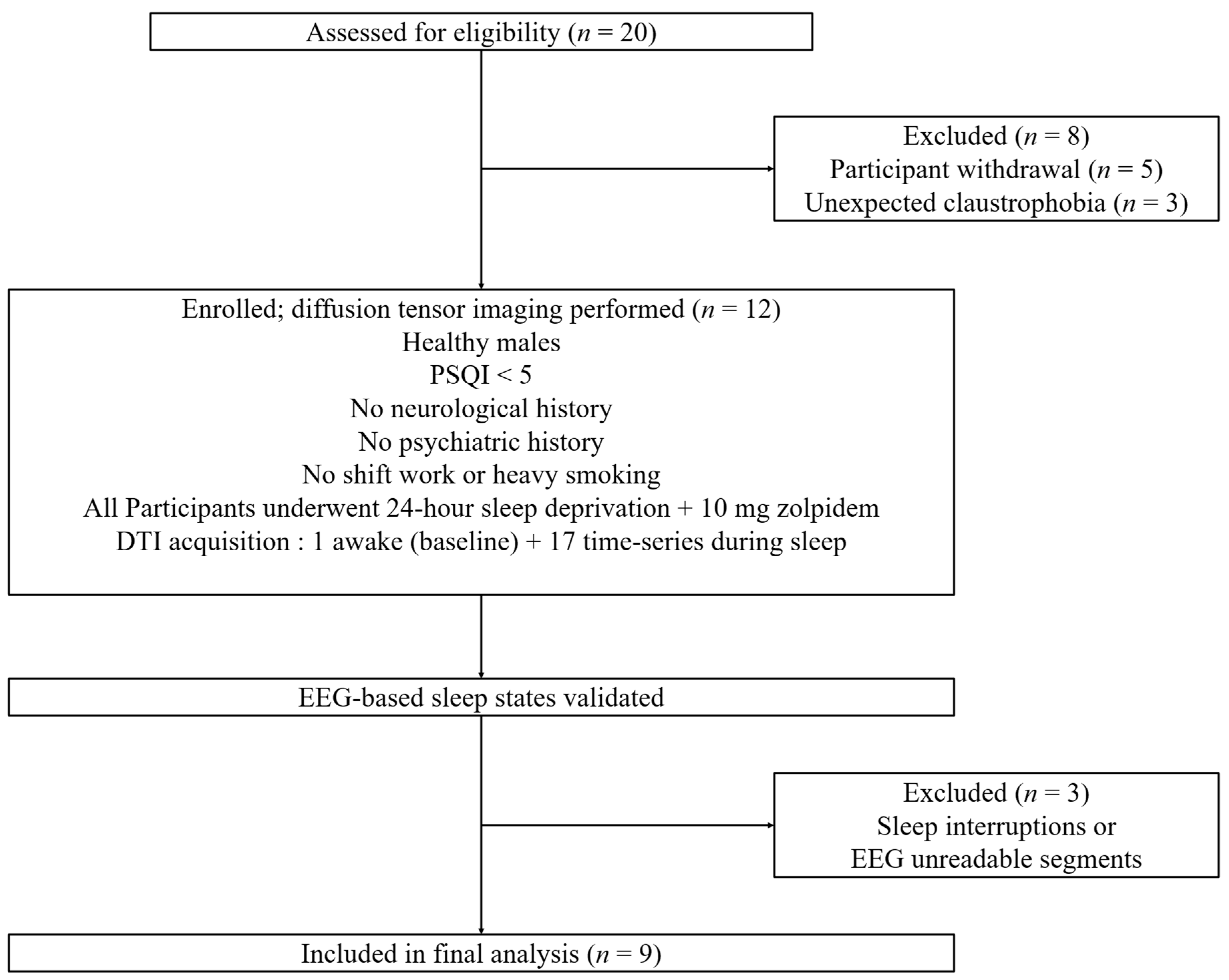
2. Materials and Methods
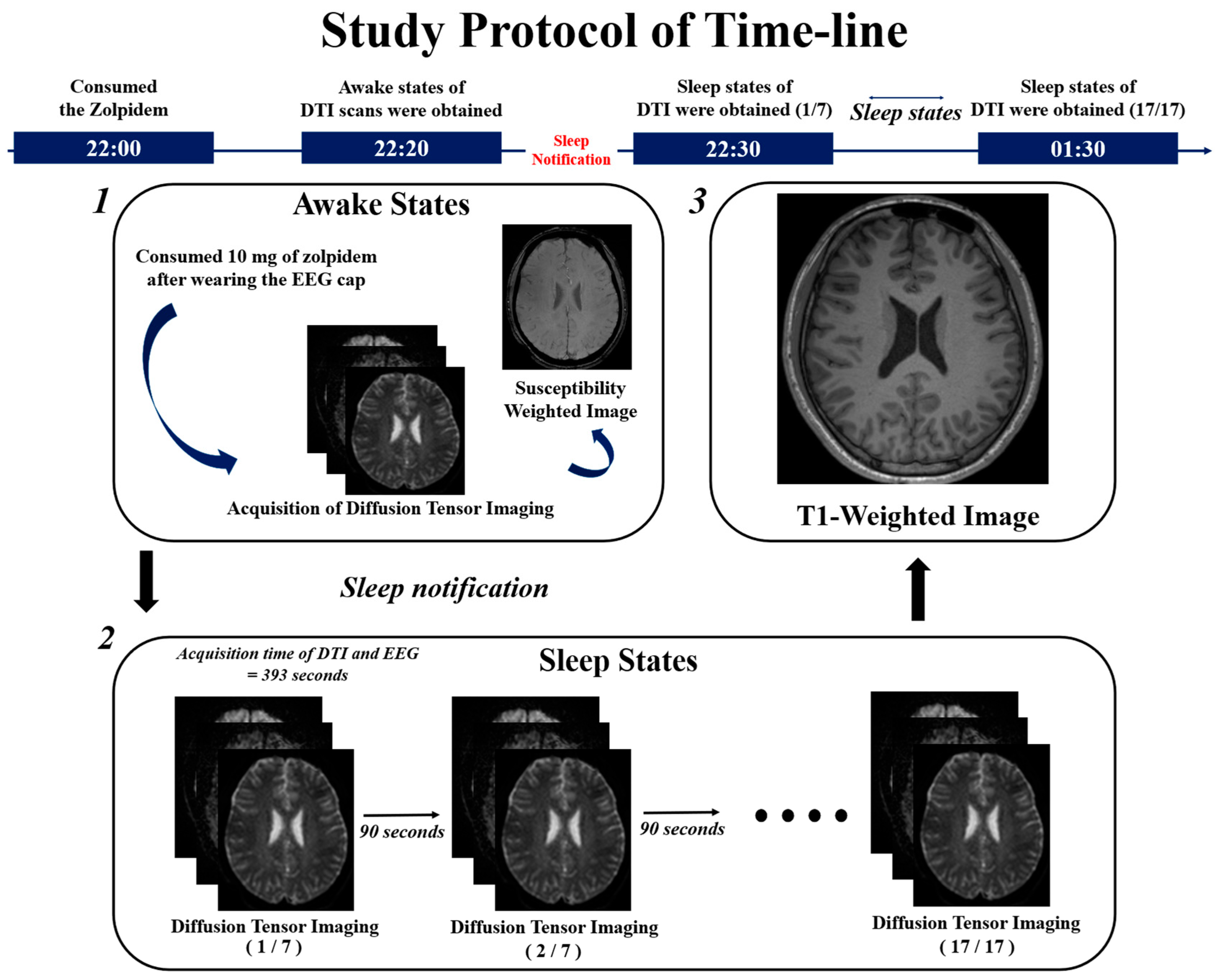
2.1. Study Design
2.2. Image Acquisition Protocol
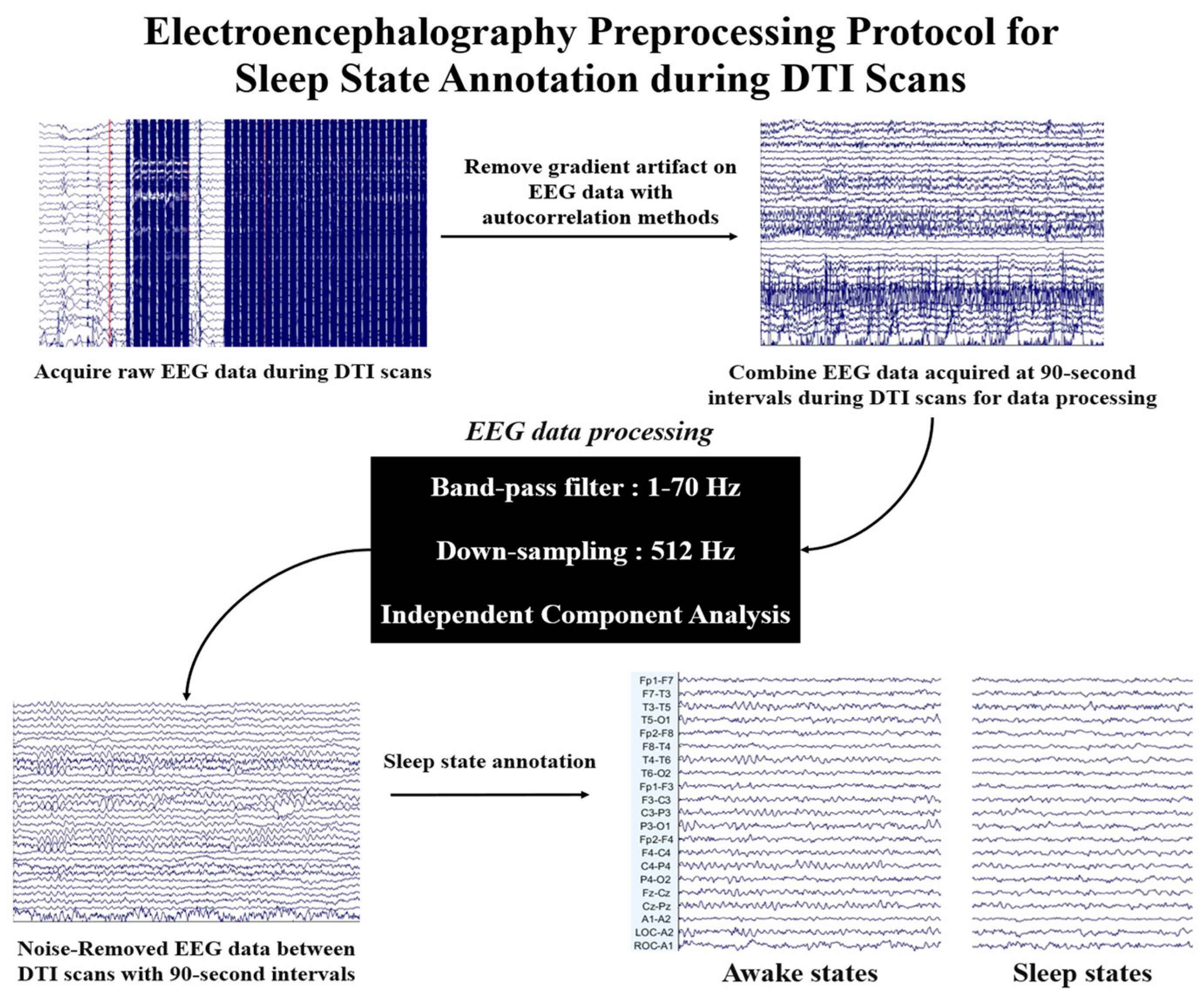
2.3. Electroencephalography Analysis
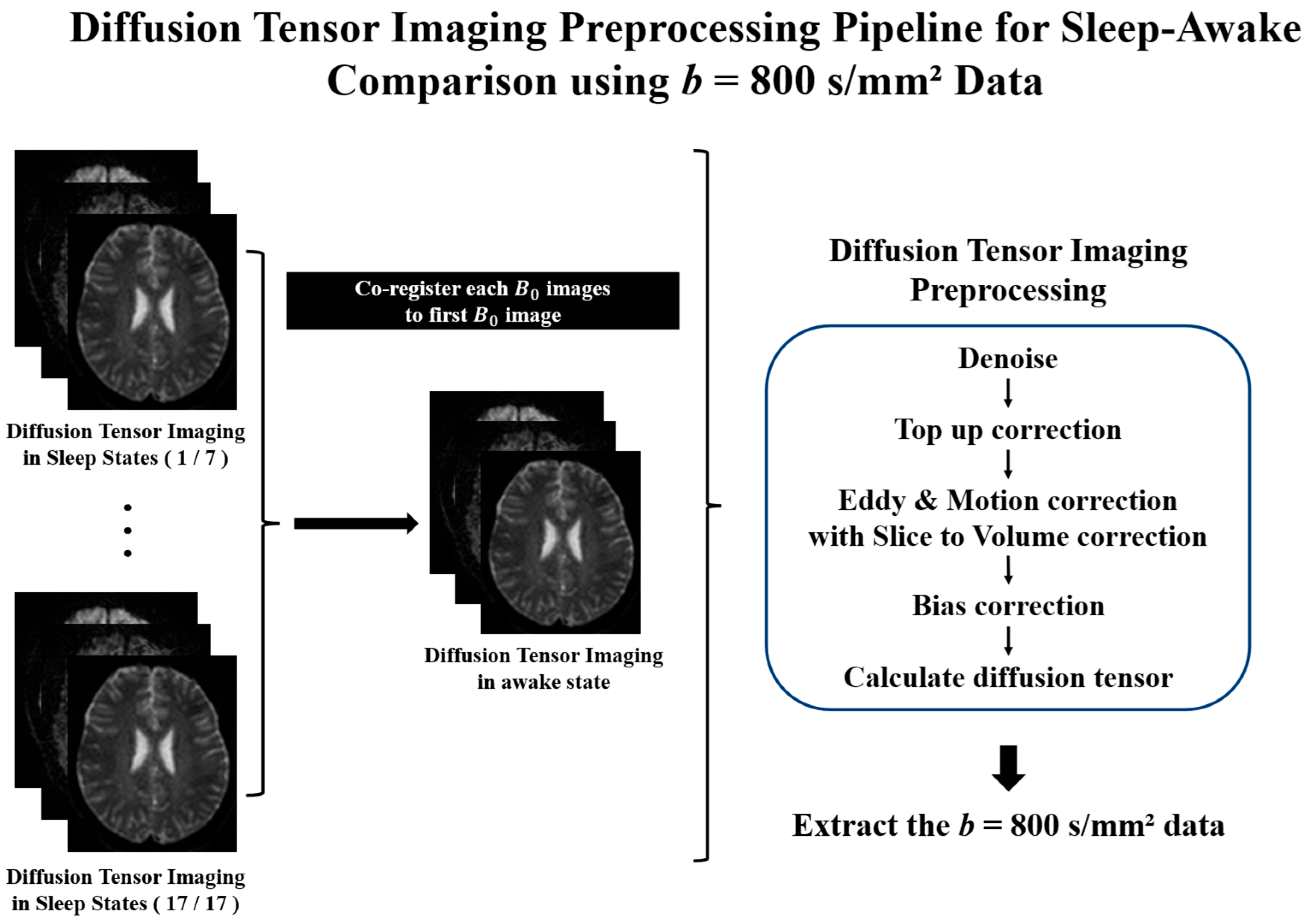
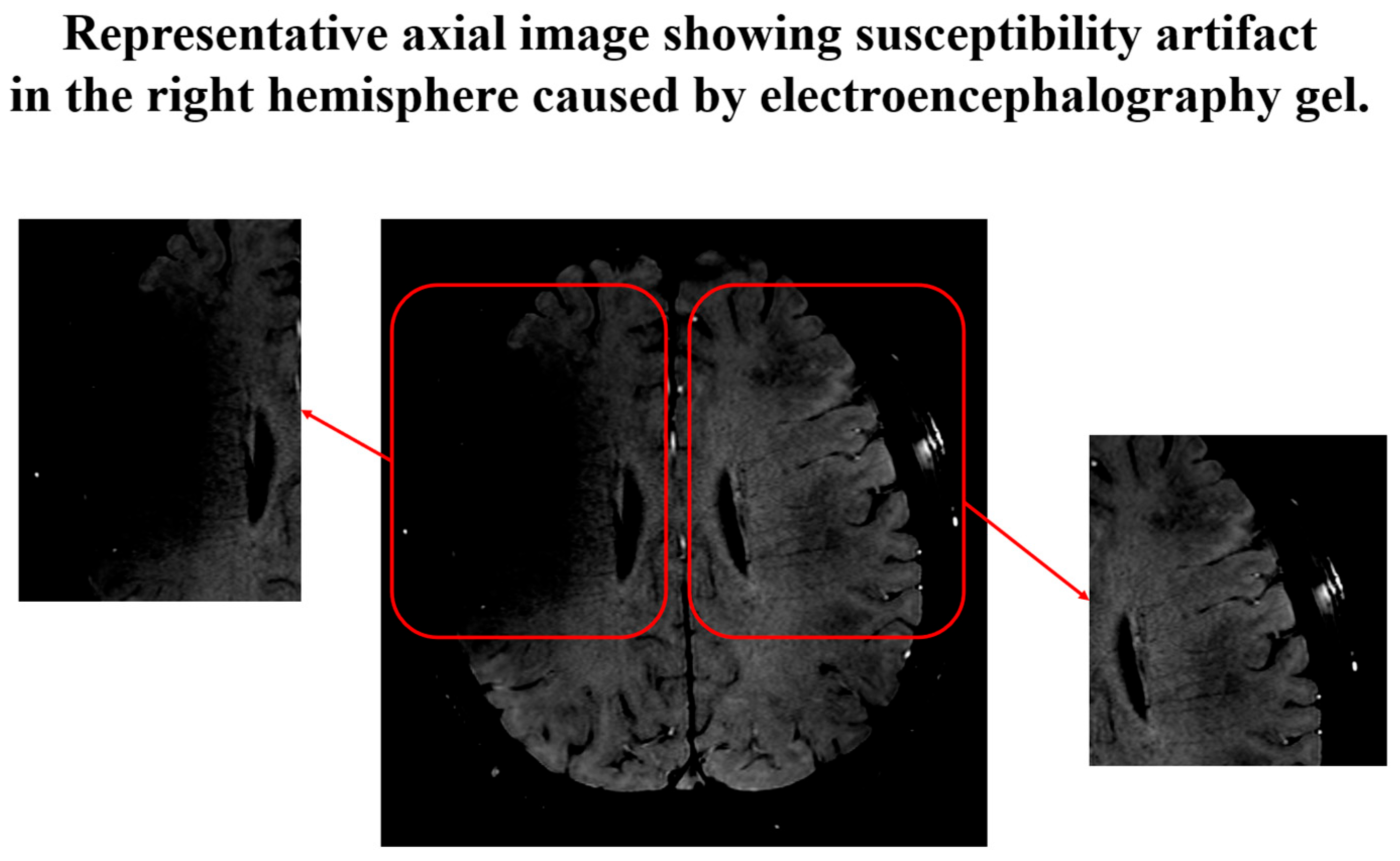
2.4. Diffusion Tensor Imaging Preprocessing
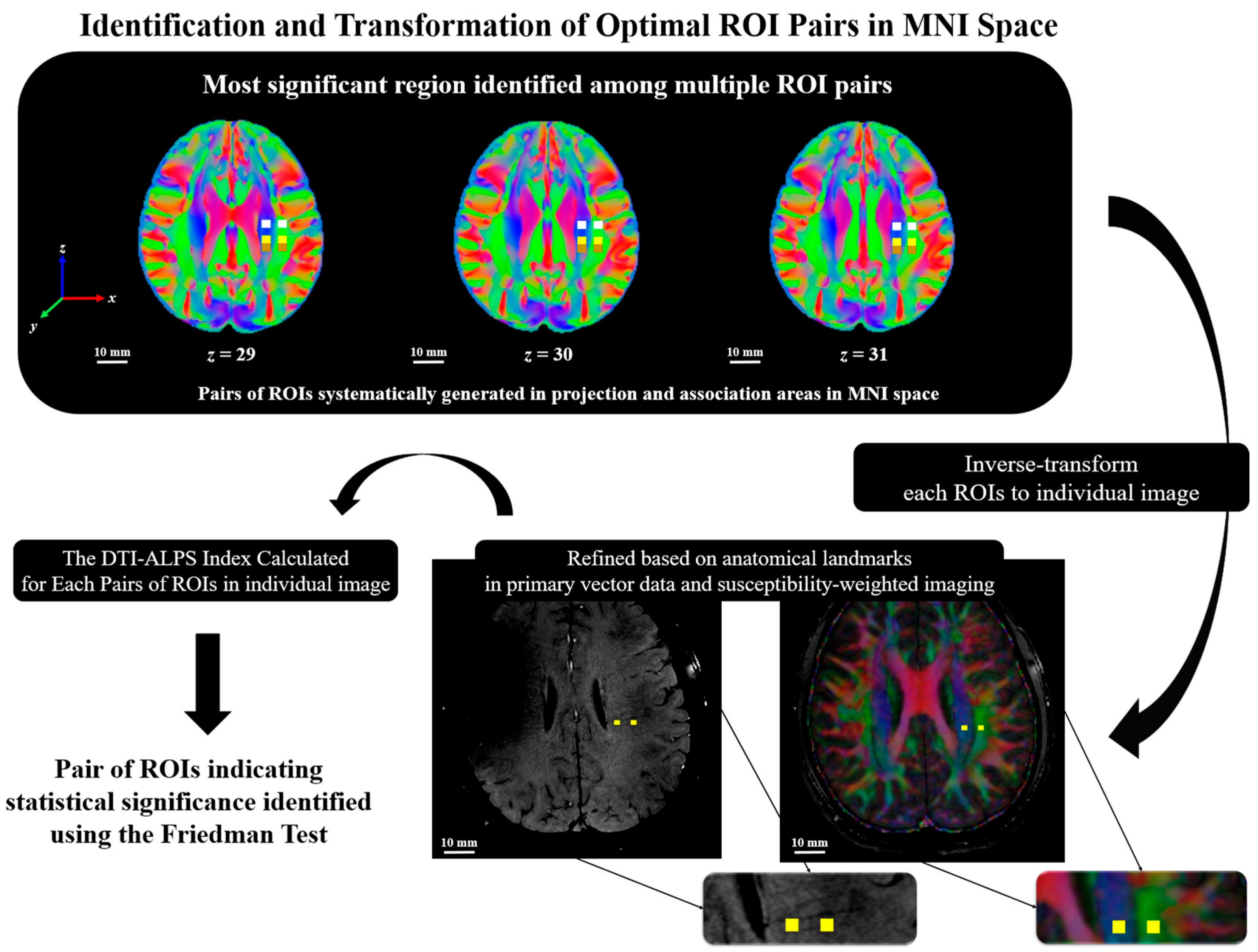
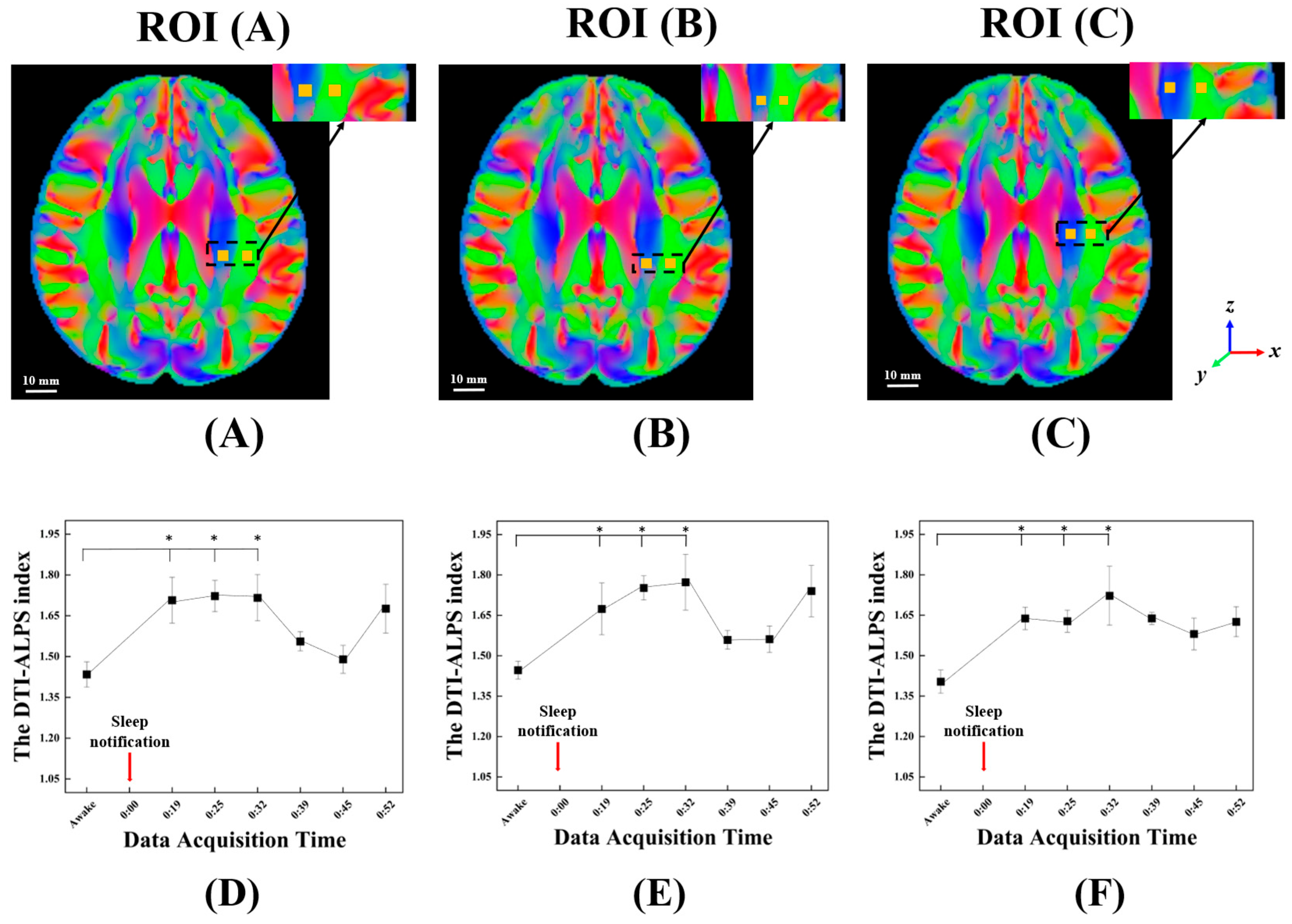
2.5. The DTI-ALPS Index with Perivascular Space
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Imeri, L.; Opp, M.R. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009, 10, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J. Sleep health: Can we define it? Does it matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, R.; Van Dongen, H.P. Sleep deprivation affects multiple distinct cognitive processes. Psychon. Bull. Rev. 2009, 16, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Mestre, H.; Mori, Y.; Nedergaard, M. The brain’s glymphatic system: Current controversies. Trends Neurosci. 2020, 43, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; Nedergaard, M. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Nunez, A.; Aysola, R.S.; Kang, D.W.; Vacas, S.; Kumar, R. Impaired glymphatic system actions in obstructive sleep apnea adults. Front. Neurosci. 2022, 16, 884234. [Google Scholar] [CrossRef] [PubMed]
- Postnov, D.; Semyachkina-Glushkovskaya, O.; Litvinenko, E.; Kurths, J.; Penzel, T. Mechanisms of activation of brain’s drainage during sleep: The nightlife of astrocytes. Cells 2023, 12, 2667. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Zhang, Y.R.; Guo, Y.; Du, J.; Ren, P.; Wu, B.S.; Feng, J.; Initiative, A.D.N.; Cheng, W.; Yu, J.T. Glymphatic system dysfunction predicts amyloid deposition, neurodegeneration, and clinical progression in Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, 3251–3269. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Tong, Y.; Zhou, X.; Dzemidzic, M.; Ho, C.Y.; Wu, Y.C. Assessing pulsatile waveforms of paravascular cerebrospinal fluid dynamics using dynamic diffusion-weighted imaging (dDWI). Neuroimage 2022, 260, 119464. [Google Scholar] [CrossRef] [PubMed]
- Kamagata, K.; Saito, Y.; Andica, C.; Uchida, W.; Takabayashi, K.; Yoshida, S.; Hagiwara, A.; Fujita, S.; Nakaya, M.; Akashi, T.; et al. Noninvasive magnetic resonance imaging measures of glymphatic system activity. J. Magn. Reson. Imaging 2024, 59, 1476–1493. [Google Scholar] [CrossRef] [PubMed]
- Taoka, T.; Naganawa, S. Glymphatic imaging using MRI. J. Magn. Reson. Imaging 2020, 51, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoo, R.E.; Choi, S.H.; Oh, S.H.; Ji, S.; Lee, J.; Hwang, I.; Kang, K.M.; Sohn, C.H. Contrast-enhanced MRI T1 mapping for quantitative evaluation of putative dynamic glymphatic activity in the human brain in sleep-wake states. Radiology 2021, 300, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Calvo, N.; Jamil, M.; Feldman, S.; Shah, A.; Nauman, F.; Ferrara, J. Neurotoxicity from intrathecal gadolinium administration: Case presentation and brief review. Neurol. Clin. Pract. 2020, 10, e7–e10. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, S.; Taoka, T. The glymphatic system: A review of the challenges in visualizing its structure and function with MR imaging. Magn. Reson. Med. Sci. 2022, 21, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Demiral, Ş.B.; Tomasi, D.; Sarlls, J.; Lee, H.; Wiers, C.E.; Zehra, A.; Volkow, N.D. Apparent diffusion coefficient changes in human brain during sleep–Does it inform on the existence of a glymphatic system? Neuroimage 2019, 185, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zhou, Y.; Zhang, K.; Jiao, B.; Hu, J.; Zhang, Y.; Lou, M.; Bai, R. Age-and time-of-day dependence of glymphatic function in the human brain measured via two diffusion MRI methods. Front. Aging Neurosci. 2023, 15, 1173221. [Google Scholar] [CrossRef] [PubMed]
- Örzsik, B.; Palombo, M.; Asllani, I.; Dijk, D.J.; Harrison, N.A.; Cercignani, M. Higher order diffusion imaging as a putative index of human sleep-related microstructural changes and glymphatic clearance. NeuroImage 2023, 274, 120124. [Google Scholar] [CrossRef] [PubMed]
- Taoka, T.; Masutani, Y.; Kawai, H.; Nakane, T.; Matsuoka, K.; Yasuno, F.; Kishimoto, T.; Naganawa, S. Evaluation of glymphatic system activity with the diffusion MR technique: Diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn. J. Radiol. 2017, 35, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Taoka, T.; Fukusumi, A.; Miyasaka, T.; Kawai, H.; Nakane, T.; Kichikawa, K.; Naganawa, S. Structure of the medullary veins of the cerebral hemisphere and related disorders. Radiographics 2017, 37, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Taoka, T.; Ito, R.; Nakamichi, R.; Nakane, T.; Kawai, H.; Naganawa, S. Diffusion tensor image analysis along the perivascular space (DTI-ALPS): Revisiting the meaning and significance of the method. Magn. Reson. Med. Sci. 2024, 23, 268–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, D.A.; Shin, K.J.; Park, K.M. Glymphatic system dysfunction in obstructive sleep apnea evidenced by DTI-ALPS. Sleep Med. 2022, 89, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Hayakawa, Y.; Kamagata, K.; Kikuta, J.; Mita, T.; Andica, C.; Taoka, T.; Uchida, W.; Takabayashi, K.; Tuerxun, R.; et al. Glymphatic system impairment in sleep disruption: Diffusion tensor image analysis along the perivascular space (DTI-ALPS). Jpn. J. Radiol. 2023, 41, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Ringstad, G. Glymphatic imaging: A critical look at the DTI-ALPS index. Neuroradiology 2024, 66, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Cai, Y.; Hong, Z.; Pan, J. Efficacy and safety of Zolpidem in the treatment of insomnia disorder for one month: A meta-analysis of a randomized controlled trial. Sleep Med. 2021, 87, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.; Jackson, G.D.; Abbott, D.F. Artifact reduction in simultaneous EEG-fMRI: A systematic review of methods and contemporary usage. Front. Neurol. 2021, 12, 622719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, B. fMRI–EEG integrated cortical source imaging by use of time-variant spatial constraints. NeuroImage 2008, 39, 1198–1214. [Google Scholar] [CrossRef] [PubMed]
- Mantini, D.; Perrucci, M.G.; Del Gratta, C.; Romani, G.L.; Corbetta, M. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. USA 2007, 104, 13170–13175. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Tangredi, M.M. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events: Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J. Clin. Sleep. Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Veraart, J.; Novikov, D.S.; Christiaens, D.; Ades-Aron, B.; Sijbers, J.; Fieremans, E. Denoising of diffusion MRI using random matrix theory. Neuroimage 2016, 142, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.L.; Graham, M.S.; Drobnjak, I.; Zhang, H.; Campbell, J. Susceptibility-induced distortion that varies due to motion: Correction in diffusion MR without acquiring additional data. Neuroimage 2018, 171, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Bastiani, M.; Cottaar, M.; Fitzgibbon, S.P.; Suri, S.; Alfaro-Almagro, F.; Sotiropoulos, S.N.; Jbabdi, S.; Andersson, J.L. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. Neuroimage 2019, 184, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.L.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016, 125, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Bojarskaite, L.; Vallet, A.; Bjørnstad, D.M.; Gullestad Binder, K.M.; Cunen, C.; Heuser, K.; Kuchta, M.; Mardal, K.-A.; Enger, R. Sleep cycle-dependent vascular dynamics in male mice and the predicted effects on perivascular cerebrospinal fluid flow and solute transport. Nat. Commun. 2023, 14, 953. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Sadeghi, N.; Nayak, A.; Trefler, A.; Sarlls, J.; Baker, C.I.; Pierpaoli, C. Impact of time-of-day on diffusivity measures of brain tissue derived from diffusion tensor imaging. Neuroimage 2018, 173, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.H. Fluid dynamics of cerebrospinal fluid flow in perivascular spaces. J. R. Soc. Interface 2019, 16, 20190572. [Google Scholar] [CrossRef] [PubMed]
- Sriram, S.; Carstens, K.; Dewing, W.; Fiacco, T.A. Astrocyte regulation of extracellular space parameters across the sleep-wake cycle. Front. Cell. Neurosci. 2024, 18, 1401698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pei, X.; Shi, Y.; Yang, Y.; Bai, X.; Chen, T.; Zhao, Y.; Yang, Q.; Ye, J.; Leng, X.; et al. Unveiling connections between venous disruption and cerebral small vessel disease using diffusion tensor image analysis along perivascular space (DTI-ALPS): A 7-T MRI study. Int. J. Stroke 2025, 20, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.M.; Wu, Y.C.; Chen, N.K.; Wen, Q. Exploring radial asymmetry in MR diffusion tensor imaging and its impact on the interpretation of glymphatic mechanisms. J. Magn. Reson. Imaging 2024, 60, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Bork, P.A.; Ladrón-de-Guevara, A.; Christensen, A.H.; Jensen, K.H.; Nedergaard, M.; Bohr, T. Astrocyte endfeet may theoretically act as valves to convert pressure oscillations to glymphatic flow. J. R. Soc. Interface 2023, 20, 20230050. [Google Scholar] [CrossRef] [PubMed]
- Lilius, T.O.; Blomqvist, K.; Hauglund, N.L.; Liu, G.; Stæger, F.F.; Bærentzen, S.; Nedergaard, M. Dexmedetomidine enhances glymphatic brain delivery of intrathecally administered drugs. J. Control. Release 2019, 304, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; O’donnell, J.; Xu, Q.; Kang, N.; Goldman, N.; Nedergaard, M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 2016, 352, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Chatelle, C.; Thibaut, A.; Gosseries, O.; Bruno, M.A.; Demertzi, A.; Bernard, C.; Hustinx, R.; Tshibanda, L.; Bahri, M.A.; Laureys, S. Changes in cerebral metabolism in patients with a minimally conscious state responding to zolpidem. Front. Hum. Neurosci. 2014, 8, 917. [Google Scholar] [CrossRef] [PubMed]
- Hauglund, N.L.; Andersen, M.; Tokarska, K.; Radovanovic, T.; Kjaerby, C.; Sørensen, F.L.; Nedergaard, M. Norepinephrine-mediated slow vasomotion drives glymphatic clearance during sleep. Cell 2025, 188, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Huh, H.; Park, S.H.; Yu, J.H.; Hong, J.; Lee, M.J.; Cho, J.E.; Lim, C.H.; Lee, H.W.; Kim, J.B.; Yang, K.-S.; et al. Quantifying the depth of anesthesia based on brain activity signal modeling. Medicine 2020, 99, e18441. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Jiao, B.; Zhang, Y.; Wang, Z.; Liang, C.; Li, Y.; Hsu, Y.-C.; Bai, R. Arterial pulsation dependence of perivascular cerebrospinal fluid flow measured by dynamic diffusion tensor imaging in the human brain. Neuroimage 2024, 297, 120653. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, L.M.; Vinitsky, H.S.; Sun, Q.; Stæger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, eaav5447. [Google Scholar] [CrossRef] [PubMed]
| Sleep State Annotations for Each Participant Across 17 DTI Acquisition Time Points | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | ||
| Subject 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | N | N | 1 | 1 | 1 | 1 | 1 | 1 | 1 | N | |
| Subject 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | N | N | N | N | N | N | N | |
| Subject 3 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | N | N | N | N | N | 1 | 1 | 1 | N | |
| Subject 4 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Subject 5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Subject 6 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | N | 0 | N | 0 | 0 | N | 0 | 1 | |
| Subject 7 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Subject 8 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | N | 1 | 1 | 1 | 1 | 1 | 1 | |
| Subject 9 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | N | N | N | N | N | N | 1 | 1 | 1 | |
| Subject 10 | 0 | 0 | 0 | 0 | N | 0 | N | N | 0 | 0 | 0 | 0 | 0 | N | 1 | 1 | 1 | |
| Subject 11 | 0 | N | N | 1 | 1 | N | 1 | 1 | 1 | 1 | N | 1 | 1 | N | N | N | N | |
| Subject 12 | 0 | N | 1 | 1 | 1 | 0 | N | N | N | 0 | N | N | 1 | 1 | 1 | 1 | N | |
| Time Since Sleep Notification (Min) | Average of the DTI-ALPS Index (Standard Error) | Increase Ratio | p-Value | Adj. p-Value | Effect Size (r) |
|---|---|---|---|---|---|
| Awake | 1.43 ± 0.05 | - | - | - | - |
| 14.46 | 1.71 ± 0.08 | 19.53% | 0.012 * | 0.024 ** | 0.640 |
| 19.46 | 1.72 ± 0.06 | 20.80% | 0.003 * | 0.018 ** | 0.887 |
| 25.46 | 1.72 ± 0.09 | 20.23% | 0.006 * | 0.018 ** | 0.587 |
| 31.45 | 1.56 ± 0.04 | 9.60% | 0.156 | 0.187 | 0.534 |
| 37.45 | 1.49 ± 0.05 | 4.57% | 0.445 | 0.445 | 0.131 |
| 43.45 | 1.68 ± 0.09 | 17.52% | 0.064 | 0.096 | 0.755 |
| Time Points (min) | Awake | 14.46 | 19.46 | 25.46 | 31.45 | 37.45 | 43.45 | |
|---|---|---|---|---|---|---|---|---|
| Percent Change in | ||||||||
| 0 | 6.33% | 3.51% | 10.57% | 5.94% | 6.39% | 4.51% | ||
| 0 | −11.05% | −14.32% | −8.04% | −3.35% | 1.74% | −9.53% | ||
| The DTI-ALPS index | 0 | 19.53% | 20.81% | 20.23% | 9.61% | 4.57% | 15.52% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, C.-S.; Sohn, C.-H.; Yeon, J.; Chung, K.-J.; Min, B.-J.; Yun, C.-H.; Han, B.S. Feasibility of Observing Glymphatic System Activity During Sleep Using Diffusion Tensor Imaging Analysis Along the Perivascular Space (DTI-ALPS) Index. Diagnostics 2025, 15, 1798. https://doi.org/10.3390/diagnostics15141798
Yun C-S, Sohn C-H, Yeon J, Chung K-J, Min B-J, Yun C-H, Han BS. Feasibility of Observing Glymphatic System Activity During Sleep Using Diffusion Tensor Imaging Analysis Along the Perivascular Space (DTI-ALPS) Index. Diagnostics. 2025; 15(14):1798. https://doi.org/10.3390/diagnostics15141798
Chicago/Turabian StyleYun, Chang-Soo, Chul-Ho Sohn, Jehyeong Yeon, Kun-Jin Chung, Byong-Ji Min, Chang-Ho Yun, and Bong Soo Han. 2025. "Feasibility of Observing Glymphatic System Activity During Sleep Using Diffusion Tensor Imaging Analysis Along the Perivascular Space (DTI-ALPS) Index" Diagnostics 15, no. 14: 1798. https://doi.org/10.3390/diagnostics15141798
APA StyleYun, C.-S., Sohn, C.-H., Yeon, J., Chung, K.-J., Min, B.-J., Yun, C.-H., & Han, B. S. (2025). Feasibility of Observing Glymphatic System Activity During Sleep Using Diffusion Tensor Imaging Analysis Along the Perivascular Space (DTI-ALPS) Index. Diagnostics, 15(14), 1798. https://doi.org/10.3390/diagnostics15141798






