Prenatal Diagnosis of Vaginal Ectopic Ureter Insertion—Case Outcome and Literature Overview
Abstract
1. Introduction
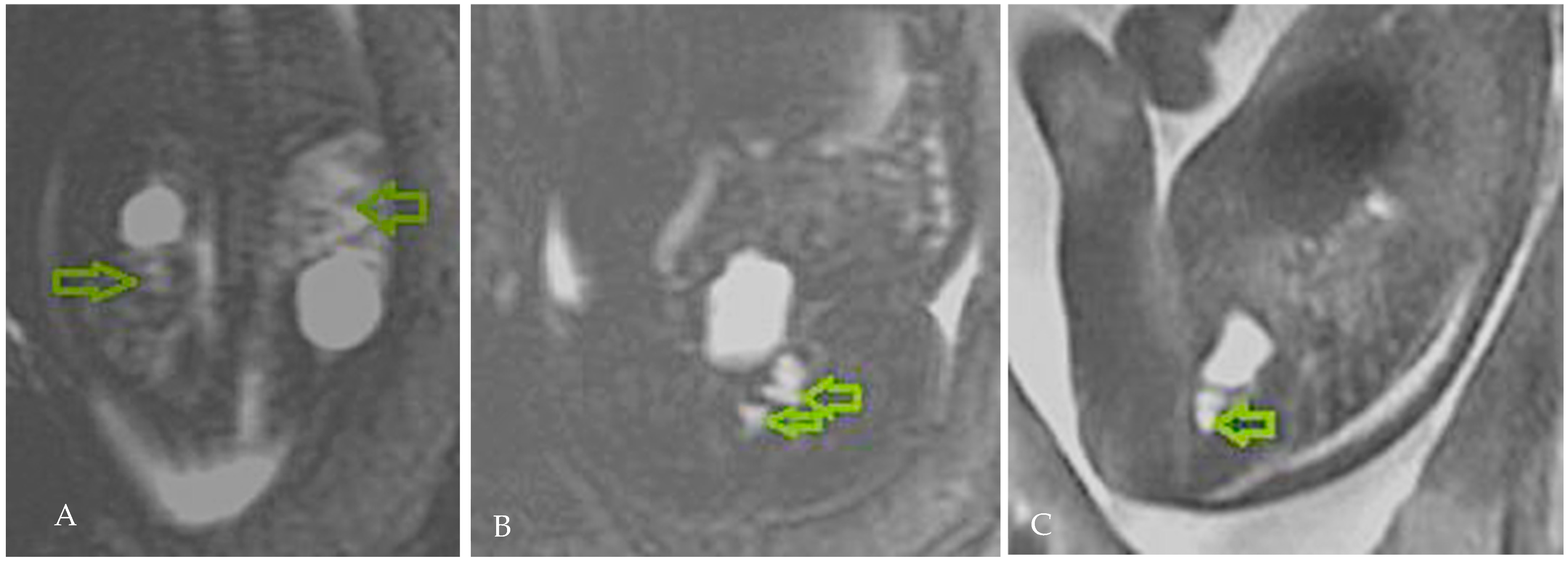
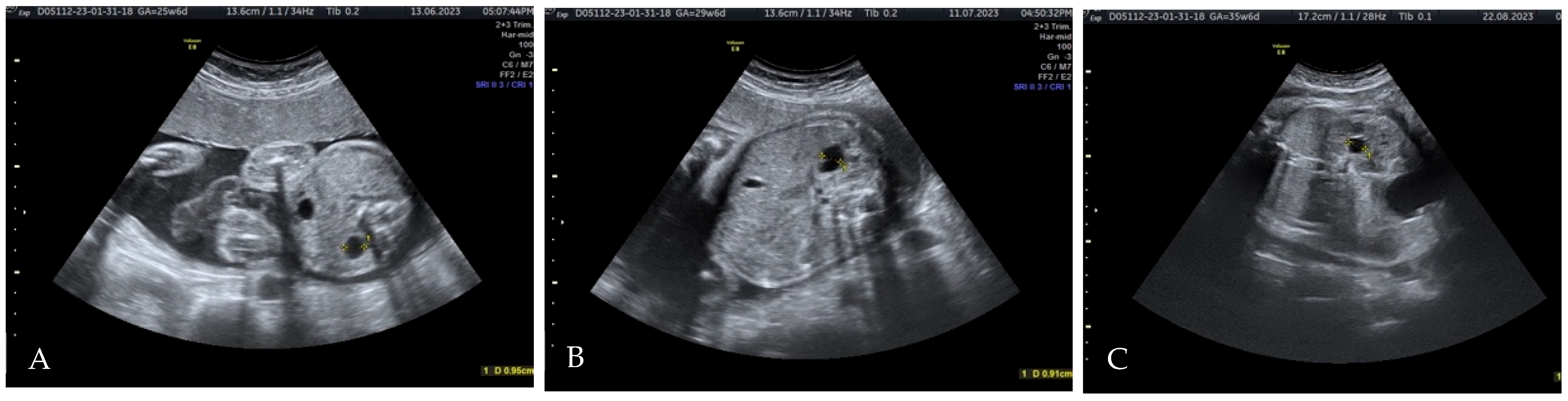
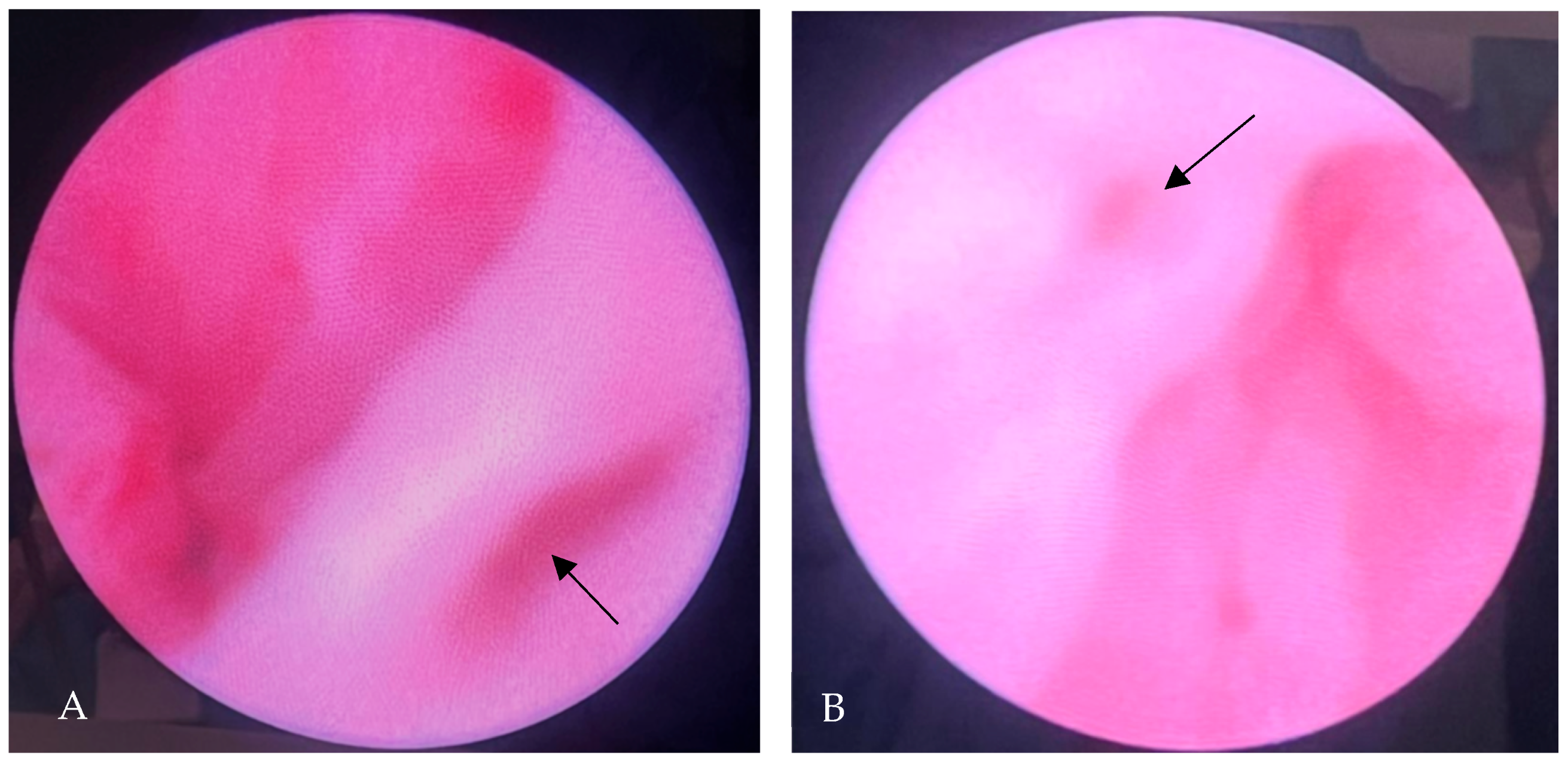
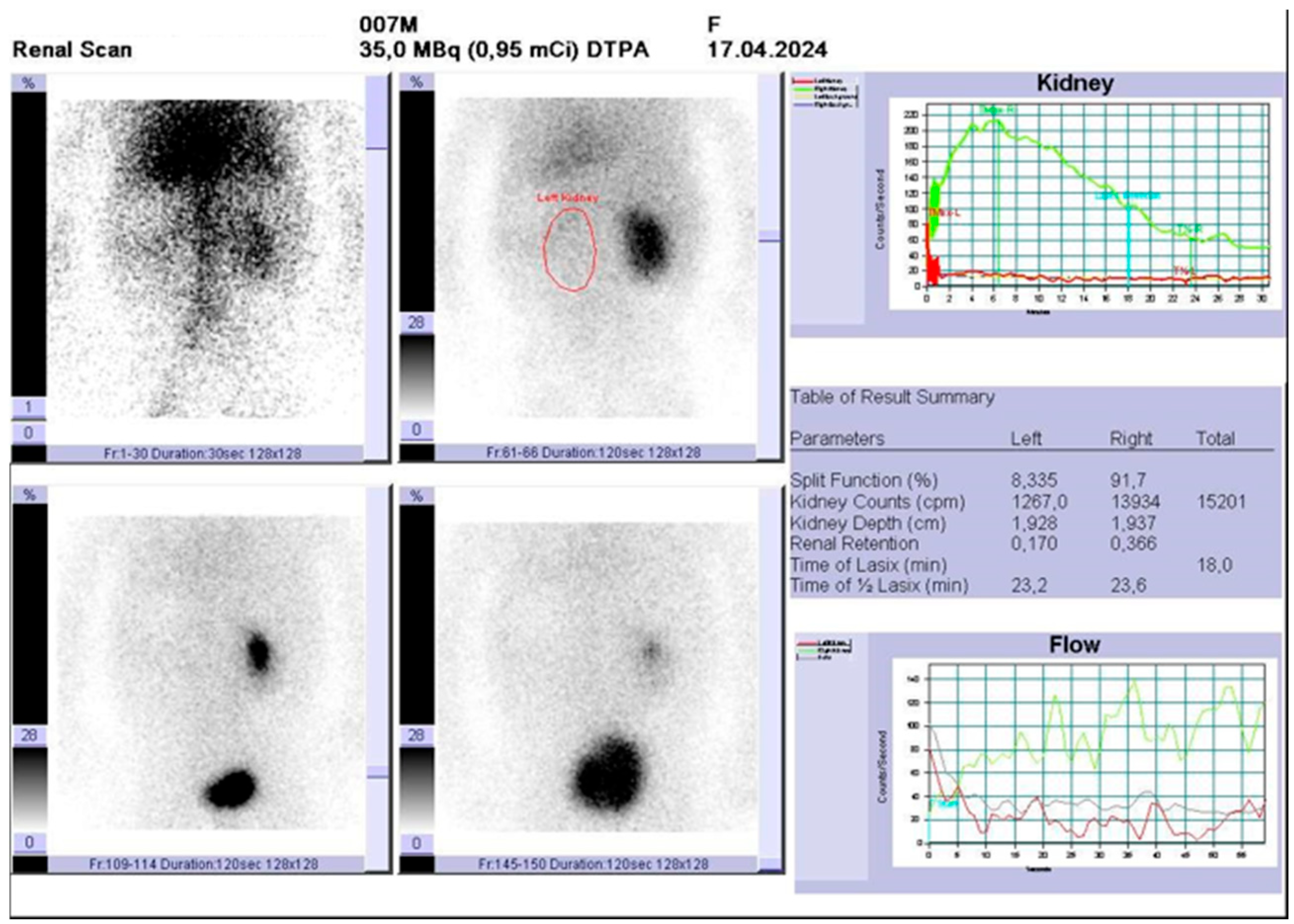
2. Case Presentation
3. Discussion
3.1. Anatomic Considerations
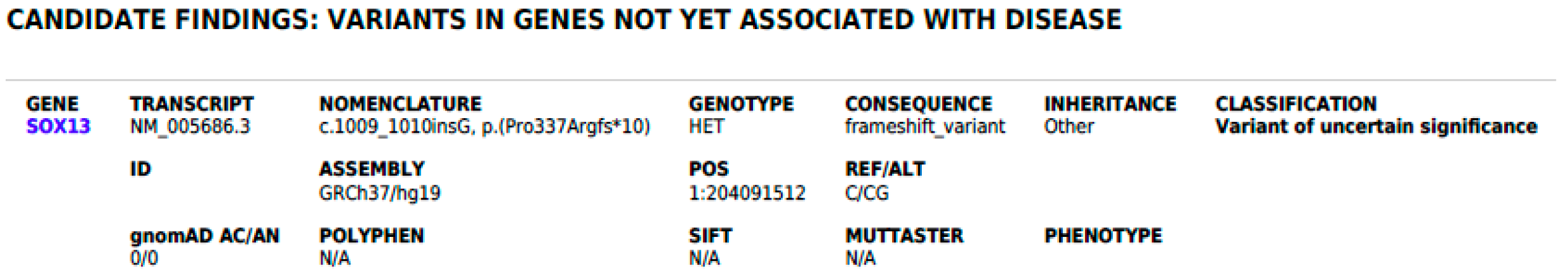
3.2. Genetic Considerations
3.3. Diagnostic Considerations
3.4. Outcome & Management Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSEU | Single system ectopic ureter |
| BSSEU | Bilateral single system ectopic ureter |
| CAKUT | Congenital anomalies of the kidney and urinary tract |
| WG | Weeks of gestation |
| MRI | Magnetic resonance imaging |
| WES | Whole exome sequencing |
| VSD | Ventricular septal defect |
| SOX13 | SRY-related HMG-box genes |
| SNVs | Single nucleotide variants |
| CNVs | Copy number variants |
| RET | Rearranged during transfection |
| FGFR2 | Fibroblast growth factor receptor 2 |
| OHVIRA | Obstructed hemivagina and ipsilateral renal anomaly |
| UTI | Urinary tract infection |
References
- Wiesel, A.; Queisser-Luft, A.; Clementi, M.; Bianca, S.; Stoll, C. Prenatal detection of congenital renal malformations by fetal ultrasonographic examination: An analysis of 709,030 births in 12 European countries. Eur. J. Med. Genet. 2005, 48, 131–144. [Google Scholar] [CrossRef]
- Walker, E.Y.X.; Winyard, P.; Marlais, M. Congenital anomalies of the kidney and urinary tract: Antenatal diagnosis, management and counselling of families. Pediatr. Nephrol. 2024, 39, 1065–1075. [Google Scholar] [CrossRef]
- Demir, M.; Çiftçi, H.; Kılıçarslan, N.; Gümüş, K.; Oğur, M.; Gülüm, M.; Yeni, E. A case of an ectopic ureter with vaginal insertion diagnosed in adulthood. Turk. J. Urol. 2015, 41, 53–55. [Google Scholar] [CrossRef]
- El-Ghar, M.A.; El-Diasty, T. Ectopic insertion of the ureter into the seminal vesicle. World J. Radiol. 2013, 5, 349–351. [Google Scholar] [CrossRef]
- Amenu, D.; Asmare, A.; Siraj, A. Congenital ureterovaginal fistula: A rare case of single-system ectopic ureter with ipsilateral ectopic kidney managed by vaginal approach: A case report. J. Med. Case Rep. 2021, 15, 617. [Google Scholar] [CrossRef] [PubMed]
- Bohnenpoll, T.; Feraric, S.; Nattkemper, M.; Weiss, A.-C.; Rudat, C.; Meuser, M.; Mark-Oliver, T.; Andreas, K. Diversification of Cell Lineages in Ureter Development. J. Am. Soc. Nephrol. 2017, 28, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, Z.; Zhang, F.; Akbar, R.; Lou, Y.; Zhou, J.; Ruan, F. Gynecological Diagnosis and Treatment of Ectopic Ureter Insertion into Vagina: Analysis of Five Cases and a Literature Review. J. Clin. Med. 2022, 11, 6267. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Takiuchi, H.; Nojima, M.; Kondoh, N.; Yoshimoto, T.; Maeda, N.; Kurachi, M.; Shima, H. Ectopic ureter in 54 children. Nihon Hinyokika Gakkai Zasshi 2001, 92, 470–473. [Google Scholar]
- Somalwar, S.A.; Chaudhary, S.E.; Joshi, S.A.; Bhalerao, A.V.; Jain, S.H.; Nagpure, M.K. Single-System Ectopic Ureter: A Late Diagnosis and Successful Management. J. Obstet. Gynecol. India 2016, 66 (Suppl. S2), 645–647. [Google Scholar] [CrossRef][Green Version]
- Ghosh, B.; Shridhar, K.; Pal, D.K.; Banerjee, M. Ectopic ureter draining into the uterus. Urol. Ann. 2016, 8, 105–107. [Google Scholar] [CrossRef]
- Balawender, K.; Pliszka, A.; Wysiadecki, G.; Walocha, J.; Likus, W.; Żytkowski, A. Complete unilateral duplication of the right ureter with ectopic orifice into the prostatic urethra found by transrectal ultrasound. Pol. Arch. Intern. Med. 2022, 132, 16249. [Google Scholar] [CrossRef] [PubMed]
- Roy Choudhury, S.; Chadha, R.; Bagga, D.; Puri, A.; Debnath, P.R. Spectrum of ectopic ureters in children. Pediatr. Surg. Int. 2008, 24, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Schulman, C.C. The single ectopic ureter. Eur. Urol. 1976, 2, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Cruciat, G.; Nemeti, G.I.; Popa-Stanila, R.; Florian, A.; Goidescu, I.G. Imaging diagnosis and legal implications of brain injury in survivors following single intrauterine fetal demise from monochorionic twins—A review of the literature. J. Perinat. Med. 2021, 49, 837–846. [Google Scholar] [CrossRef]
- Goidescu, I.G.; Nemeti, G.; Preda, A.; Kovacs, T.; Surcel, M.; Eniu, D.T.; Cruciat, G.; Mureșan, D. Krukenberg tumor in pregnancy: A rare case and review of the literature. J. Matern. Fetal Neonatal Med. 2021, 35, 7290–7295. [Google Scholar] [CrossRef]
- Stein, D.; McNamara, E. Congenital Anomalies of the Kidneys and Urinary Tract. Clin. Perinatol. 2022, 49, 791–798. [Google Scholar] [CrossRef]
- Hecht, S.; Hall, J.; Ketzer, J.; Walker, J.; Trecartin, A.; Wilcox, D.; Peña, A.; Bischoff, A. Ectopic ureters in anorectal malformations. Pediatr. Surg. Int. 2019, 35, 1005–1008. [Google Scholar] [CrossRef]
- Wang, Z.J.; Daldrup-Link, H.; Coakley, F.V.; Yeh, B.M. Ectopic ureter associated with uterine didelphys and obstructed hemivagina: Preoperative diagnosis by MRI. Pediatr. Radiol. 2010, 40, 358–360. [Google Scholar] [CrossRef]
- Agarwal, S.; Yadav, R.N.; Kumar, M.; Sankhwar, S. Horseshoe kidney with unilateral single ectopic ureter. BMJ Case Rep. 2018, 2018, bcr-2017-223913. [Google Scholar] [CrossRef]
- Tanagho, E.A. Embryologic basis for lower ureteral anomalies: A hypothesis. Urology 1976, 7, 451–464. [Google Scholar] [CrossRef]
- Chowdhary, S.K.; Lander, A.; Parashar, K.; Corkery, J.J. Single-system ectopic ureter: A 15-year review. Pediatr. Surg. Int. 2001, 17, 638–641. [Google Scholar] [CrossRef]
- Schulman, C.C. Ectopic implantations of the ureter. Acta Urol. Belg. 1972, 40, 201–478. [Google Scholar]
- Johnston, J.H.; Davenport, T.J. The single ectopic ureter. Br. J. Urol. 1969, 41, 428–433. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L. Dysplastic kidney and ectopic ureter in association with obstructed hemivagina and ipsilateral renal anomaly. Int. Urogynecol. J. 2020, 31, 1707–1709. [Google Scholar] [CrossRef]
- Santos, X.M.; Dietrich, J.E. Obstructed Hemivagina with Ipsilateral Renal Anomaly. J. Pediatr. Adolesc. Gynecol. 2016, 29, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Schlomer, B.; Rodriguez, E.; Baskin, L. Obstructed hemivagina and ipsilateral renal agenesis (OHVIRA) syndrome should be redefined as ipsilateral renal anomalies: Cases of symptomatic atrophic and dysplastic kidney with ectopic ureter to obstructed hemivagina. J. Pediatr. Urol. 2015, 11, 77.e1–77.e6. [Google Scholar] [CrossRef] [PubMed]
- Aswani, Y.; Varma, R.; Choudhary, P.; Gupta, R.B. Wolffian Origin of Vagina Unfolds the Embryopathogenesis of OHVIRA (Obstructed Hemivagina and Ipsilateral Renal Anomaly) Syndrome and Places OHVIRA as a Female Counterpart of Zinner Syndrome in Males. Polish J. Radiol. 2016, 81, 549–556. [Google Scholar] [CrossRef]
- Mehra, S.; Ranjan, R.; Garga, U.C. Zinner syndrome-a rare developmental anomaly of the mesonephric duct diagnosed on magnetic resonance imaging. Radiol. Case Rep. 2016, 11, 313–317. [Google Scholar] [CrossRef]
- Duicu, C.; Kiss, E.; Simu, I.; Aldea, C. A Rare Case of Double-System With Ectopic Ureteral Openings Into Vagina. Front. Pediatr. 2018, 6, 176. [Google Scholar] [CrossRef]
- Mackie, G.G.; Stephens, F.D. Duplex kidneys: A correlation of renal dysplasia with position of the ureteric orifice. Birth Defects Orig. Artic. Ser. 1977, 13, 313–321. [Google Scholar] [CrossRef]
- Meyer, R. Normal and abnormal development of the ureter in the human embryo; a mechanistic consideration. Anat. Rec. 1946, 96, 355–371. [Google Scholar] [CrossRef]
- Chia, I.; Grote, D.; Marcotte, M.; Batourina, E.; Mendelsohn, C.; Bouchard, M. Nephric duct insertion is a crucial step in urinary tract maturation that is regulated by a Gata3-Raldh2-Ret molecular network in mice. Development 2011, 138, 2089–2097. [Google Scholar] [CrossRef]
- Grote, D.; Boualia, S.K.; Souabni, A.; Merkel, C.; Chi, X.; Costantini, F.; Carroll, T.; Bouchard, M. Gata3 acts downstream of beta-catenin signaling to prevent ectopic metanephric kidney induction. PLoS Genet. 2008, 4, e1000316. [Google Scholar] [CrossRef]
- Jain, S.; Chen, F. Developmental pathology of congenital kidney and urinary tract anomalies. Clin. Kidney J. 2019, 12, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Baez, L.; Kirsch, A. Single ectopic ureter inserting into a duplicated imperforate hemivagina: A rare cause of a fetal abdominal mass. Urology 2003, 62, 144. [Google Scholar] [CrossRef]
- Coroado, M.; Dias, J.; Veloso, H.; Inocêncio, G.; Rodrigues, M.D.C. Prenatal diagnosis of fetal hydrocolpos with duplicated vaginal ectopic ureter: Case report. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, e200. [Google Scholar] [CrossRef]
- Cassart, M.; Majoub, N.; Irtan, S.; Jouannic, J.-M.; Ducou le Pointe, H.; Blondiaux, E.; Garel, C. Prenatal Evaluation and Postnatal Follow-Up of Ureteral Ectopic Insertion in Multicystic Dysplastic Kidneys. Fetal Diagn. Ther. 2019, 45, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Athey, P.A.; Carpenter, R.J.; Hadlock, F.P.; Hedrick, T.D. Ultrasonic demonstration of ectopic ureterocele. Pediatrics 1983, 71, 568–571. [Google Scholar] [CrossRef]
- Caire, J.T.; Ramus, R.M.; Magee, K.P.; Fullington, B.K.; Ewalt, D.H.; Twickler, D.M. MRI of fetal genitourinary anomalies. AJR Am. J. Roentgenol. 2003, 181, 1381–1385. [Google Scholar] [CrossRef]
- Chen, C.-P.; Liu, Y.-P.; Huang, J.-P.; Chang, T.-Y.; Tsai, F.-J.; Tsai, J.-D.; Sheu, J.-C.; Wang, W. Prenatal evaluation with magnetic resonance imaging of a giant blind ectopic ureter associated with a duplex kidney. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008, 31, 360–362. [Google Scholar] [CrossRef]
- Dhale, A.; Pendkar, R.; Hatwar, G.; Dharamshi, J.D.; Trivedi, Y. Ectopic Ureter Opening in Vagina: A Rare Cause of Nonfunctional Kidney and Urinary Incontinence in a Pediatric Patient. Cureus 2024, 16, e60052. [Google Scholar] [CrossRef]
- Lee, Y.S.; Im, Y.J.; Kim, S.W.; Kim, M.-J.; Lee, M.-J.; Lim, N.L.; Han, S.W. The vagaries of proper imaging in diagnosing single-system ectopic ureter in children with continuous incontinence and outcomes of simple nephrectomy. J. Pediatr. Surg. 2016, 51, 469–474. [Google Scholar] [CrossRef]
- Ray, J.G.; Vermeulen, M.J.; Bharatha, A.; Montanera, W.J.; Park, A.L. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA 2016, 316, 952–961. [Google Scholar] [CrossRef]
- Goidescu, I.G.; Nemeti, G.; Caracostea, G.; Eniu, D.T.; Chiorean, A.; Pintican, R.; Cruciat, G.; Muresan, D. The role of imaging techniques in the diagnosis, staging and choice of therapeutic conduct in pregnancy associated breast cancer. Med. Ultrason. Soc. Romana Ultrason. Med. Biol. 2019, 21, 336–343. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goidescu, I.G.; Nemeti, G.; Staicu, A.; Surcel, M.; Goidescu, C.M.; Rotar, I.C.; Cruciat, G.; Muresan, D. Prenatal Diagnosis of Vaginal Ectopic Ureter Insertion—Case Outcome and Literature Overview. Diagnostics 2025, 15, 1788. https://doi.org/10.3390/diagnostics15141788
Goidescu IG, Nemeti G, Staicu A, Surcel M, Goidescu CM, Rotar IC, Cruciat G, Muresan D. Prenatal Diagnosis of Vaginal Ectopic Ureter Insertion—Case Outcome and Literature Overview. Diagnostics. 2025; 15(14):1788. https://doi.org/10.3390/diagnostics15141788
Chicago/Turabian StyleGoidescu, Iulian Gabriel, Georgiana Nemeti, Adelina Staicu, Mihai Surcel, Cerasela Mihaela Goidescu, Ioana Cristina Rotar, Gheorghe Cruciat, and Daniel Muresan. 2025. "Prenatal Diagnosis of Vaginal Ectopic Ureter Insertion—Case Outcome and Literature Overview" Diagnostics 15, no. 14: 1788. https://doi.org/10.3390/diagnostics15141788
APA StyleGoidescu, I. G., Nemeti, G., Staicu, A., Surcel, M., Goidescu, C. M., Rotar, I. C., Cruciat, G., & Muresan, D. (2025). Prenatal Diagnosis of Vaginal Ectopic Ureter Insertion—Case Outcome and Literature Overview. Diagnostics, 15(14), 1788. https://doi.org/10.3390/diagnostics15141788





