A Comparability Study Between Intravenous Contrast-Enhanced Cone-Beam Computed Tomography (CBCT) and Magnetic Resonance Angiography (MRA) on the Post-Treatment Follow-Up of Intracranial Aneurysms: A Single-Center Prospective Cohort Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- -
- The presence of large or dense coil packing, as seen in those with giant aneurysm (aneurysm > 2.5 cm) or used for trapping.
- -
- Patients with a modified Rankin score of 4 or 5.
- -
- Illiterate and mentally incapacitated patients.
- -
- Patients with severe chronic renal failure with an eGFR < 30 mL/min/1.73 m2 or those requiring renal replacement therapy.
- -
- Patients with allergy to either iodinated or gadolinium contrast.
2.2. Imaging Protocol
- -
- Injection rate: 5 mL/s.
- -
- Injection volume: 100 mL Omnipaque 350 (iodinated contrast medium) administered at full strength.
2.3. Qualitative Image Review
- -
- Assessment of residual aneurysmal neck.
- -
- Parent vessel status.
- -
- Degree of artifacts of any form.
- -
- Stent apposition to vessel wall.
- -
- Delineation of stent struts.
- -
- Vessel wall status of stented segment.
- Unacceptable.
- Poor.
- Acceptable (acceptable for diagnostic use but with minor issues).
- Good.
- Excellent.
- Massive artifacts, significant distortion, parent vessel not differentiable, no diagnostic value.
- Severe artifacts, moderate distortion, parent vessel poorly differentiable.
- Moderate artifacts, mild distortion, satisfactory assessment of parent vessel status.
- Mild artifacts without obvious distortion, parent vessel well differentiable.
- Minimal or no artifacts.
2.4. Statistical Analysis
3. Results
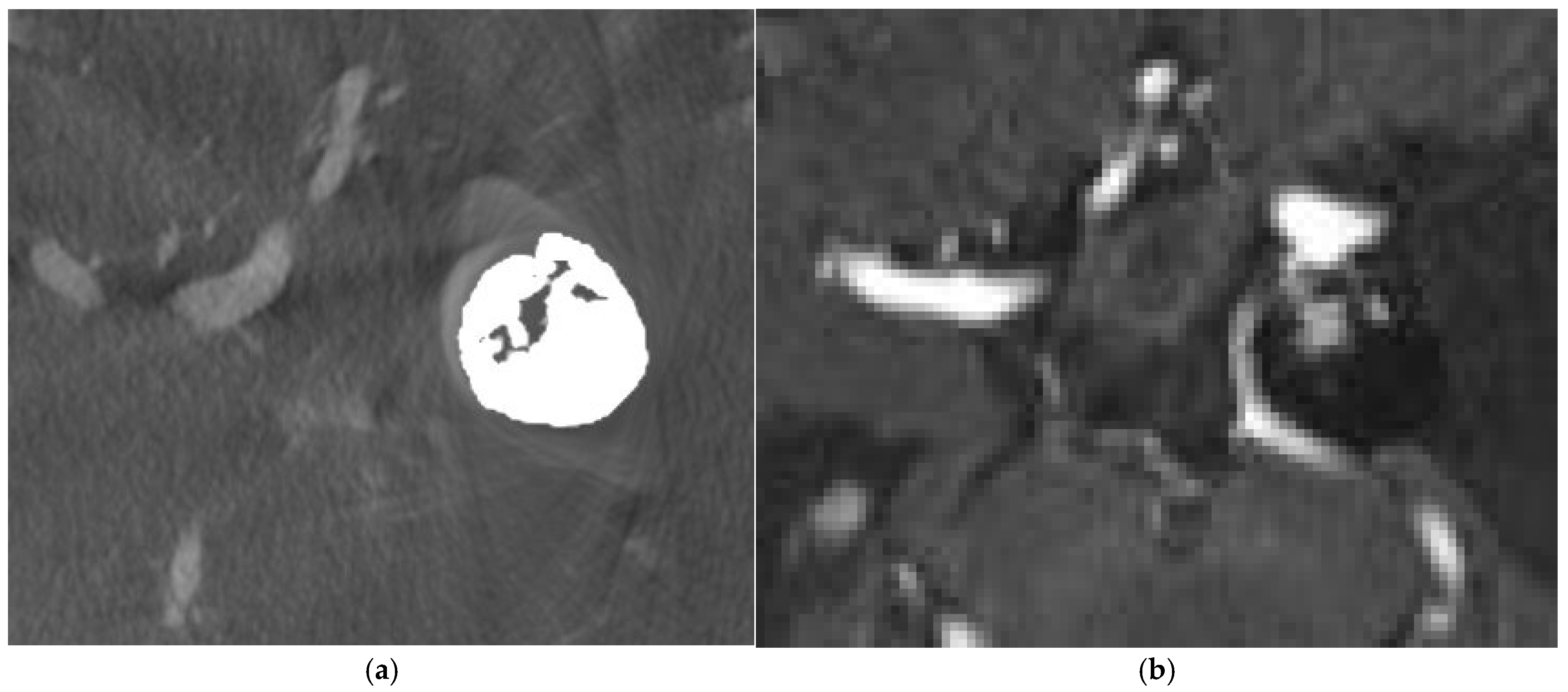
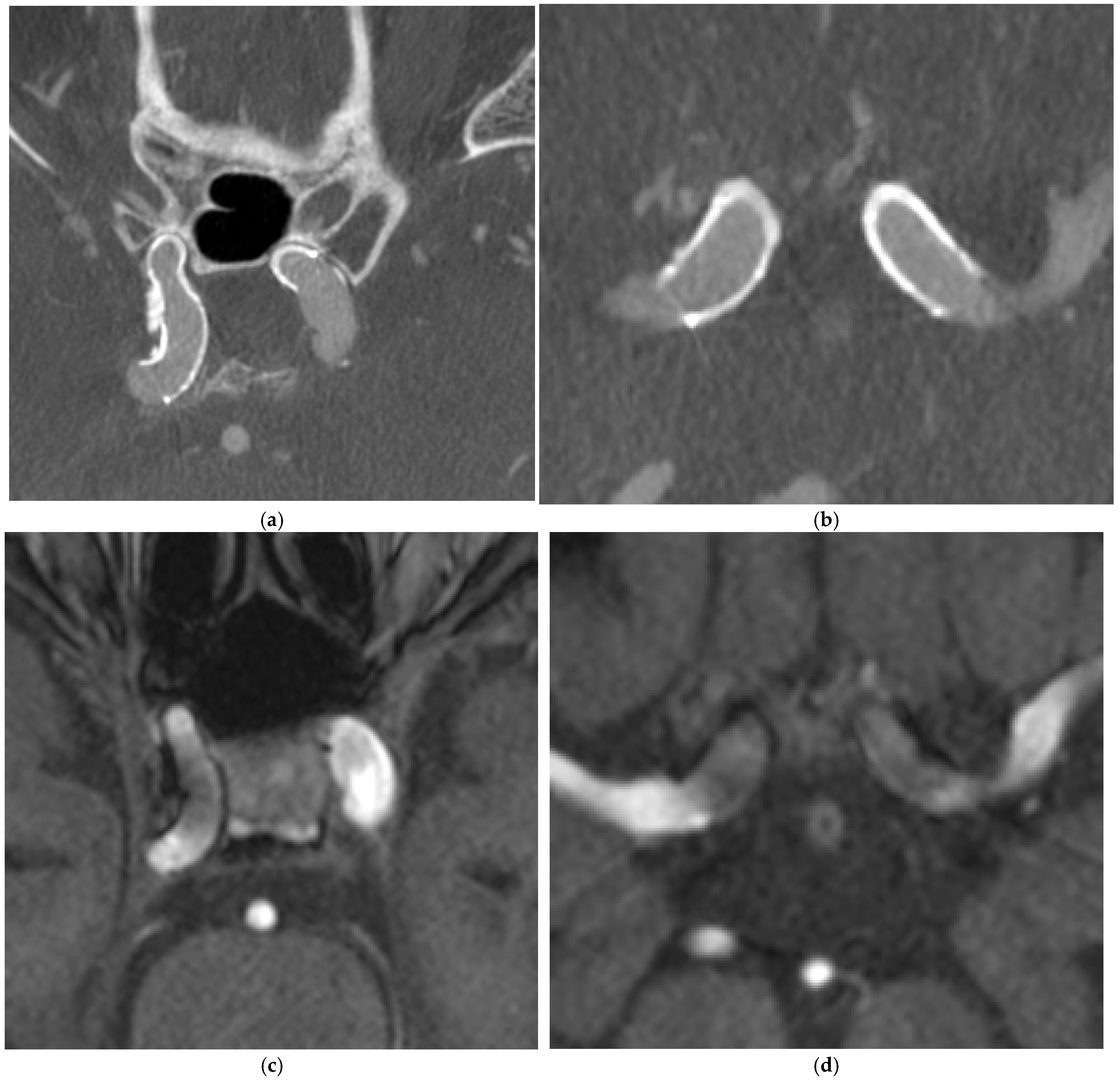
4. Illustrative Cases
4.1. Case 1
4.2. Case 2
4.3. Case 3
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cognard, C.; Weill, A.; Castaings, L.; Rey, A.; Moret, J. Intracranial berry aneurysms: Angiographic and clinical results after endovascular treatment. Radiology 1998, 206, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, A.J.; Kerr, R.S.; Yu, L.M.; Clarke, M.; Sneade, M.; Yarnold, J.A.; Sandercock, P.; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005, 366, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Moret, J.; Pierot, L.; Boulin, A.; Castaings, L.; Rey, A. Endovascular treatment of anterior communicating artery aneurysms using Guglielmi detachable coils. Neuroradiology 1996, 38, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Viñuela, F.; Duckwiler, G.; Mawad, M. Guglielmi detachable coil embolization of acute intracranial aneurysm: Perioperative anatomical and clinical outcome in 403 patients. 1997. J. Neurosurg. 2008, 108, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Boulin, A.; Pierot, L. Follow-up of intracranial aneurysms treated with detachable coils: Comparison of gadolinium-enhanced 3D time-of-flight MR angiography and digital subtraction angiography. Radiology 2001, 219, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Gönner, F.; Heid, O.; Remonda, L.; Nicoli, G.; Baumgartner, R.W.; Godoy, N.; Schroth, G. MR angiography with ultrashort echo time in cerebral aneurysms treated with Guglielmi detachable coils. Am. J. Neuroradiol. 1998, 19, 1324–1328. [Google Scholar] [PubMed] [PubMed Central]
- Lövblad, K.O.; Yilmaz, H.; Chouiter, A.; San Millan Ruiz, D.; Abdo, G.; Bijlenga, P.; de Tribolet, N.; Ruefenacht, D.A. Intracranial aneurysm stenting: Follow-up with MR angiography. J. Magn. Reson. Imaging 2006, 24, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.E.; Wise, J.; Burke, E.M.; Tekle, W.G. Visualization of flow diverter stent wall apposition during intracranial aneurysm treatment using a virtually diluted cone beam CT technique (Vessel ASSIST). Neuroradiology 2021, 63, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Yuki, I.; Ishibashi, T.; Ikemura, A.; Kan, I.; Nishimura, K.; Kodama, T.; Kaku, S.; Abe, Y.; Otani, K.; et al. Visualization of stent apposition after stent-assisted coiling of intracranial aneurysms using high resolution 3D fusion images acquired by C-arm CT. J. NeuroInterv. Surg. 2020, 12, 192–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prell, D.; Kyriakou, Y.; Struffert, T.; Dörfler, A.; Kalender, W.A. Metal artifact reduction for clipping and coiling in interventional C-arm CT. AJNR Am. J. Neuroradiol. 2010, 31, 634–639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Struffert, T.; Saake, M.; Ott, S.; Engelhorn, T.; Gölitz, P.; Kloska, S.; Doelken, M.; Doerfler, A. Intravenous flat detector CT angiography for non-invasive visualisation of intracranial flow diverter: Technical feasibility. Eur. Radiol. 2011, 21, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.C.; Lee, K.T.; Lau, T.W.; Wong, G.K.; Pang, V.K.; Chan, K.Y. Intravenous C-Arm Conebeam CT Angiography following Long-Term Flow-Diverter Implantation: Technologic Evaluation and Preliminary Results. AJNR Am. J. Neuroradiol. 2016, 37, 481–486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, S.; Shi, C.C.; Ma, J.; Wang, Y.; Zhu, M.; Bao-Ma Ren, J.Z.; Han, X.W.; Li, T.F. Clinical evaluation of high-resolution cone-beam computed tomography for the implantation of flow-diverter stents in intracranial aneurysms. J. Clin. Neurosci. 2022, 103, 14–19. [Google Scholar] [CrossRef] [PubMed]
- ESNR 2024. Neuroradiology 2024, 66 (Suppl. 1), 1–179. [CrossRef]
- Raz, E.; Nossek, E.; Sahlein, D.H.; Sharashidze, V.; Narayan, V.; Ali, A.; Esparza, R.; Peschillo, S.; Chung, C.; Diana, F.; et al. Principles, techniques and applications of high resolution cone beam CT angiography in the neuroangio suite. J. NeuroInterv. Surg. 2023, 15, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, M.N.; Scholz, B.; Rohkohl, C.; Kyriakou, Y.; Mohr, A.; Schramm, P.; Wachter, D.; Wasser, K.; Knauth, M. Impact of a new metal artefact reduction algorithm in the noninvasive follow-up of intracranial clips, coils, and stents with flat-panel angiographic CTA: Initial results. Neuroradiology 2013, 55, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Van der Bom, I.M.; Hou, S.Y.; Puri, A.S.; Spilberg, G.; Ruijters, D.; van de Haar, P.; Carelsen, B.; Vedantham, S.; Gounis, M.J.; Wakhloo, A.K. Reduction of coil mass artifacts in high-resolution flat detector conebeam CT of cerebral stent-assisted coiling. AJNR Am. J. Neuroradiol. 2013, 34, 2163–2170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cancelliere, N.M.; Hummel, E.; van Nijnatten, F.; van de Haar, P.; Withagen, P.; van Vlimmeren, M.; Hallacoglu, B.; Agid, R.; Nicholson, P.; Mendes Pereira, V. The butterfly effect: Improving brain cone-beam CT image artifacts for stroke assessment using a novel dual-axis trajectory. J. NeuroInterv. Surg. 2023, 15, 283–287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hosoo, H.; Ito, Y.; Marushima, A.; Hayakawa, M.; Masumoto, T.; Ishikawa, E.; Matsumaru, Y. Image quality improvements for brain soft tissue in neuro-endovascular treatments: A novel dual-axis “butterfly” trajectory for optimized Cone-Beam CT. Eur. J. Radiol. 2023, 160, 110713. [Google Scholar] [CrossRef] [PubMed]

| TOF MRA | CE MRA | |
|---|---|---|
| TR (ms) | 24 | 20 |
| TE (ms) | 3.43 | 2.95 |
| Flip angle | 18 | 18 |
| Acquisition matrix | 319 × 384 | 319 × 384 |
| Field of view | 200 mm | 200 mm |
| Slice thickness | 0.8 mm | 0.8 mm |
| Total acquisition time | 2 min and 54 s | 3 min and 1 s |
| CBCT | MRA | |||||||
|---|---|---|---|---|---|---|---|---|
| Assessment Parameters | Overall Agreement, % | Score 1–2 Agreement, % | Score ≥ 3 Agreement, % | k | Overall Agreement, % | Score 1–2 Agreement, % | Score ≥ 3 Agreement, % | k |
| Residual aneurysmal neck OR = 0.4 95% CI = 0.2–1.0 p = 0.05 | 75.4 | 15.8 | 83.3 | 0.803 | 82.5 | 7.0 | 92.1 | 0.791 |
| Parent vessel status OR = 0.9 95% CI = 0.3–2.3 p = 0.79 | 80.7 | 9.6 | 84.2 | 0.821 | 75.4 | 8.8 | 87.7 | 0.755 |
| Degree of artifacts OR = 1.2 95%CI = 0.5–2.9 p = 0.689 | 73.7 | 9.6 | 80.7 | 0.723 | 71.1 | 12.3 | 86.0 | 0.734 |
| Stent apposition to vessel wall OR = 10.0 95% CI = 4.3–23.4 p < 0.001 | 75.6 | 7.3 | 87.8 | 0.781 | 82.9 | 43.9 | 51.2 | 0.834 |
| Stent strut delineation OR > 1000 p < 0.001 | 82.9 | 14.6 | 80.5 | 0.833 | 100 | 100 | 0 | 1.0 |
| Vessel wall status of stented segment OR > 1000 p < 0.001 | 78.0 | 14.6 | 82.9 | 0.805 | 95.1 | 100 | 0 | 0.828 |
| CBCT (02 vs. 03) | MRA (Q1–3: 01 vs. 03; Q4–6: 02 vs. 03) | |||||||
|---|---|---|---|---|---|---|---|---|
| Assessment Parameters | Overall Agreement, % | Score 1–2 Agreement, % | Score ≥ 3 Agreement, % | k | Overall Agreement, % | Score 1–2 Agreement, % | Score ≥ 3 Agreement, % | k |
| Residual aneurysmal neck OR = 16.0 95% CI = 7.6–34.0 p < 0.001 | 77.8 | 11.1 | 88.9 | 0.775 | 88.9 | 66.7 | 33.3 | 0.800 |
| Parent vessel status OR = 15.1 95% CI = 6.7–33.6 p < 0.001 | 55.6 | 11.1 | 55.6 | 0.595 | 77.8 | 66.7 | 22.2 | 0.804 |
| Degree of artifacts OR > 100 p < 0.001 | 44.4 | 22.2 | 55.6 | 0.372 | 77.8 | 77.8 | 0 | 0.455 |
| CBCT (02 vs. 03) | MRA (Q1–3: 01 vs. 03; Q4–6: 02 vs. 03) | |||||||
|---|---|---|---|---|---|---|---|---|
| Assessment Parameters | Overall Agreement, % | Score 1–2 Agreement, % | Score ≥ 3 Agreement, % | k | Overall Agreement, % | Score 1–2 Agreement, % | Score ≥ 3 Agreement, % | k |
| Residual aneurysmal neck OR > 20 p = 0.002 | 95.0 | 0 | 100 | 0.857 | 70.0 | 10.0 | 90.0 | 0.613 |
| Parent vessel status OR > 20 p = 0.002 | 90.0 | 0 | 100 | 0.783 | 65.0 | 10.0 | 90.0 | 0.565 |
| Degree of artifacts OR > 20 p = 0.002 | 85.0 | 0 | 100 | 0.736 | 70.0 | 10.0 | 90.0 | 0.630 |
| Stent apposition to vessel wall OR > 100 p < 0.001 | 85.0 | 0 | 100 | 0.348 | 75.0 | 35.0 | 55.0 | 0.734 |
| Stent strut delineation | 75.0 | 5.0 | 85.0 | 0.606 | 100 | 100 | 0 | 1.0 |
| Vessel wall status of stented segment | 75.0 | 0 | 100 | 0.464 | 95.0 | 100 | 0 | 0.857 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.C.; Fung, K.H.; Liu, S.H.; Ko, K.W.S.; Chan, N.L.; Mahboobani, N.R.; Shek, K.W.; Poon, T.L.; Poon, W.L. A Comparability Study Between Intravenous Contrast-Enhanced Cone-Beam Computed Tomography (CBCT) and Magnetic Resonance Angiography (MRA) on the Post-Treatment Follow-Up of Intracranial Aneurysms: A Single-Center Prospective Cohort Study. Diagnostics 2025, 15, 1774. https://doi.org/10.3390/diagnostics15141774
Lee MC, Fung KH, Liu SH, Ko KWS, Chan NL, Mahboobani NR, Shek KW, Poon TL, Poon WL. A Comparability Study Between Intravenous Contrast-Enhanced Cone-Beam Computed Tomography (CBCT) and Magnetic Resonance Angiography (MRA) on the Post-Treatment Follow-Up of Intracranial Aneurysms: A Single-Center Prospective Cohort Study. Diagnostics. 2025; 15(14):1774. https://doi.org/10.3390/diagnostics15141774
Chicago/Turabian StyleLee, Man Cho, King Him Fung, Shing Him Liu, Koel Wei Sum Ko, Nok Lun Chan, Neeraj Ramesh Mahboobani, Ka Wai Shek, Tak Lap Poon, and Wai Lun Poon. 2025. "A Comparability Study Between Intravenous Contrast-Enhanced Cone-Beam Computed Tomography (CBCT) and Magnetic Resonance Angiography (MRA) on the Post-Treatment Follow-Up of Intracranial Aneurysms: A Single-Center Prospective Cohort Study" Diagnostics 15, no. 14: 1774. https://doi.org/10.3390/diagnostics15141774
APA StyleLee, M. C., Fung, K. H., Liu, S. H., Ko, K. W. S., Chan, N. L., Mahboobani, N. R., Shek, K. W., Poon, T. L., & Poon, W. L. (2025). A Comparability Study Between Intravenous Contrast-Enhanced Cone-Beam Computed Tomography (CBCT) and Magnetic Resonance Angiography (MRA) on the Post-Treatment Follow-Up of Intracranial Aneurysms: A Single-Center Prospective Cohort Study. Diagnostics, 15(14), 1774. https://doi.org/10.3390/diagnostics15141774






